Is There a Difference in Brain Functional Connectivity between Chinese Coal Mine Workers Who Have Engaged in Unsafe Behavior and Those Who Have Not?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Demographic Information of the Subjects
2.2. Data Acquisition
2.3. Data Preprocessing
2.4. Resting-State Functional Connectivity Analysis
2.4.1. Pearson’s Correlation Coefficient and t-Test
2.4.2. Brain Network Analysis
3. Results
3.1. Demographic Information
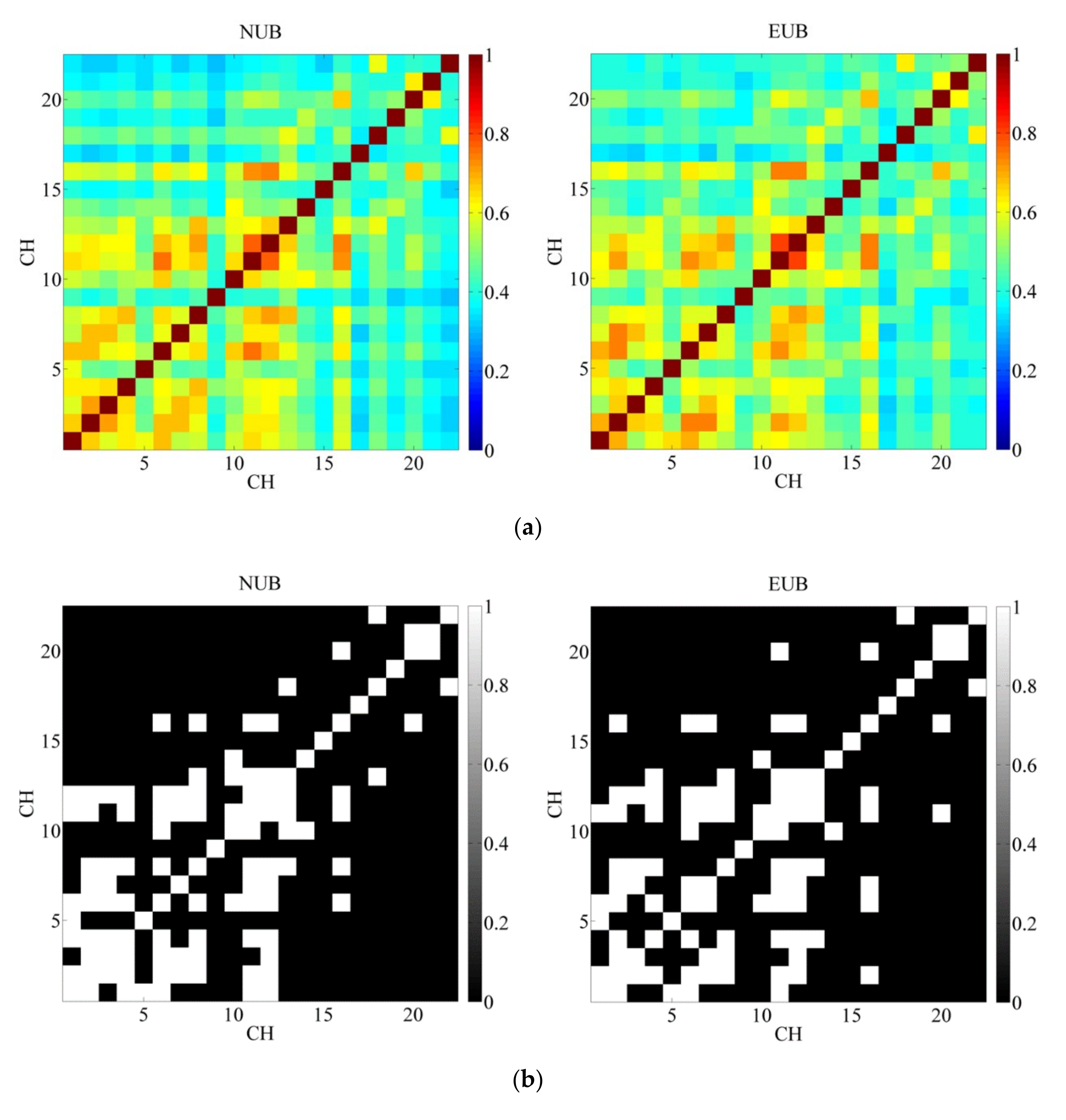
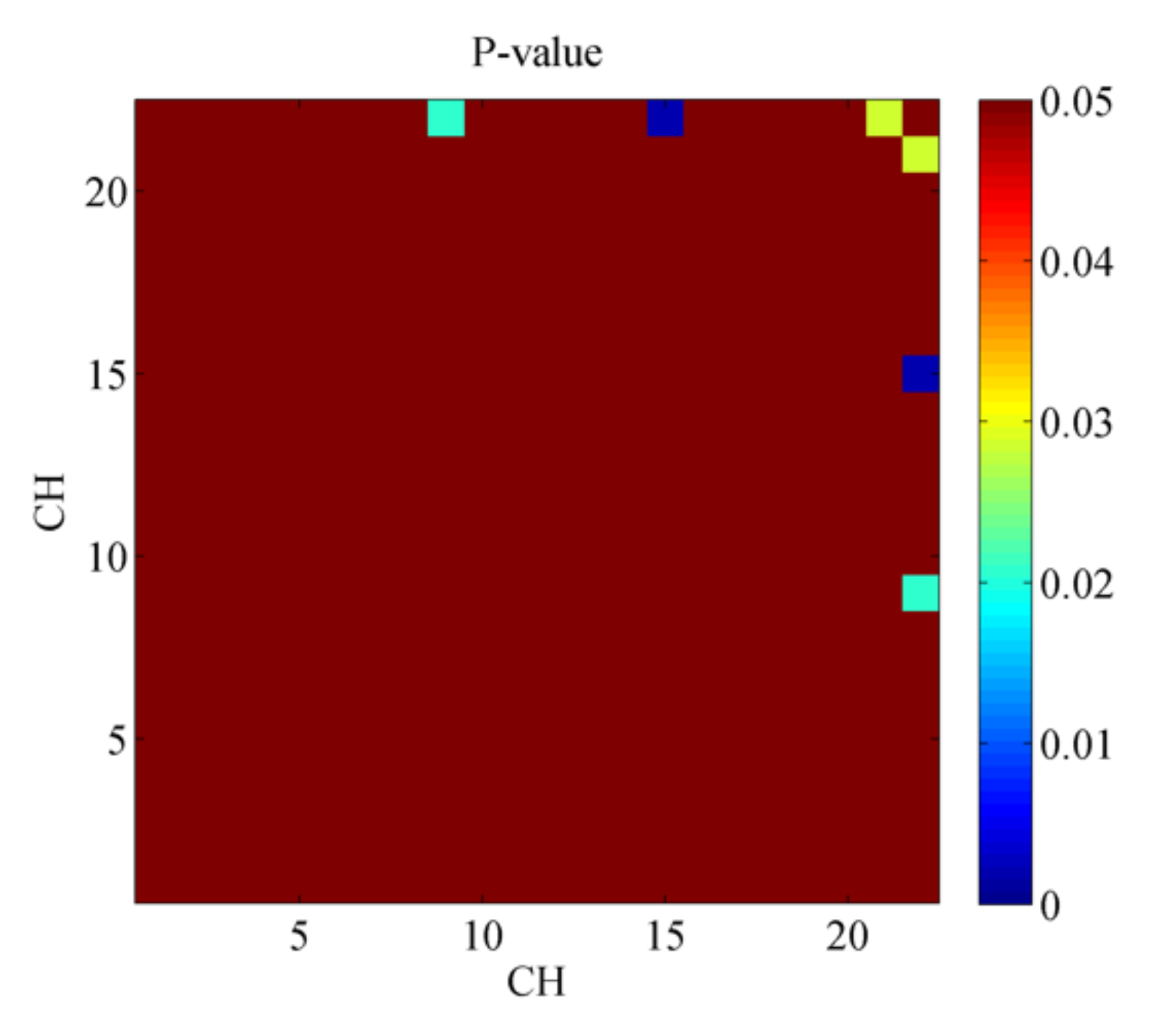
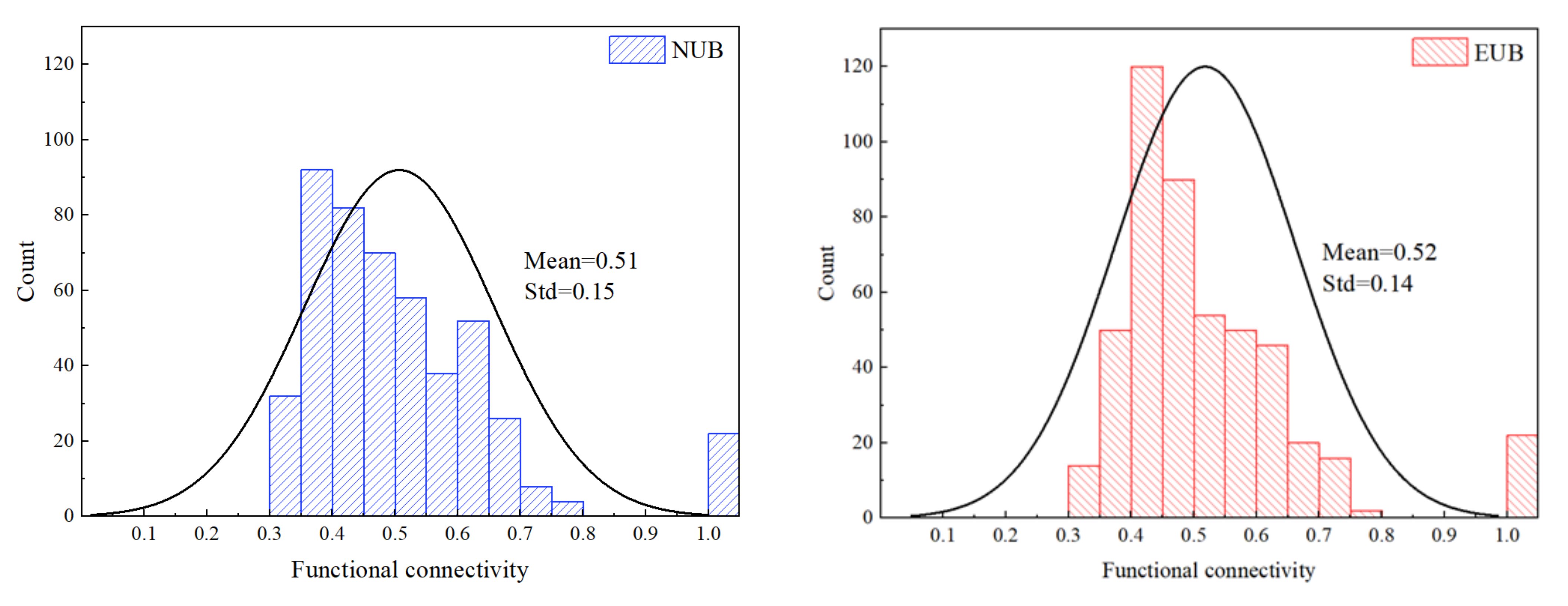
3.2. Pearson’s Correlation Coefficient and t-Test
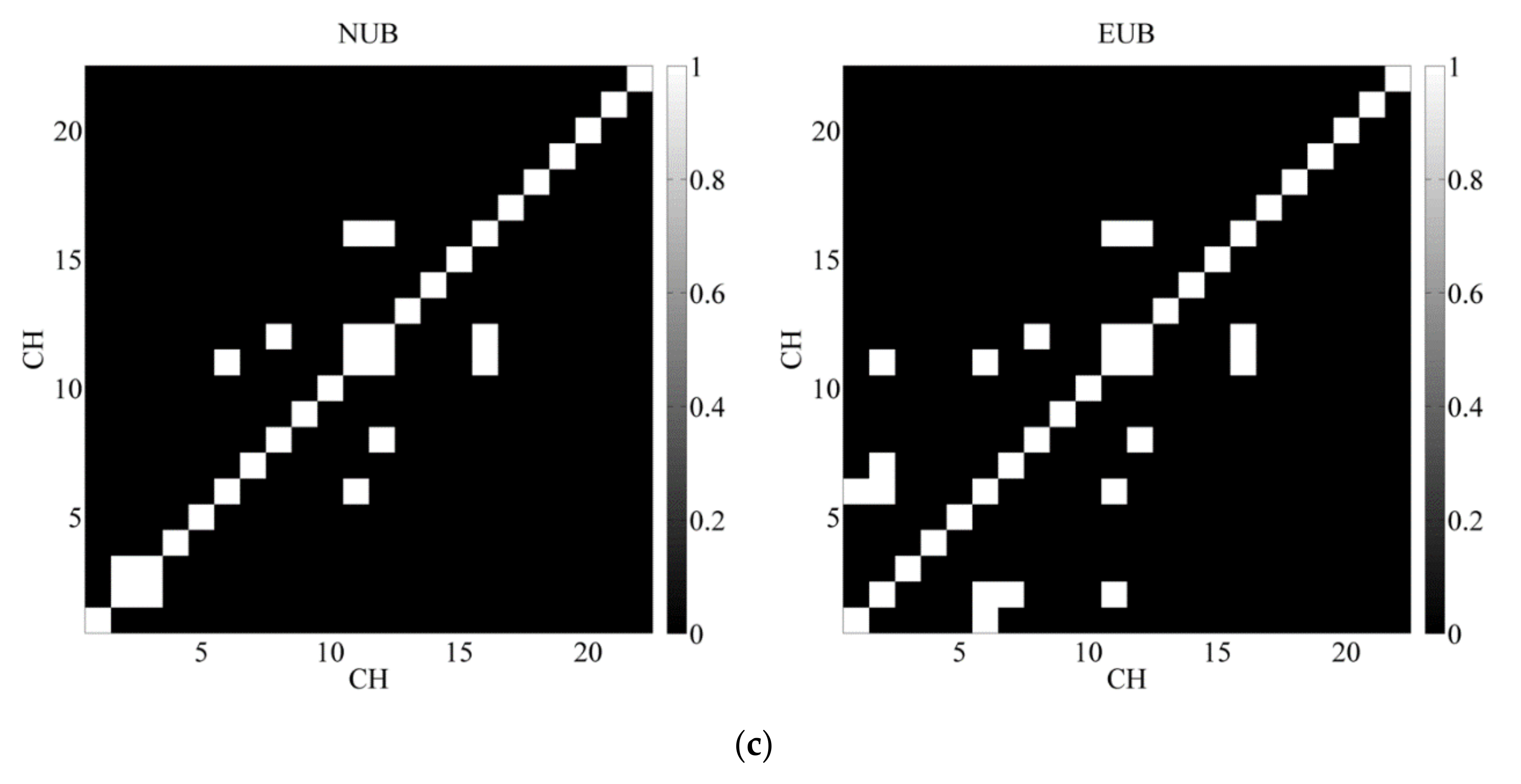
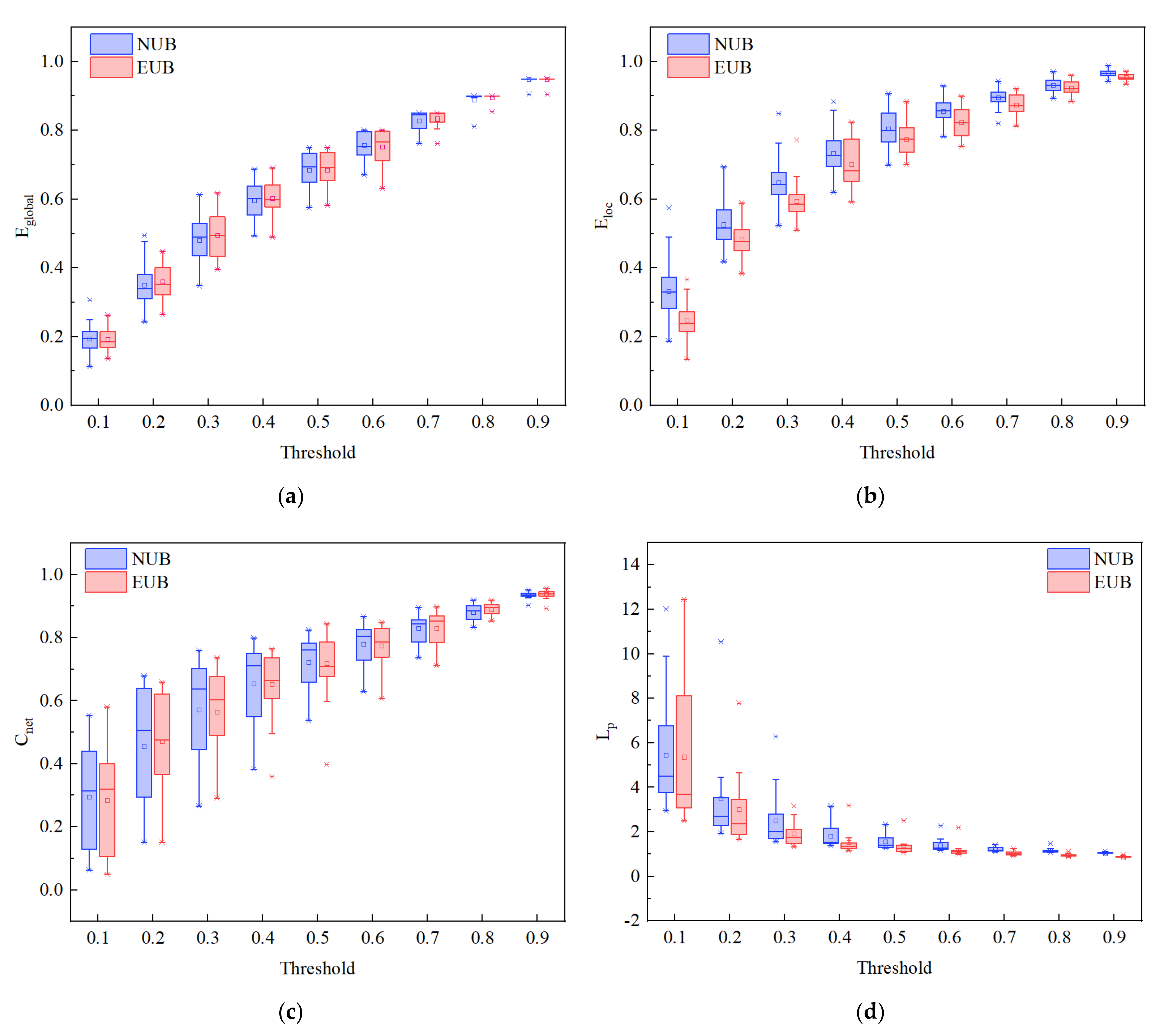
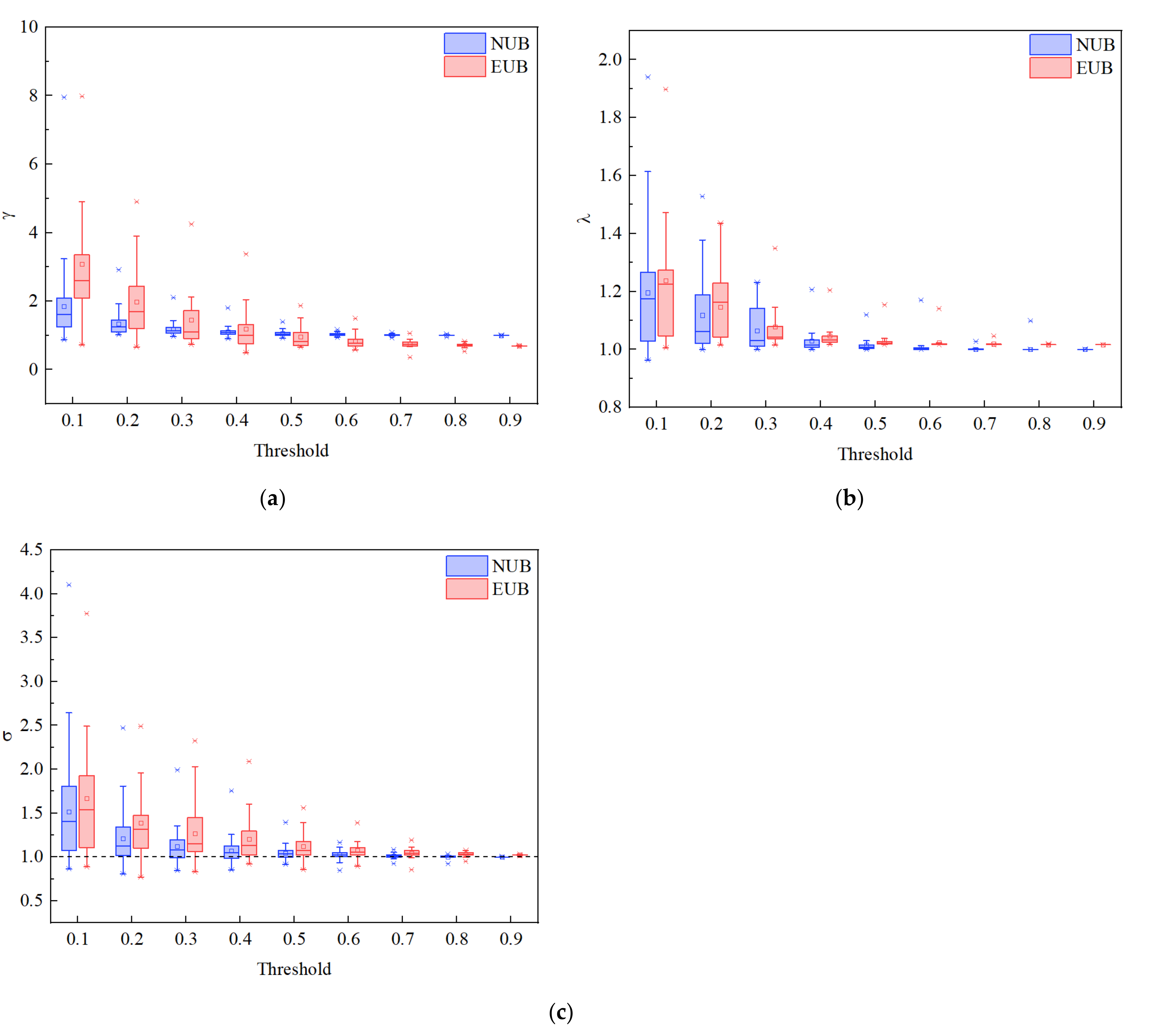
3.3. Brain Network Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goh, Y.M.; Ubeynarayana, C.U.; Wong, K.L.X.; Guo, B.H.W. Factors influencing unsafe behaviors: A supervised learning approach. Accid. Anal. Prev. 2018, 118, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, H.W. Industrial Accident Prevention. A Scientific Approach; McGraw-Hill Book Company, Inc.: New York, NY, USA; London, UK, 1941. [Google Scholar]
- Tong, R.; Yang, X.; Li, H.; Li, J. Dual process management of coal miners’ unsafe behaviour in the Chinese context: Evidence from a meta-analysis and inspired by the JD-R model. Resour. Policy 2019, 62, 205–217. [Google Scholar] [CrossRef]
- Chen, H.; Qi, H.; Long, R.; Zhang, M. Research on 10-year tendency of China coal mine accidents and the characteristics of human factors. Saf. Sci. 2012, 50, 745–750. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, J.; Hao, H.; Fu, G.; Nie, F.; Zhang, W. Root causes of coal mine accidents: Characteristics of safety culture deficiencies based on accident statistics. Process Saf. Environ. Prot. 2020, 136, 78–91. [Google Scholar] [CrossRef]
- Khanzode, V.V.; Maiti, J.; Ray, P.K. A methodology for evaluation and monitoring of recurring hazards in underground coal mining. Saf. Sci. 2011, 49, 1172–1179. [Google Scholar] [CrossRef]
- Shappell, S.A.; Wiegmann, D.A. A Human Error Approach to Aviation Accident Analysis; Ashgate Pub, Co.: Farnham, UK, 2003; ISBN 9780754618737. [Google Scholar]
- Shappell, S.; Detwiler, C.; Holcomb, K.; Hackworth, C.; Boquet, A.; Wiegmann, D.A. Human Error and Commercial Aviation Accidents: An Analysis Using the Human Factors Analysis and Classification System. Hum. Factors 2007, 49, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.M.; Shappell, S.A. Operator error and system deficiencies: Analysis of 508 mining incidents and accidents from Queensland, Australia using HFACS. Accid. Anal. Prev. 2010, 42, 1379–1385. [Google Scholar] [CrossRef]
- Bullmore, E.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef]
- Carlén, M. What constitutes the prefrontal cortex? Science 2017, 358, 478–482. [Google Scholar] [CrossRef] [Green Version]
- Herold, F.; Wiegel, P.; Scholkmann, F.; Müller, N.G. Applications of Functional Near-Infrared Spectroscopy (fNIRS) Neuroimaging in Exercise–Cognition Science: A Systematic, Methodology-Focused Review. J. Clin. Med. 2018, 7, 466. [Google Scholar] [CrossRef] [Green Version]
- Berkman, E.T.; Falk, E.B. Beyond Brain Mapping: Using Neural Measures to Predict Real-World Outcomes. Curr. Dir. Psychol. Sci. 2013, 22, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Borragán, G.; Peigneux, P. Applications of Functional Near-Infrared Spectroscopy in Fatigue, Sleep Deprivation, and Social Cognition. Brain Topogr. 2019, 32, 998–1012. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, M.; Quaresima, V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage 2012, 63, 921–935. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, G.; Dong, Q.; Niu, H. Applications of Resting-State fNIRS in the Developing Brain: A Review From the Connectome Perspective. Front. Neurosci. 2020, 14, 476. [Google Scholar] [CrossRef]
- Scholkmann, F.; Kleiser, S.; Metz, A.J.; Zimmermann, R.; Mata Pavia, J.; Wolf, U.; Wolf, M. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage 2014, 85, 6–27. [Google Scholar] [CrossRef]
- Wu, S.-T.; Rubianes Silva, J.A.I.; Novi, S.L.; de Souza, N.G.S.R.; Forero, E.J.; Mesquita, R.C. Accurate Image-guided (Re)Placement of NIRS Probes. Comput. Methods Programs Biomed. 2020, 200, 105844. [Google Scholar] [CrossRef]
- Aydin, E.A. Subject-Specific feature selection for near infrared spectroscopy based brain-computer interfaces. Comput. Methods Programs Biomed. 2020, 195, 105535. [Google Scholar] [CrossRef]
- Phillips, K.C.; Verbrigghe, D.; Gabe, A.; Jauquet, B.; Eischer, C.; Yoon, T. The Influence of Thermal Alterations on Prefrontal Cortex Activation and Neuromuscular Function during a Fatiguing Task. Int. J. Environ. Res. Public Health 2020, 17, 7194. [Google Scholar] [CrossRef]
- Blasi, A.; Lloyd-Fox, S.; Katus, L.; Elwell, C.E. fNIRS for Tracking Brain Development in the Context of Global Health Projects. Photonics 2019, 6, 89. [Google Scholar] [CrossRef] [Green Version]
- Lloyd-Fox, S.; Blasi, A.; McCann, S.; Rozhko, M.; Katus, L.; Mason, L.; Austin, T.; Moore, S.E.; Elwell, C.E.; Bright Project Team. Habituation and novelty detection fNIRS brain responses in 5- and 8-month-old infants: The Gambia and UK. Dev. Sci. 2019, 22, e12817. [Google Scholar] [CrossRef] [Green Version]
- Geng, S.J.; Liu, X.Y.; Biswal, B.B.; Niu, H.J. Effect of Resting-State fNIRS Scanning Duration on Functional Brain Connectivity and Graph Theory Metrics of Brain Network. Front. Neurosci. 2017, 11, 12. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Hu, Z.; Liu, L.; Li, H.; Qian, Q.; Niu, H. Disrupted functional brain connectivity networks in children with attention-deficit/hyperactivity disorder: Evidence from resting-state functional near-infrared spectroscopy. Neurophotonics 2020, 7, 1–13. [Google Scholar] [CrossRef]
- Ahn, S.; Nguyen, T.; Jang, H.; Kim, J.G.; Jun, S.C. Exploring Neuro-Physiological Correlates of Drivers’ Mental Fatigue Caused by Sleep Deprivation Using Simultaneous EEG, ECG, and fNIRS Data. Front. Hum. Neurosci. 2016, 10, 219. [Google Scholar] [CrossRef]
- Pinti, P.; Aichelburg, C.; Lind, F.; Power, S.; Swingler, E.; Merla, A.; Hamilton, A.; Gilbert, S.; Burgess, P.; Tachtsidis, I. Using Fiberless, Wearable fNIRS to Monitor Brain Activity in Real-world Cognitive Tasks. J. Vis. Exp. 2015, 106, e53336. [Google Scholar] [CrossRef] [Green Version]
- Fan, S.; Blanco-Davis, E.; Zhang, J.; Bury, A.; Warren, J.; Yang, Z.; Yan, X.; Wang, J.; Fairclough, S. The Role of the Prefrontal Cortex and Functional Connectivity during Maritime Operations: An fNIRS study. Brain Behav. 2021, 11, e01910. [Google Scholar] [CrossRef]
- Liu, T.; Pelowski, M.; Pang, C.; Zhou, Y.; Cai, J. Near-infrared spectroscopy as a tool for driving research. Ergonomics 2016, 59, 368–379. [Google Scholar] [CrossRef]
- Liu, T. Positive correlation between drowsiness and prefrontal activation during a simulated speed-control driving task. Neuroreport 2014, 25, 1316–1319. [Google Scholar] [CrossRef]
- Lohani, M.; Payne, B.R.; Strayer, D.L. A Review of Psychophysiological Measures to Assess Cognitive States in Real-World Driving. Front. Hum. Neurosci. 2019, 13, 57. [Google Scholar] [CrossRef]
- Tsunashima, H.; Yanagisawa, K. Measurement of Brain Function of Car Driver Using Functional Near-Infrared Spectroscopy (fNIRS). Comput. Intell. Neurosci. 2009, 2009, 164958. [Google Scholar] [CrossRef]
- Xu, L.; Wang, B.; Xu, G.; Wang, W.; Liu, Z.; Li, Z. Functional connectivity analysis using fNIRS in healthy subjects during prolonged simulated driving. Neurosci. Lett. 2017, 640, 21–28. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Xu, G.; Huo, C.; Tan, Q.; Li, Z.; Yuan, Q. Effective Connectivity Analysis of the Brain Network in Drivers during Actual Driving Using Near-Infrared Spectroscopy. Front. Behav. Neurosci. 2017, 11, 211. [Google Scholar] [CrossRef] [PubMed]
- Perpetuini, D.; Cardone, D.; Filippini, C.; Spadolini, E.; Mancini, L.; Chiarelli, A.M.; Merla, A. Can Functional Infrared Thermal Imaging Estimate Mental Workload in Drivers as Evaluated by Sample Entropy of the fNIRS Signal? BT, Proceedings of the 8th European Medical and Biological Engineering Conference, Portorož, Slovenia, 29 November—3 December 2020; Jarm, T., Cvetkoska, A., Mahnič-Kalamiza, S., Miklavcic, D., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 223–232. [Google Scholar]
- Hu, M.; Shealy, T.; Hallowell, M.; Hardison, D. Advancing Construction Hazard Recognition through Neuroscience: Measuring Cognitive Response to Hazards Using Functional near Infrared Spectroscopy. In Proceedings of the Construction Research Congress 2018: Safety and Disaster Management, New Orleans, LA, USA, 2–4 April 2018; pp. 134–143, Selected Papers from the Construction Research Congress 2018.. [Google Scholar]
- Shi, Y.; Zhu, Y.; Mehta, R.K.; Du, J. A neurophysiological approach to assess training outcome under stress: A virtual reality experiment of industrial shutdown maintenance using Functional Near-Infrared Spectroscopy (fNIRS). Adv. Eng. Informatics 2020, 46, 101153. [Google Scholar] [CrossRef]
- Dehais, F.; Dupres, A.; Di Flumeri, G.; Verdiere, K.; Borghini, G.; Babiloni, F.; Roy, R. Monitoring Pilot’s Cognitive Fatigue with Engagement Features in Simulated and Actual Flight Conditions Using an Hybrid fNIRS-EEG Passive BCI. In Proceedings of the 2018 IEEE International Conference on Systems, Man and Cybernetics (SMC), Miyazaki, Japan, 7–10 October 2018; pp. 544–549. [Google Scholar]
- Verdière, K.J.; Roy, R.N.; Dehais, F. Detecting Pilot’s Engagement Using fNIRS Connectivity Features in an Automated vs. Manual Landing Scenario. Front. Hum. Neurosci. 2018, 12, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, A.; Li, B.; Wang, X.; Zhang, S.; Zhu, Y.; Liu, W. NeuroDesignScience: An fNIRS-Based System Designed to Help Pilots Sustain Attention During Transmeridian Flights BT, Proceedings of the Intelligent Human Systems Integration 2021, Palermo, Italy, 22–24 February 2021; Russo, D., Ahram, T., Karwowski, W., di Bucchianico, G., Taiar, R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 165–170. [Google Scholar]
- Liu, R.; Cheng, W.; Yu, Y.; Xu, Q. Human factors analysis of major coal mine accidents in China based on the HFACS-CM model and AHP method. Int. J. Ind. Ergon. 2018, 68, 270–279. [Google Scholar] [CrossRef]
- Qiao, W.; Liu, Q.; Li, X.; Luo, X.; Wan, Y. Using data mining techniques to analyze the influencing factor of unsafe behaviors in Chinese underground coal mines. Resour. Policy 2018, 59, 210–216. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Wang, X.; Yu, H.; Bai, L.; Sun, Q. Exploring the impacts of factors contributing to unsafe behavior of coal miners. Saf. Sci. 2019, 115, 339–348. [Google Scholar] [CrossRef]
- Quick, B.L.; Stephenson, M.T.; Witte, K.; Vaught, C.; Booth-Butterfield, S.; Patel, D. An examination of antecedents to coal miners’ hearing protection behaviors: A test of the theory of planned behavior. J. Safety Res. 2008, 39, 329–338. [Google Scholar] [CrossRef]
- Harris, D.; Li, W.-C. Using Neural Networks to predict HFACS unsafe acts from the pre-conditions of unsafe acts. Ergonomics 2019, 62, 181–191. [Google Scholar] [CrossRef]
- Fuster, J.M. Chapter 10 The Prefrontal Cortex and Its Relation to Behavior. In Role of The Forebrain in Sensation and Behavior; Holstege, G.B.T.-P., Ed.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 87, pp. 201–211. ISBN 0079-6123. [Google Scholar]
- Szczepanski, S.M.; Knight, R.T. Insights into Human Behavior from Lesions to the Prefrontal Cortex. Neuron 2014, 83, 1002–1018. [Google Scholar] [CrossRef] [Green Version]
- Miller, E.K.; Cohen, J.D. An Integrative Theory of Prefrontal Cortex Function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Dai, C.; Shao, Y.; Wang, C.; Zhou, Q. Changes in ventromedial prefrontal cortex functional connectivity are correlated with increased risk-taking after total sleep deprivation. Behav. Brain Res. 2022, 418, 113674. [Google Scholar] [CrossRef]
- Borragán, G.; Guerrero-Mosquera, C.; Guillaume, C.; Slama, H.; Peigneux, P. Decreased prefrontal connectivity parallels cognitive fatigue-related performance decline after sleep deprivation. An optical imaging study. Biol. Psychol. 2019, 144, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Radel, R.; Brisswalter, J.; Perrey, S. Saving mental effort to maintain physical effort: A shift of activity within the prefrontal cortex in anticipation of prolonged exercise. Cogn. Affect. Behav. Neurosci. 2017, 17, 305–314. [Google Scholar] [CrossRef]
- Myrden, A.; Chau, T. Effects of user mental state on EEG-BCI performance. Front. Hum. Neurosci. 2015, 9, 308. [Google Scholar] [CrossRef] [Green Version]
- Dehais, F.; Hodgetts, H.M.; Causse, M.; Behrend, J.; Durantin, G.; Tremblay, S. Momentary lapse of control: A cognitive continuum approach to understanding and mitigating perseveration in human error. Neurosci. Biobehav. Rev. 2019, 100, 252–262. [Google Scholar] [CrossRef] [Green Version]
- Aghajani, H.; Garbey, M.; Omurtag, A. Measuring Mental Workload with EEG+fNIRS. Front. Hum. Neurosci. 2017, 11, 359. [Google Scholar] [CrossRef] [Green Version]
- Nosrati, R.; Vesely, K.; Schweizer, T.A.; Toronov, V. Event-related changes of the prefrontal cortex oxygen delivery and metabolism during driving measured by hyperspectral fNIRS. Biomed. Opt. Express 2016, 7, 1323–1335. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Zhang, M.; Wang, Y.; Liu, Z.; Huo, C.; Li, Z.; Huo, M. Functional connectivity analysis of distracted drivers based on the wavelet phase coherence of functional near-infrared spectroscopy signals. PLoS ONE 2017, 12, e0188329. [Google Scholar] [CrossRef] [Green Version]
- Mirelman, A.; Maidan, I.; Bernad-Elazari, H.; Nieuwhof, F.; Reelick, M.; Giladi, N.; Hausdorff, J.M. Increased frontal brain activation during walking while dual tasking: An fNIRS study in healthy young adults. J. Neuroeng. Rehabil. 2014, 11, 85. [Google Scholar] [CrossRef] [Green Version]
- Gateau, T.; Durantin, G.; Lancelot, F.; Scannella, S.; Dehais, F. Real-Time State Estimation in a Flight Simulator Using fNIRS. PLoS ONE 2015, 10, e0121279. [Google Scholar] [CrossRef]
- Fishburn, F.; Norr, M.; Medvedev, A.; Vaidya, C. Sensitivity of fNIRS to cognitive state and load. Front. Hum. Neurosci. 2014, 8, 76. [Google Scholar] [CrossRef]
- Adrian, C.; Hasan, A. The Age of Neuroergonomics: Towards Ubiquitous and Continuous Measurement of Brain Function with fNIRS. Jpn. Psychol. Res. 2018, 60, 374–386. [Google Scholar] [CrossRef]
- Khan, M.J.; Hong, K.-S. Passive BCI based on drowsiness detection: An fNIRS study. Biomed. Opt. Express 2015, 6, 4063–4078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.; Babawale, O.; Kim, T.; Jo, H.J.; Liu, H.; Kim, J.G. Exploring brain functional connectivity in rest and sleep states: A fNIRS study. Sci. Rep. 2018, 8, 16144. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Xu, J.; Li, J.; Peng, H.; Cai, T.; Li, X.; Wu, S.; Cao, W.; He, S. Decreased functional connectivity and disrupted neural network in the prefrontal cortex of affective disorders: A resting-state fNIRS study. J. Affect. Disord. 2017, 221, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wu, X.; Zhang, T.; Lin, F.; Sun, H.; Li, J. Narrowband Resting-State fNIRS Functional Connectivity in Autism Spectrum Disorder. Front. Hum. Neurosci. 2021, 15, 294. [Google Scholar] [CrossRef]
- Bassett, D.S.; Bullmore, E.T. Human brain networks in health and disease. Curr. Opin. Neurol. 2009, 22, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Qi, P.; Ru, H.; Gao, L.; Zhang, X.; Zhou, T.; Tian, Y.; Thakor, N.; Bezerianos, A.; Li, J.; Sun, Y. Neural Mechanisms of Mental Fatigue Revisited: New Insights from the Brain Connectome. Engineering 2019, 5, 276–286. [Google Scholar] [CrossRef]
- Qing-gui, C.; Kai, L.; Ye-jiao, L.; Qi-hua, S.; Jian, Z. Risk management and workers’ safety behavior control in coal mine. Saf. Sci. 2012, 50, 909–913. [Google Scholar] [CrossRef]
- China, C.P.G. of the P.R Special Provisions of the State Council on the Prevention of Production Safety Accidents in Coal Mines. Available online: http://www.gov.cn/gongbao/content/2005/content_91201.htm (accessed on 11 November 2021).
- Villringer, A.; Chance, B. Non-invasive optical spectroscopy and imaging of human brain function. Trends Neurosci. 1997, 20, 435–442. [Google Scholar] [CrossRef]
- Wang, J.; Dong, Q.; Niu, H. The minimum resting-state fNIRS imaging duration for accurate and stable mapping of brain connectivity network in children. Sci. Rep. 2017, 7, 6461. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Yuan, Z.; Niu, H. Reliability evaluation on weighted graph metrics of fNIRS brain networks. Quant. Imaging Med. Surg. 2019, 9, 832–841. [Google Scholar] [CrossRef]
- Urquhart, E.L.; Wang, X.; Liu, H.; Fadel, P.J.; Alexandrakis, G. Differences in Net Information Flow and Dynamic Connectivity Metrics Between Physically Active and Inactive Subjects Measured by Functional Near-Infrared Spectroscopy (fNIRS) During a Fatiguing Handgrip Task. Front. Neurosci. 2020, 14, 167. [Google Scholar] [CrossRef]
- Ye, J.C.; Tak, S.; Jang, K.E.; Jung, J.; Jang, J. NIRS-SPM: Statistical parametric mapping for near-infrared spectroscopy. Neuroimage 2009, 44, 428–447. [Google Scholar] [CrossRef]
- Yaqub, M.A.; Woo, S.-W.; Hong, K.-S. Effects of HD-tDCS on Resting-State Functional Connectivity in the Prefrontal Cortex: An fNIRS Study. Complexity 2018, 2018, 1613402. [Google Scholar] [CrossRef]
- Cope, M.; Delpy, D.T. System for long-term measurement of cerebral blood and tissue oxygenation on newborn infants by near infra-red transillumination. Med. Biol. Eng. Comput. 1988, 26, 289–294. [Google Scholar] [CrossRef]
- Duncan, A.; Meek, J.H.; Clemence, M.; Elwell, C.E.; Tyszczuk, L.; Cope, M.; Delpy, D. Optical pathlength measurements on adult head, calf and forearm and the head of the newborn infant using phase resolved optical spectroscopy. Phys. Med. Biol. 1995, 40, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Molavi, B.; Dumont, G.A. Wavelet-based motion artifact removal for functional near-infrared spectroscopy. Physiol. Meas. 2012, 33, 259–270. [Google Scholar] [CrossRef]
- Ji, X.; Quan, W.; Yang, L.; Chen, J.; Wang, J.; Wu, T. Classification of Schizophrenia by Seed-based Functional Connectivity using Prefronto-Temporal Functional Near Infrared Spectroscopy. J. Neurosci. Methods 2020, 344, 108874. [Google Scholar] [CrossRef]
- Sasai, S.; Homae, F.; Watanabe, H.; Sasaki, A.T.; Tanabe, H.C.; Sadato, N.; Taga, G. A NIRS–fMRI study of resting state network. Neuroimage 2012, 63, 179–193. [Google Scholar] [CrossRef]
- Fu, G.; Mondloch, C.J.; Ding, X.P.; Short, L.A.; Sun, L.; Lee, K. The neural correlates of the face attractiveness aftereffect: A functional near-infrared spectroscopy (fNIRS) study. Neuroimage 2014, 85, 363–371. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.-Y.; Lu, F.-M.; Wang, M.-Y.; Hu, Z.-S.; Zhang, J.; Chen, Z.-Y.; Armada-da-Silva, P.A.S.; Yuan, Z. Altered Functional Connectivity in the Motor and Prefrontal Cortex for Children with Down’s Syndrome: An fNIRS Study. Front. Hum. Neurosci. 2020, 14, 6. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Xu, S.; Ieong, H.F.-H.; Yuan, Z. Optical mapping of prefrontal activity in pathological gamblers. Appl. Opt. 2017, 56, 5948–5953. [Google Scholar] [CrossRef]
- Aarabi, A.; Huppert, T.J. Assessment of the effect of data length on the reliability of resting-state fNIRS connectivity measures and graph metrics. Biomed. Signal Process. Control 2019, 54, 101612. [Google Scholar] [CrossRef]
- Racz, F.S.; Mukli, P.; Nagy, Z.; Eke, A. Increased prefrontal cortex connectivity during cognitive challenge assessed by fNIRS imaging. Biomed. Opt. Express 2017, 8, 3842–3855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Lin, X.; Fu, G.; Sai, L.; Chen, H.; Yang, J.; Wang, M.; Liu, Q.; Yang, G.; Zhang, J.; et al. Mapping the small-world properties of brain networks in deception with functional near-infrared spectroscopy. Sci. Rep. 2016, 6, 25297. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.L.; Ung, W.C.; Lim, L.G.; Lu, C.-K.; Kiguchi, M.; Tang, T.B. Automated Thresholding Method for fNIRS-Based Functional Connectivity Analysis: Validation With a Case Study on Alzheimer’s Disease. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, A.; Lewis, C.M.; Committeri, G.; Snyder, A.Z.; Romani, G.L.; Corbetta, M. Individual variability in functional connectivity predicts performance of a perceptual task. Proc. Natl. Acad. Sci. USA 2012, 109, 3516–3521. [Google Scholar] [CrossRef] [Green Version]
- Humphries, M.D.; Gurney, K. Network ‘Small-World-Ness’: A Quantitative Method for Determining Canonical Network Equivalence. PLoS ONE 2008, 3, e0002051. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Xia, M.; Liao, X.; Evans, A.; He, Y. GRETNA: A graph theoretical network analysis toolbox for imaging connectomics. Front. Hum. Neurosci. 2015, 9, 386. [Google Scholar] [CrossRef] [Green Version]
- Niu, H.; Wang, J.; Zhao, T.; Shu, N.; He, Y. Revealing Topological Organization of Human Brain Functional Networks with Resting-State Functional near Infrared Spectroscopy. PLoS ONE 2012, 7, e45771. [Google Scholar] [CrossRef] [Green Version]
- Niu, H.; Li, Z.; Liao, X.; Wang, J.; Zhao, T.; Shu, N.; Zhao, X.; He, Y. Test-Retest Reliability of Graph Metrics in Functional Brain Networks: A Resting-State fNIRS Study. PLoS ONE 2013, 8, e72425. [Google Scholar] [CrossRef] [Green Version]
- Einalou, Z.; Maghooli, K.; Setarehdan, S.K.; Akin, A. Graph theoretical approach to functional connectivity in prefrontal cortex via fNIRS. Neurophotonics 2017, 4, 1–8. [Google Scholar] [CrossRef]
- Cao, W.; Zhu, H.; Li, Y.; Wang, Y.; Bai, W.; Lao, U.; Zhang, Y.; Ji, Y.; He, S.; Zou, X. The Development of Brain Network in Males with Autism Spectrum Disorders from Childhood to Adolescence: Evidence from fNIRS Study. Brain Sci. 2021, 11, 120. [Google Scholar] [CrossRef]
- Wang, M.-Y.; Zhang, J.; Lu, F.-M.; Xiang, Y.-T.; Yuan, Z. Neuroticism and conscientiousness respectively positively and negatively correlated with the network characteristic path length in dorsal lateral prefrontal cortex: A resting-state fNIRS study. Brain Behav. 2018, 8, e01074. [Google Scholar] [CrossRef]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef]
- Wang, M.-Y.; Lu, F.-M.; Hu, Z.; Zhang, J.; Yuan, Z. Optical mapping of prefrontal brain connectivity and activation during emotion anticipation. Behav. Brain Res. 2018, 350, 122–128. [Google Scholar] [CrossRef]
- Watts, D.J.; Strogatz, S.H. Collective dynamics of ‘small-world’ networks. Nature 1998, 393, 440–442. [Google Scholar] [CrossRef]
- Maslov, S.; Sneppen, K. Specificity and Stability in Topology of Protein Networks. Science 2002, 296, 910–913. [Google Scholar] [CrossRef] [Green Version]
- Sporns, O.; Zwi, J.D. The small world of the cerebral cortex. Neuroinformatics 2004, 2, 145–162. [Google Scholar] [CrossRef]
- Latora, V.; Marchiori, M. Efficient Behavior of Small-World Networks. Phys. Rev. Lett. 2001, 87, 198701. [Google Scholar] [CrossRef] [Green Version]
- Ieong, H.F.; Yuan, Z. Abnormal resting-state functional connectivity in the orbitofrontal cortex of heroin users and its relationship with anxiety: A pilot fNIRS study. Sci. Rep. 2017, 7, 46522. [Google Scholar] [CrossRef] [Green Version]
- Ieong, H.F.; Yuan, Z. Emotion recognition and its relation to prefrontal function and network in heroin plus nicotine dependence: A pilot study. Neurophotonics 2018, 5, 1–15. [Google Scholar] [CrossRef]
- Luo, J.; Li, H.; Yeung, P.S.; Chang, C. The association between media multitasking and executive function in Chinese adolescents: Evidence from self-reported, behavioral and fNIRS data. Cyberpsychology J. Psychosoc. Res. Cybersp. 2021, 15. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, J.; Jiang, X.; Zhou, G.; Chen, G.; Ding, X.P.; Fu, G.; Lee, K. Linking Resting-State Networks in the Prefrontal Cortex to Executive Function: A Functional Near Infrared Spectroscopy Study. Front. Neurosci. 2016, 10, 452. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Zhao, C.; Pan, X.; Qu, J.; Wei, J.; Li, C.; Liang, Y.; Zhang, X. Decoding different working memory states during an operation span task from prefrontal fNIRS signals. Biomed. Opt. Express 2021, 12, 3495–3511. [Google Scholar] [CrossRef]
- Curtis, C.E.; D’Esposito, M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn. Sci. 2003, 7, 415–423. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, R.H.; Whitfield-Gabrieli, S.; Dillon, D.G.; Goer, F.; Beltzer, M.; Minkel, J.; Smoski, M.; Dichter, G.; Pizzagalli, D.A. Dynamic Resting-State Functional Connectivity in Major Depression. Neuropsychopharmacology 2016, 41, 1822–1830. [Google Scholar] [CrossRef] [Green Version]
- Korponay, C.; Pujara, M.; Deming, P.; Philippi, C.; Decety, J.; Kosson, D.S.; Kiehl, K.A.; Koenigs, M. Impulsive-antisocial psychopathic traits linked to increased volume and functional connectivity within prefrontal cortex. Soc. Cogn. Affect. Neurosci. 2017, 12, 1169–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strotzer, M. One Century of Brain Mapping Using Brodmann Areas*. Clin. Neuroradiol. 2009, 19, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Heim, S.; Eickhoff, S.B.; Ischebeck, A.K.; Friederici, A.D.; Stephan, K.E.; Amunts, K. Effective connectivity of the left BA 44, BA 45, and inferior temporal gyrus during lexical and phonological decisions identified with DCM. Hum. Brain Mapp. 2009, 30, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Shallice, T.; Broadbent, D.E.; Weiskrantz, L. Specific impairments of planning. Philos. Trans. R. Soc. London. B, Biol. Sci. 1982, 298, 199–209. [Google Scholar] [CrossRef]
- Dimitrakopoulos, G.N.; Kakkos, I.; Dai, Z.; Wang, H.; Sgarbas, K.; Thakor, N.; Bezerianos, A.; Sun, Y. Functional Connectivity Analysis of Mental Fatigue Reveals Different Network Topological Alterations Between Driving and Vigilance Tasks. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Alavash, M.; Doebler, P.; Holling, H.; Thiel, C.M.; Gießing, C. Is functional integration of resting state brain networks an unspecific biomarker for working memory performance? Neuroimage 2015, 108, 182–193. [Google Scholar] [CrossRef]

| NUB (n = 80) | EUB (n = 26) | Chi-Square Test | One-Way ANOVA | |||
|---|---|---|---|---|---|---|
| Mean ± Std | Mean ± Std | χ2 | P1 | P2 | F | |
| Length of service/year | 9.00 ± 7.06 | 9.76 ± 7.02 | 0.831 | 1.000 | 0.961 | 0.154 |
| Height/cm | 172.88 ± 5.01 | 171.71 ± 4.49 | 1.319 | 0.966 | 0.304 | 1.226 |
| Age/year | 34.89 ± 6.77 | 36.38 ± 6.40 | 1.855 | 0.562 | 0.540 | 0.724 |
| Weight/kg | 69.50 ± 7.04 | 67.73 ± 7.97 | 1.306 | 0.802 | 0.199 | 1.579 |
| Marital status | - | - | 0.283 | 0.868 | 0.812 | 0.209 |
| Education information | - | - | 1.442 | 0.780 | 0.831 | 0.368 |
| NUB (n = 80) | UW (n = 26) | |||
|---|---|---|---|---|
| n | % | n | % | |
| Marital status | ||||
| Divorced | 1 | 1.2 | 0 | 0 |
| Married | 71 | 88.8 | 21 | 80.8 |
| Unmarried | 8 | 10.0 | 5 | 19.2 |
| Education information | ||||
| Bachelor’s degree | 6 | 7.5 | 3 | 11.5 |
| College | 12 | 15.0 | 5 | 19.2 |
| High school | 39 | 48.8 | 9 | 34.6 |
| Junior high school | 1 | 1.3 | 0 | 0 |
| Technical secondary school | 22 | 27.5 | 9 | 34.6 |
| CH | Brodmann Area | MNI Coordinates | Probability | ||
|---|---|---|---|---|---|
| x | y | z | |||
| CH01 | * 9—Dorsolateral prefrontal cortex | 31 | 48 | 42 | 0.7547 |
| CH02 | * 9—Dorsolateral prefrontal cortex | 11 | 58 | 41 | 0.9958 |
| CH03 | * 9—Dorsolateral prefrontal cortex | −12 | 58 | 41 | 1.0000 |
| CH04 | * 9—Dorsolateral prefrontal cortex | −29 | 48 | 39 | 0.6406 |
| CH05 | * 46—Dorsolateral prefrontal cortex | 43 | 51 | 28 | 0.6368 |
| CH06 | * 10—Frontopolar area | 21 | 65 | 28 | 0.6324 |
| CH07 | * 10—Frontopolar area | −1 | 64 | 26 | 0.8878 |
| CH08 | * 46—Dorsolateral prefrontal cortex | −22 | 63 | 28 | 0.8619 |
| CH09 | 45—pars triangularis Broca’s area | −39 | 51 | 27 | 0.9889 |
| CH10 | * 10—Frontopolar area | 31 | 67 | 14 | 0.8810 |
| CH11 | * 10—Frontopolar area | 12 | 72 | 16 | 1.0000 |
| CH12 | * 10—Frontopolar area | −13 | 72 | 15 | 1.0000 |
| CH13 | * 46—Dorsolateral prefrontal cortex | −31 | 64 | 16 | 0.7677 |
| CH14 | * 46—Dorsolateral prefrontal cortex | 40 | 64 | 0 | 0.3726 |
| CH15 | * 10—Frontopolar area | 21 | 72 | 3 | 0.4762 |
| CH16 | * 10—Frontopolar area | −4 | 71 | 4 | 0.7357 |
| CH17 | * 11—Orbitofrontal area | −21 | 72 | 5 | 0.6826 |
| CH18 | * 46—Dorsolateral prefrontal cortex | −39 | 62 | 3 | 0.9173 |
| CH19 | * 11—Orbitofrontal area | 29 | 69 | −8 | 0.8404 |
| CH20 | * 11—Orbitofrontal area | 11 | 73 | −7 | 0.9443 |
| CH21 | * 11—Orbitofrontal area | −13 | 72 | −6 | 0.9964 |
| CH22 | * 11—Orbitofrontal area | −32 | 66 | −6 | 0.5135 |
| Clustering Coefficient | Nodal Efficiency | Nodal Local Efficiency | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ROI | CH | Mean ± Sd | T-Value | p-Value | Mean ± Sd | T-Value | p-Value | Mean ± Sd | T-Value | p-Value | |||
| NUB | EUB | NUB | EUB | NUB | EUB | ||||||||
| * 9—Dorsolateral prefrontal cortex | 01 | 0.6 ± 0.1 | 0.59 ± 0.11 | 0.4812 | 0.6314 | 0.56 ± 0.01 | 0.57 ± 0.09 | −0.3753 | 0.7082 | 0.66 ± 0.11 | 0.65 ± 0.12 | 0.3351 | 0.7382 |
| * 9—Dorsolateral prefrontal cortex | 02 | 0.64 ± 0.1 | 0.62 ± 0.08 | 0.8237 | 0.4120 | 0.56 ± 0.01 | 0.59 ± 0.07 | −1.7021 | 0.0917 | 0.69 ± 0.09 | 0.69 ± 0.06 | 0.0159 | 0.9873 |
| * 9—Dorsolateral prefrontal cortex | 03 | 0.64 ± 0.12 | 0.61 ± 0.15 | 0.7971 | 0.4272 | 0.54 ± 0.01 | 0.51 ± 0.09 | 1.4707 | 0.1444 | 0.68 ± 0.12 | 0.65 ± 0.15 | 1.1885 | 0.2374 |
| * 9—Dorsolateral prefrontal cortex | 04 | 0.6 ± 0.11 | 0.6 ± 0.1 | −0.0185 | 0.9853 | 0.57 ± 0.01 | 0.55 ± 0.09 | 1.0231 | 0.3086 | 0.66 ± 0.11 | 0.65 ± 0.1 | 0.4286 | 0.6691 |
| * 46—Dorsolateral prefrontal cortex | 05 | 0.53 ± 0.16 | 0.54 ± 0.14 | −0.1626 | 0.8712 | 0.48 ± 0.02 | 0.5 ± 0.11 | −0.5690 | 0.5706 | 0.57 ± 0.17 | 0.58 ± 0.15 | −0.2154 | 0.8299 |
| * 10—Frontopolar area | 06 | 0.62 ± 0.07 | 0.6 ± 0.08 | 1.0348 | 0.3032 | 0.6 ± 001 | 0.6 ± 0.04 | −0.7637 | 0.4468 | 0.69 ± 0.06 | 0.68 ± 0.06 | 0.7059 | 0.4818 |
| * 10—Frontopolar area | 07 | 0.62 ± 0.13 | 0.63 ± 0.16 | −0.2504 | 0.8028 | 0.53 ± 0.01 | 0.54 ± 0.11 | −0.3812 | 0.7039 | 0.67 ± 0.14 | 0.68 ± 0.16 | −0.3188 | 0.7505 |
| * 46—Dorsolateral prefrontal cortex | 08 | 0.64 ± 0.07 | 0.56 ± 0.13 | 3.6304 | 0.0004 * | 0.58 ± 0.01 | 0.54 ± 0.12 | 2.0967 | 0.0384 * | 0.7 ± 0.06 | 0.63 ± 0.15 | 3.6598 | 0.0004 * |
| 45—pars triangularis Broca’s area | 09 | 0.45 ± 0.2 | 0.48 ± 0.17 | −0.7149 | 0.4763 | 0.41 ± 0.02 | 0.44 ± 0.16 | −0.7777 | 0.4385 | 0.48 ± 0.22 | 0.52 ± 0.19 | −0.7838 | 0.4350 |
| * 10—Frontopolar area | 10 | 0.58 ± 0.1 | 0.58 ± 0.1 | −0.2765 | 0.7827 | 0.56 ± 0.01 | 0.56 ± 0.07 | 0.2711 | 0.7869 | 0.64 ± 0.1 | 0.64 ± 0.1 | −0.2684 | 0.7889 |
| * 10—Frontopolar area | 11 | 0.61 ± 0.08 | 0.62 ± 0.05 | −0.4760 | 0.6351 | 0.6 ± 0.01 | 0.61 ± 0.02 | −1.1720 | 0.2439 | 0.69 ± 0.06 | 0.7 ± 0.03 | −0.6768 | 0.5000 |
| * 10—Frontopolar area | 12 | 0.62 ± 0.08 | 0.62 ± 0.08 | 0.0643 | 0.9489 | 0.6 ± 0.01 | 0.59 ± 0.06 | 0.5009 | 0.6175 | 0.69 ± 0.07 | 0.69 ± 0.08 | 0.2394 | 0.8113 |
| * 46—Dorsolateral prefrontal cortex | 13 | 0.58 ± 0.09 | 0.55 ± 0.12 | 1.4942 | 0.1382 | 0.58 ± 0.01 | 0.58 ± 0.06 | −0.0972 | 0.9228 | 0.65 ± 0.08 | 0.62 ± 0.11 | 1.5247 | 0.1304 |
| * 46—Dorsolateral prefrontal cortex | 14 | 0.53 ± 0.13 | 0.51 ± 0.16 | 0.4713 | 0.6384 | 0.5 ± 0.01 | 0.48 ± 0.13 | 0.9824 | 0.3282 | 0.58 ± 0.14 | 0.56 ± 0.17 | 0.4336 | 0.6655 |
| * 10—Frontopolar area | 15 | 0.47 ± 0.18 | 0.51 ± 0.17 | −1.0407 | 0.3004 | 0.43 ± 0.02 | 0.48 ± 0.14 | −1.4789 | 0.1422 | 0.51 ± 0.19 | 0.56 ± 0.18 | −1.1735 | 0.2433 |
| * 10—Frontopolar area | 16 | 0.61 ± 0.07 | 0.63 ± 0.09 | −1.1105 | 0.2694 | 0.6 ± 0.01 | 0.59 ± 0.05 | 0.6103 | 0.5430 | 0.68 ± 0.05 | 0.69 ± 0.07 | −0.9391 | 0.3499 |
| * 11—Orbitofrontal area | 17 | 0.44 ± 0.19 | 0.41 ± 0.2 | 0.7440 | 0.4585 | 0.39 ± 0.02 | 0.36 ± 0.16 | 0.9054 | 0.3673 | 0.48 ± 0.21 | 0.43 ± 0.22 | 0.9364 | 0.3513 |
| * 46—Dorsolateral prefrontal cortex | 18 | 0.53 ± 0.14 | 0.51 ± 0.18 | 0.5957 | 0.5527 | 0.5 ± 0.01 | 0.48 ± 0.14 | 0.8376 | 0.4042 | 0.57 ± 0.15 | 0.55 ± 0.19 | 0.6609 | 0.5101 |
| * 11—Orbitofrontal area | 19 | 0.46 ± 0.16 | 0.48 ± 0.19 | −0.4376 | 0.6626 | 0.43 ± 0.02 | 0.45 ± 0.13 | −0.4082 | 0.6840 | 0.5 ± 0.18 | 0.52 ± 0.19 | −0.3948 | 0.6938 |
| * 11—Orbitofrontal area | 20 | 0.52 ± 0.13 | 0.56 ± 0.13 | −1.3365 | 0.1843 | 0.52 ± 0.01 | 0.54 ± 0.1 | −0.9959 | 0.3216 | 0.58 ± 0.14 | 0.62 ± 0.13 | −1.2797 | 0.2035 |
| * 11—Orbitofrontal area | 21 | 0.44 ± 0.17 | 0.48 ± 0.19 | −1.0392 | 0.3011 | 0.42 ± 0.02 | 0.43 ± 0.15 | −0.3884 | 0.6985 | 0.47 ± 0.19 | 0.51 ± 0.2 | −0.9037 | 0.3682 |
| * 11—Orbitofrontal area | 22 | 0.41 ± 0.18 | 0.44 ± 0.16 | −0.6393 | 0.5241 | 0.38 ± 0.02 | 0.44 ± 0.13 | −1.7044 | 0.0913 | 0.44 ± 0.2 | 0.48 ± 0.18 | −0.8278 | 0.4097 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, F.; Li, H.; Tian, S.; Tian, C.; Shao, J. Is There a Difference in Brain Functional Connectivity between Chinese Coal Mine Workers Who Have Engaged in Unsafe Behavior and Those Who Have Not? Int. J. Environ. Res. Public Health 2022, 19, 509. https://doi.org/10.3390/ijerph19010509
Tian F, Li H, Tian S, Tian C, Shao J. Is There a Difference in Brain Functional Connectivity between Chinese Coal Mine Workers Who Have Engaged in Unsafe Behavior and Those Who Have Not? International Journal of Environmental Research and Public Health. 2022; 19(1):509. https://doi.org/10.3390/ijerph19010509
Chicago/Turabian StyleTian, Fangyuan, Hongxia Li, Shuicheng Tian, Chenning Tian, and Jiang Shao. 2022. "Is There a Difference in Brain Functional Connectivity between Chinese Coal Mine Workers Who Have Engaged in Unsafe Behavior and Those Who Have Not?" International Journal of Environmental Research and Public Health 19, no. 1: 509. https://doi.org/10.3390/ijerph19010509








