Abstract
Background: Lung cancer is the most common cancer worldwide. Evidence suggests self-management (SM) interventions benefit cancer patients. This review aims to determine the effectiveness of SM interventions for lung cancer patients. Method: Searches occurred in PubMed, Cinahl, ProQuest, Psych Info, Scopus, and Medline, using predefined criteria, assessing randomised controlled trials (RCTs). Results: Five hundred and eighty-seven studies were yielded, 10 RCTs met criteria. Of the total patient pool, 1001 of 1089 had Non-Small Cell Lung Cancer (NSCLC). Six studies tested home-based SM exercise, two studies SM education, and one each for diary utilisation and symptom reporting. Fatigue was the most targeted function. Other functions targeted included exercise capacity, anxiety, depression, quality of life (QoL), sleep quality, and symptom burden. Six studies met their primary endpoints (five SM exercise, one SM education). Positive outcomes are described for fatigue, anxiety/depression, sleep quality, self-efficacy, and exercise capacity. With exception to fatigue, early-stage NSCLC, younger age, female, never smokers, partnered patients experienced increased treatment effect. Conclusions: SM interventions improve outcomes among some lung cancer patients. Interventions targeting fatigue yield benefit despite histology, stage or gender and could encourage broader cohort engagement. Consideration of patient characteristics may predict SM effect. Effectiveness of home-based SM exercise by NSCLC stage and SM tailored to sociodemographic variables requires further research.
1. Introduction
Lung cancer is the most common cancer and leading cause of cancer death worldwide, contributing 11.6% of total cancer diagnosis and 18.4% of total cancer deaths [1].
Patients with lung cancer present as highly symptomatic with complex needs [2]. Pain, symptom distress, anxiety, dyspnoea, fatigue, and appetite loss can be found in over 90% of lung cancer patients [3]. Lung cancer treatment adds further complexities to the patient’s symptom burden. Treatment related adverse events for lung cancer include haematological abnormalities, hypertension, pneumonia, and treatment fatigue [4].
Cancer associated costs can further impact patient outcomes [5,6]. Patients managed in both third-party payer and free public health systems experience out of pocket expenses and indirect financial burden such as income loss [7]. The sequalae associated with financial burden for cancer patients sees a reduction in leisure activity, higher dependency on savings and selling of possessions resulting in lowered treatment compliance and higher stress [5,7]. Treatment evolution for lung cancer has shifted this disease to be considered as chronic in several situations [8]. Lung cancer as a chronic disease can exacerbate anxiety, intensify fear of recurrence, hinder life plans, and increase stigma-related challenges [8]. Several guidelines currently exist, focusing on the treatment of lung cancer. Examples include guidelines from the European Society for Medical Oncology (ESMO) and the National Comprehensive Cancer Network (NCCN). These guidelines mainly focus on lung cancer screening, and surgical, radiation and systemic interventions appropriate for treatment of specific histology and stages of lung cancer disease [9,10,11] The “Optimal Care Pathway for People Living with Lung Cancer” endorsed by Cancer Australia, acknowledge the need for supportive care in the management of physical, mental and spiritual associated with lung cancer [12]. None of the mentioned guidelines specifically discuss the role of SM interventions within the lung cancer cohort.
SM intervention among cancer patients is increasing. There has been a shift from the traditional provider to patient relationship. Individuals are increasingly playing a key role in their care [13,14,15]. SM is described as a person’s ability to manage their disease symptoms including treatment, physical, social and lifestyle changes [16,17,18,19]. SM has five identified components that include problem solving, decision making, resource utilisation, patients/provider relationship development and taking action [20,21]. Studies have shown that SM leads to better health, better healthcare, better doctor–patient relationships, and communication, as well as reductions in depression, fatigue, pain, and emergency room visits [22,23,24,25]. In the context of SM and cancer, the National Cancer Research Institute (NCRI) define SM for cancer patients as “Approaches used by the individual affected by cancer (or life limiting illness) and its effects to optimise living (with the illness and its effects)” [26].
The main body of evidence for SM as a tool to manage chronic disease has largely been explored in non-malignant disease, although data are emerging that cancer patients utilising SM experience better disease management and improved quality of life [27]. A literature review of SM programmes targeting cancer patients identified six established SM programmes which centred around SM education and SM guided exercise. These programmes were mainly used in patients with breast or prostate cancer due to incidence rates and high survivorship at five years post diagnosis [28]. Nonetheless, SM interventions for cancer patients are lagging other chronic conditions, possibly due to the complex nature of the disease [27]. To the best of our knowledge, there is no other systematic review which assesses the efficacy of SM interventions among those with lung cancer. This systematic review aims to fill this gap by collating all data about the effectiveness of SM interventions on patient outcomes among people living with lung cancer using RCTs.
2. Materials and Methods
This systematic review was registered with PROSPERO (registration number CRD42021253619). The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) framework was used for this review. The population, intervention, control, outcome framework forms the primary question “how effective are SM interventions at improving outcomes among patients with lung cancer?” The effectiveness of the SM interventions was determined by the result of the effect measure described in the respective studies. Secondary objectives assessed outcomes by intervention type, stage and histology of disease, patient socio-demographics, and influence of family/partners participation in SM activity. The inclusion and exclusion criteria are described in Table 1.

Table 1.
Study Criteria.
2.1. Search Strategy
Databases searched were EBSCOHOST (CINAHL, Medline, Psych Info, Ipswich, MA, USA) Pro Quest, Scopus, and PubMed. Keywords, Boolean operators, and truncation terms used were “lung cancer” or “lung malignancy” or “thoracic malignancy” or “lung tum*” or “lung aden*” or “lung carcinoma” or “thoracic cancer” or “NSCLC” or “lung neoplasm” and “self manage*” or “self-manage*” or “self car*” or “self-car*” or “self-efficacy*” or “self efficacy*” or “home-base*” or “home base*” or “self-regulat*” or “self regulat*”. Searches occurred in July and August 2021, and keywords commanded to appear in “title” and “title and abstract”. Searches aimed to be identical across all databases to maintain authenticity. No date parameters were enforced to allow for a comprehensive search.
2.2. Data Extraction
Endnote (Clarivate Analytics, Philadelphia, PA, USA) and Covidence (Covidence, Melbourne, Australia) software packages were used for collation and extraction purposes. Two reviewers (RR and HH) assessed studies prior to inclusion within this review. The Critical Appraisal Skills Programme (CASP) for RCTs was used to appraise the selected articles. RR conducted the initial appraisals for all studies. Reviewer HH and RR performed final checks on extraction and appraised data. Table 2 outlines the extracted data for each RCT. Table 3 outlines the quality assessment outcomes of the 10 RCTs using CASP for RCTs checklist.

Table 2.
Extraction Details of Included RCTs.

Table 3.
CASP Checklist for RCTs.
3. Results
The primary aim of this review is to establish how effective SM interventions are at improving patient outcomes among people with lung cancer. Due to the variability in the endpoints and their associated effect measures, a narrative synthesis approach has been adopted to describe the findings from the included studies. The heterogenous nature of the interventions and their associated effect measure prohibited the utility of a meta-analysis for this review.
3.1. Database Record Yield
Database searches yielded 587 studies; 252 duplications and 201 irrelevant articles were removed. One hundred and thirty-three articles were reviewed for inclusion. Eighty-two percent of studies were the wrong design (n = 109), eight RCTs met the inclusion criteria, and a further two studies were identified via reference list pearling. Search findings are documented in the PRISMA algorithm (Figure 1).
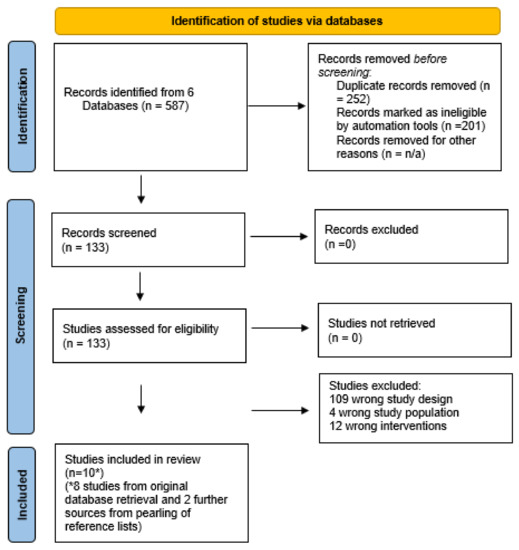
Figure 1.
Prisma Flow Chart. * confirms that two further studies were yielded via database pearling.
3.2. Quality Assessment of Included Studies
All studies provided a clear focus of research and were randomly assigned. Seven of the ten studies accounted for all patients at conclusion, one study had failure to follow up in five patients [33] and two studies could not be determined [36,39]. Due to the nature of studies, blinding was not possible for all stakeholders. All groups were treated equally and baseline characteristics, overall, were evenly distributed between control and intervention arms. All studies clearly defined a primary endpoint. Effect measures for endpoints were clearly outlined in all studies with results documented indicating if intervention was significant via a p value. Half of the studies specified a 95% confidence interval (CI). All results could be applied to local populations and consideration had been given to the outcomes appropriate for this cohort. One study found the primary endpoint yielded a negative outcome, which could be considered harmful in the intervention arm [34]. Eight of the studies stated that sample size was adequate, and two studies could not be determined. Two of the eight studies stating adequate sample size raised uncertainty of being sufficiently powered to detect an effect within their discussion. Seven of the studies recruited from single centres and three studies recruited over three sites each.
3.3. Effectiveness of SM Intervention among Patients with Lung Cancer
The total patient pool was 1089 patients. Baseline targeted function details were recorded for each study and at subsequent time points during the intervention period and at completion. Six of the 10 studies measured effect after the completion of the SM intervention [29,30,31,33,35,36]. Thirty days post intervention was the shortest follow up period [33] and six months from baseline was the longest follow up period [29,30,31]. The SM education studies were executed in the hospital setting [35,36] with Schofield et al. allowing for home-based education in the event a patient was not well enough to attend clinic. The remaining eight studies were conducted in the participant’s home or community. Six of the 10 studies met their primary endpoint which, on balance, supports the hypothesis that SM interventions utilised among patients with lung cancer are effective.
3.4. Outcomes by SM Intervention Type
Four intervention types were identified, targeting eight different patient functions. Exercise was the intervention of choice in 60% of the studies. Two studies utilised SM education [35,36], one study used a telephone-based symptom reporting tool [37] and the remaining study adopted a QoL diary [34]. Five of the six studies adopting exercise as the intervention met their primary endpoint. Telephone symptom reporting and a QoL diary failed to meet their primary endpoints. SM education provided mixed results, with one study meeting their primary endpoint.
Fatigue was the targeted function in three studies, all meeting their primary endpoints. One of the studies primarily targeting fatigue also had a primary endpoint for self-efficacy which also demonstrated significance [39]. Two studies focused on exercise capacity, with one study meeting its endpoint [33] and the other did not show significance [31]. The remaining five studies focused on various functions, with differing primary endpoint outcomes. Chen [29,30] demonstrated positive outcomes for anxiety/depression and sleep quality. The studies by Chen et al., were conducted in the same patient sample. In contrast, studies focusing on QoL, unmet needs, and symptom burden did not meet their primary endpoint [34,35,37]. Table 4 outlines these findings.

Table 4.
Function and Intervention Outcomes.
3.5. Outcomes for Studies including and Excluding Early-Stage NSCLC
Lung cancer histology was available for 1077 of the 1089 patients. NSCLC was reported for 92% of those 1077 patients (n = 1001). Staging for the 1001 NSCLC patients was documented in 8 of the 10 studies as stage I-IV, although one study amalgamated stage of disease for SCLC and NSCLC participants [38]. Definitive staging of NSCLC was available for 702 of the 1001 pooled NSCLC cohort (70%). Stage I-II accounted for 41% of all definitively staged NSCLC disease (n = 294) 25.5% was stage III NSCLC disease (n = 182) and 33% was stage IV NSCLC disease (n = 235).
Six of the 8 NSCLC staged studies indicated the inclusion of early-stage NSCLC disease (stage I-II), with five of the six studies availing the exact numerical breakdown by NSCLC stage. Of the 462 participants included within the 5 trials demonstrating a clear breakdown by NSCLC stage, most patients (63.6% n = 294) presented with early-stage disease. Only one of these five studies reported no significant difference between the control and intervention group for the primary endpoint [31]. This study accounted for only 1% (n = 3) of early-stage disease, with most participants having advanced disease. The four studies that account for the remaining 291 early-stage patients demonstrated significance across all primary endpoints, which included benefits to subjective and objective sleep [30], anxiety and depression [29], perioperative functional capacity [33] and fatigue [39]. The study where staging for SCLC and NSCLC was amalgamated [38], NSCLC accounted for most of the patient population (82% n = 75) and the study met its primary endpoint. All six studies including early-stage disease utilised physical activity as the primary intervention (with supplementary education and diary utilisation provided in five of these studies) to influence the effect measure. On balance, 83% of studies including early-stage disease met their primary endpoint.
Four studies excluded early-stage NSCLC, or their inclusion could not be determined [34,35,36,37], two of the studies staged their NSCLC participants (stage III-IV). The remaining studies stated “inoperable” NSCLC, which is more common in later stage (IIIb-c/IV) disease [40]. Three of these four studies included patients with SCLC and two for mesothelioma. Nonetheless, 78% of patients had NSCLC (n = 419). In contrast to interventions explored in studies including early-stage disease, interventions were more heterogenous in this group. Interventions included SM education [35,36], self-reporting of symptoms [37], and keeping a diary [34]. Outcomes for studies excluding early disease demonstrated mixed results. Only one of these four studies demonstrated benefit to the intervention group [36], this study was exclusively stage III-IV NSCLC patients and targeting fatigue. The remaining three studies which included SCLC, mesothelioma, and advanced NSCLC, failed to meet their primary endpoint. These three studies included patients with lower performance status when compared to Wangnum et al. and did not target fatigue. The study assessing the utility of keeping a diary demonstrated a decline in QoL in the intervention group suggesting the intervention had a negative effect on patient outcomes [34].
3.6. Outcomes for SCLC and Mesothelioma
SCLC and mesothelioma accounted for 6.2% and <1% of confirmed histology patients, respectively (n = 68 and n = 8). SCLC was represented in four of the 10 studies, with only one of those studies meeting the primary endpoint, which targeted fatigue [38]. The two studies including mesothelioma did not reach their endpoint [34,35]. The representation of SCLC and mesothelioma was small in comparison to NSCLC (92% versus 7%) therefore a degree of caution should be used in applying the findings to these cohorts.
3.7. Outcomes by Operability Status
Five of the 10 studies stipulated operability within their inclusion criteria. Three studies listing inoperable lung cancer as part of their inclusion criteria derived no benefit in the intervention group [31,34,35]. In contrast, studies where operable lung cancer made up part of the inclusion criteria, described a benefit in the intervention arm [33,39]. The latter two studies were restricted to patients with stages I-III NSCLC.
Overall, studies including early-stage NSCLC (I-II) and/or operable disease were more likely to meet their primary endpoint, which contrasted with studies excluding early-stage disease, including SCLC and mesothelioma and inoperable disease.
3.8. Patient Socio-Demographics and SM Outcomes
Socio-demographic variables describe the characteristics associated with individuals. Age, sex, education, smoking status, relationship/living arrangements have been identified as variables that can influence health outcomes [41,42,43] Background details for each variable is documented in Supplementary Table S1. Table 5 tabulates sociodemographic variables for each study included in this review.

Table 5.
Socio-Demographic Variables by Study.
3.9. Age
Participant age was recorded for all trials, with eight of the 10 studies recording a mean age for control and intervention groups. The pooled mean age for these eight studies is 61.86 years, which is almost one decade younger than the median age of lung cancer presentation [40]. The remaining two studies categorised age as ≤60 y/61–70 y/≥70 y [34] and ≤60 y/≥60 y [38]. Mills et al. had 32.5% of participants aged over 70 y. In contrast, age 70 y and above could not be determined in Zhang et al., although most patients (56%) were 60 years or younger. One study excluded patients over the age of 65 y [36] and another excluded patients over 70 y [33]. Both studies recorded positive outcomes in the intervention arms. In contrast, Mills et al., with one third of patients over 70 years did not demonstrate a positive outcome with the intervention. Of the six trials which showed a significant outcome, one excluded people over 65 years, one excluded over 70 years and one study included most participants less than 60 years. This review highlights two findings in reference to age. The first is that participants, overall, are younger than the average median age of lung cancer presentation which supports the argument of study outcomes being difficult to extrapolate to older cohorts. Secondly, where significance was reached, three studies were weighted to patients under 60 years. The latter suggests that younger patients may derive a larger benefit than their older counterparts.
3.10. Sex
Participant gender was evenly split when studies were pooled together. Female participants equated to 50.1% (n = 546). Of the six studies demonstrating significance, four contained more females, with a mean percentage of 59% [29,30,39]. This suggests that females have a larger benefit when utilising SM interventions compared to males. The remaining two studies with significance [36,38] weighted more to male participants. The latter studies were largely represented by participants under 60 years of age. This raises the question of younger age mitigating any adverse association with being male, having lung cancer and utilising SM interventions?
3.11. Education
Seven of the 10 studies documented participants’ level of education attainment. Five of these studies had significant outcomes, although the level of education was mixed and makes any association with education attainment and SM outcomes inconclusive. Further, the additional two studies that have not shown significance, most participants had completed high school or higher, thus further supporting an inconclusive finding.
3.12. Smoking Status
Three of the included studies recorded smoking status for participants. Two studies demonstrated significance and most patients were never smokers [33,38]. In contrast, Eadebrooke et al., contained a majority of current and former smokers and did not meet its endpoint.
3.13. Relationship Status and Living Arrangements
Half of the RCTs documented the martial and/or living arrangements for participants. All five studies documented most patients were either married and/or not living alone. Eighty percent of these studies (n = 4) met their primary endpoint.
3.14. SM Interventions Involving Family and Caregivers
None of the included studies focused on the involvement of family caregivers participating in SM interventions for patients with lung cancer. The inclusion criteria specified that interventions needed to be tested against a standard care control arm. Database searches yielded two RCTs that included family caregivers in the SM of patients with lung cancer. Both studies were excluded due to the wrong comparator being used. The studies were comparing the effectiveness of two interventions and did not include a standard care arm [44,45]. Both studies document a benefit to the involvement of family caregivers and SM interventions among patients with lung cancer, although the lack of a standard care arm makes it difficult to measure the degree of effect. This was acknowledged by Porter et al. [45].
4. Discussion
This review aims to assess the effectiveness of SM interventions among people with lung cancer. Overall, the utility of SM within this cohort appears to yield a positive effect. From the 587 database results, 453 results were removed (252 duplications and 201 irrelevant articles) of the remaining 133 screened articles, and pearling of references, 10 RCTs were included within this review. Overall, the majority of the included RCTs demonstrated that the intervention of SM among lung cancer patients exhibited a positive effect on patient outcomes and therefore, at first glance, favours the hypothesis that SM interventions are beneficial among patients with lung cancer. The studies however, tested four intervention types, targeting eight different functions, and included patients with three different histology, various disease staging, operability, and different sociodemographic variables. The heterogenous nature of the review makes it difficult to affiliate the hypothesis as a blanket rule for all lung cancer patients and a more tailored consideration is required.
All included articles provide sound rationale for their study, with clear commentary of their research aims and primary endpoints. Results were provided in conjunction with a p value. Further, all studies have clear randomisation and overall, equal distribution of characteristics are seen between the intervention and control arms. Eight of the studies specified that the sample size was adequate to detect an effect, although two of these studies commented on their uncertainty of being sufficiently powered [31,35]. The remaining two studies could not be determined [38,39]. When a study is not adequately powered, it can prohibit the research question from being answered as accurately as possible, which may be the case for up to four of the studies included within this review [46].
Seven of the studies were performed from single centres, which raises the question of result validity. Interestingly, all multiple site trials did not meet their primary endpoint and six of the seven single centres did meet their endpoints. Half of the studies had less than 100 participants. Small sample sizes increase the risk of distorting the results [47]. Single centre and small sample size were common themes identified as limitations throughout this review.
Due to the nature of the intervention, none of the studies were fully blinded, which introduces an element of bias to this review. A systematic review of RCTs that used patient reported outcomes which included blinded and non-blinded patients found that the effect size was exaggerated among unblinded patients [48].
Physical exercise has been proven in other studies covering various malignancies to reduce fatigue, depression, improve sleep, and improve clinical/functional outcomes which is documented in other systematic reviews with meta-analyses [49,50]. This review builds upon existing evidence that exercise yields improved patient outcomes among people with lung cancer. All 6 studies utilising SM exercise included early-stage NSCLC patients, and this cohort accounted for most of the pooled patients. There is existing data that exercise in early-stage lung cancer has positive effects [51]. This review however supports home-based exercise in this cohort which could be considered a cost-effective alternative to hospital-based programmes. Advanced NSCLC representation was not seen in all SM exercise studies. This makes it difficult to say with confidence if the effect of SM exercise was equally effective in early and later stage NSCLC. Compared to early stage disease, evidence suggests exercise outcomes are less certain in later stage disease, which should be considered in light of this review [51]. A systematic review of RCTs for hospital-based exercise in advanced lung cancer demonstrated a significant improvement for exercise capacity and QoL, but no improvement for fatigue, depression, and anxiety [52]. This finding contrasts to the current review for advanced lung cancer patients. The intervention setting was different between the two reviews (hospital based versus home-based) and the current review includes early-stage patients, which may have influenced the outcomes.
Beyond early-stage disease, SM exercise trials that were weighted to the female gender and evidence of being partnered demonstrated improved outcomes. Evidence states that there are some genetic and hormonal factors that influence cancer between the sexes [53]. Females experience better survival when surgery, radiotherapy and chemotherapy modalities are utilised when compared to men [53]. Marriage appears to have a desirable effect on cancer mortality rates. Further, social deprivation might be a factor leading to more advanced disease, co-morbidity, treatment morbidity and treatment access [54] It could be argued that the benefits of female gender and partnership yield a larger effect from exercise intervention.
In contrast to SM exercise studies, non-exercise studies tested a range of interventions across patients with more advanced and/or inoperable disease and various histology. The heterogeneity in interventions makes it difficult to draw a definitive conclusion. Only one of the non-exercise-based SM interventions met its primary endpoint (fatigue), which further supports that SM interventions are more effective in earlier stage NSCLC. Further, non-exercise interventions contained more males which supports the finding that SM interventions work better in females.
Early stage lung cancer is diagnosed in approximately 40% of patients, with locally advanced and advanced stage equating to 20% and 40%, respectively [40]. Goals of treatment are often different among these cohorts, ranging from a surgical and curative intent approach among stage I-IIIa NSCLC, to a palliative approach in the advanced setting [40]. SCLC, which accounts for approximately 13% of all lung cancers, and mesothelioma (incidence varies globally) both have poor prognosis with the aim of treatment being palliative care [40]. Patients with lung cancer often present with several co-morbidities which may influence function [55] It could be argued that different disease presentation and associated treatment pathways may influence how effective SM interventions among those with lung cancer, and possibly warrants further research.
Smoking is the biggest risk factor for developing lung cancer [56] and this may explain why only three of the trials included smoking status within their patients characteristics. Not surprisingly, studies represented by majority non-smokers met their endpoint which contrasted to the study containing majority smokers. Evidence for patients developing lung cancer and have a smoking history experience more guilt, shame, and perceived stigma than their non-smoking counterparts [57], further, evidence shows that ever smokers have a poorer performance status when compared to never smokers [58]. Smoking history is scant within this review. The trend seen for effective SM outcomes in non-smokers, however, raises the need to develop SM interventions that mitigates any prohibitive effect of being a current or former smoker.
The mean age could be determined for 80% of the pooled patients and shown to be almost a decade lower than the median age at diagnosis for lung cancer; consideration should be given to the extrapolation of this review to elderly patients. This limitation is not exclusive to this review, studies world over tend to recruit subjects that are aged 18 to 65 years, despite the elderly being one of the fastest growing populations worldwide. Fortunately, the sea of change is approaching with medical agencies in Europe, Canada and India making recommendations for adequate geriatric representation in medical trials moving forward [59].
Half of the studies recorded if patients were partnered or lived alone, with most participants identifying as married or not living alone. This review supports evidence that there are improved outcomes in cancer among those with a partner [60]. This review failed to test if partner/family involvement with SM intervention improved outcomes. Two studies identified in the search included family participation when utilising SM interventions. The studies however compared the effect of two interventions and no comparison was made against a control arm, which excluded them from this review. Family involvement of SM interventions tested against a control arm warrants further research to establish the true effect of their role in SM interventions for people with lung cancer.
Irrespective of histology, disease stage, and some sociodemographic presentation, fatigue as a primary endpoint was significantly improved in all three studies included in this review. Fatigue is the most frequently reported adverse event associated with lung cancer and is seen from diagnosis until end of life in 57–100% of lung cancer patients. Fatigue experienced by patients with lung cancer can have a negative impact on QoL [55]. This finding could help incentivise patients to adopt SM interventions as a proven way to mitigate the effects of fatigue and extends the benefit of SM beyond those with early-stage NSCLC disease, female gender, never smokers and partnered.
This systematic review has several limitations. All studies were not fully blinded, which may lead to bias. Small sample size and single centre trials were evident in 50% and 70%, respectively, and may distort the validity of the outcomes. None of the studies had follow up beyond six months which limits the durability of the SM interventions beyond this time frame. Two of the studies highlighted the low-cost low-technology natures of their trials, which may have introduced administrative errors. Two studies reported difficulty in patient recruitment and high attrition rates suggesting the engagement with this cohort is challenging. Three studies highlighted the challenges in overseeing activities in the control arm of their respective studies and one study highlighted that relying on patient adherence to the intervention and any subsequent patient reporting was difficult. These confounding factors may have influenced the results within this review.
5. Conclusions
This review demonstrates that the role of SM in lung cancer appears to have a positive effect on improving patient outcomes. Exercise was the most used intervention and was largely effective in at improving features of fatigue, sleep quality, anxiety, depression, and exercise capacity. The effect of SM interventions appears to be most pronounced in early-stage NSCLC disease. Being female, partnered and a non-smoker also appear to support improved SM intervention outcomes. Fatigue yielded positive outcomes irrespective of patient features and interventions, demonstrating utility across the broad spectrum of this disease, which is clinically meaningful. More research is needed to determine effectiveness of home-based SM exercise by NSCLC disease stage. Further, this review identifies that the development of tailored SM interventions which consider disease stage/histology, gender, partner status, smoking history and age should be explored to enhance a more precise SM intervention effect for all lung cancer patients.
Supplementary Materials
The following is available online at https://www.mdpi.com/article/10.3390/ijerph19010536/s1, Table S1: Socio-demographic Variables.
Author Contributions
Both authors conceptualized and designed the study. R.A.R. performed searches and assessed the eligibility of the papers. H.H. critically assessed the eligible studies and extracted data. R.A.R. analyzed and interpreted the data and drafted the manuscript. Both authors critically reviewed the manuscript. H.H. reviewed and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
Thank you to Shalini Vinod, Radiation Oncologist, Liverpool Hospital, NSW, Australia and Melvin Chin, Medical Oncologist, Prince of Wales Hospital, NSW, Australia for their expert guidance in developing this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Cooley, M.E. Symptoms in Adults with Lung Cancer: A Systematic Research Review. J. Pain Symptom Manag. 2000, 19, 137–153. [Google Scholar] [CrossRef]
- Iyer, S.; Taylor-Stokes, G.; Roughley, A. Symptom burden and quality of life in advanced non-small cell lung cancer patients in France and Germany. Lung Cancer 2013, 81, 288–293. [Google Scholar] [CrossRef]
- Bittoni, M.A.; Arunachalam, A.; Li, H.; Camacho, R.; He, J.; Zhong, Y.; Lubiniecki, G.M.; Carbone, D.P. Real-World Treatment Patterns, Overall Survival, and Occurrence and Costs of Adverse Events Associated with First-line Therapies for Medicare Patients 65 Years and Older With Advanced Non–small-cell Lung Cancer: A Retrospective Study. Clin. Lung Cancer 2018, 19, e629–e645. [Google Scholar] [CrossRef]
- De Souza, J.A.; Yap, B.J.; Hlubocky, F.J.; Wroblewski, K.; Ratain, M.J.; Cella, D.; Daugherty, C.K. The development of a financial toxicity patient-reported outcome in cancer: The COST Measure. Cancer 2014, 120, 3245–3253. [Google Scholar] [CrossRef]
- Cerni, J.; Rhee, J.; Hosseinzadeh, H. End-of-Life Cancer Care Resource Utilisation in Rural Versus Urban Settings: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 4955. [Google Scholar] [CrossRef]
- Ezeife, D.A.; Morganstein, B.J.; Lau, S.; Law, J.H.; Le, L.W.; Bredle, J.; Cella, D.; Doherty, M.K.; Bradbury, P.; Liu, G.; et al. Financial Burden Among Patients With Lung Cancer in a Publically Funded Health Care System. Clin. Lung Cancer 2019, 20, 231–236. [Google Scholar] [CrossRef]
- Rigney, M. ES05.03 From Living Longer to Also Living Better; Managing Lung Cancer as a Chronic Disease—The Principle of Survivorship. J. Thorac. Oncol. 2019, 14, S25. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. NCCN Guidelines Insights: Non–Small Cell Lung Cancer, Version 2.2021: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2021, 19, 254–266. [Google Scholar] [CrossRef]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef]
- Vansteenkiste, J.; De Ruysscher, D.; Eberhardt, W.E.; Lim, E.; Senan, S.; Felip, E.; Peters, S.; ESMO Guidelines Working Group. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24, 89–98. [Google Scholar] [CrossRef]
- Victoria, C.C. Optimal Care Pathway for People with Lung Cancer; Cancer Council Victoria and Department of Health Victoria: Melbourne, Australia, 2021. [Google Scholar]
- Grady, P.A.; Gough, L.L. Self-management: A comprehensive approach to management of chronic conditions. Am. J. Public Health 2014, 104, e25–e31. [Google Scholar] [CrossRef]
- Ansari, R.M.; Harris, M.; Hosseinzadeh, H.; Zwar, N. Self-management experiences among middle-aged population of rural area of Pakistan with type 2 diabetes: A qualitative analysis. Clin. Epidemiol. Glob. Health 2019, 7, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Yadav, U.N.; Hosseinzadeh, H.; Baral, K.P. Self-management and patient activation in COPD patients: An evidence summary of randomized controlled trials. Clin. Epidemiol. Glob. Health 2018, 6, 148–154. [Google Scholar] [CrossRef] [Green Version]
- Barlow, J.; Wright, C.; Sheasby, J.; Turner, A.; Hainsworth, J. Self-management approaches for people with chronic conditions: A review. Patient Educ. Couns. 2002, 48, 177–187. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Shnaigat, M. Effectiveness of chronic obstructive pulmonary disease self-management interventions in primary care settings: A systematic review. Aust. J. Prim. Health 2019, 25, 195–204. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Verma, I.; Gopaldasani, V. Patient activation and Type 2 diabetes mellitus self-management: A systematic review and meta-analysis. Aust. J. Prim. Health 2020, 26, 431–442. [Google Scholar] [CrossRef]
- Yadav, U.N.; Lloyd, J.; Hosseinzadeh, H.; Baral, K.P.; Bhatta, N.; Harris, M.F. Levels and determinants of health literacy and patient activation among multi-morbid COPD people in rural Nepal: Findings from a cross-sectional study. PLoS ONE 2020, 15, e0233488. [Google Scholar] [CrossRef]
- Lorig, K.R.; Holman, H.R. Self-management education: History, definition, outcomes, and mechanisms. Ann. Behav. Med. 2003, 26, 1–7. [Google Scholar] [CrossRef]
- Ghofranipour, F.; Ghaffarifar, S.; Ahmadi, F.; Hosseinzadeh, H.; Akbarzadeh, A. Improving interns’ patient–physician communication skills: Application of self-efficacy theory, a pilot study. Cogent Psychol. 2018, 5, 1524083. [Google Scholar] [CrossRef]
- Ory, M.G.; Ahn, S.; Jiang, L.; Smith, M.L.; Ritter, P.L.; Whitelaw, N.; Lorig, K. Successes of a National Study of the Chronic Disease Self-Management Program: Meeting the Triple Aim of Health Care Reform. Med. Care 2013, 51, 992–998. [Google Scholar] [CrossRef]
- Ansari, R.M.; Harris, M.; Hosseinzadeh, H.; Zwar, N. Healthcare Professionals’ Perspectives of Patients’ Experiences of the Self-Management of Type 2 Diabetes in the Rural Areas of Pakistan: A Qualitative Analysis. Int. J. Environ. Res. Public Health 2021, 18, 9869. [Google Scholar] [CrossRef]
- Niknami, M.; Mirbalouchzehi, A.; Zareban, I.; Kalkalinia, E.; Rikhtgarha, G.; Hosseinzadeh, H. Association of health literacy with type 2 diabetes mellitus self-management and clinical outcomes within the primary care setting of Iran. Aust. J. Prim. Health 2018, 24, 162–170. [Google Scholar] [CrossRef]
- Shnaigat, M.; Downie, S.; Hosseinzadeh, H. Effectiveness of Health Literacy Interventions on COPD Self-Management Outcomes in Outpatient Settings: A Systematic Review. COPD J. Chronic Obstr. Pulm. Dis. 2021, 18, 367–373. [Google Scholar] [CrossRef]
- Foster, C.; Brown, J.; Killen, M.; Brearley, S. The NCRI Cancer Experiences Collaborative: Defining self management. Eur. J. Oncol. Nurs. 2007, 11, 295–297. [Google Scholar] [CrossRef]
- Howell, D.; Richardson, A.; May, C.; Calman, L.; Fazelzad, R.; Moradian, S.; Foster, C. Implementation of self-management support in cancer care and normalization into routine practice: A systematic scoping literature review protocol. Syst. Rev. 2019, 8, 37–47. [Google Scholar] [CrossRef]
- Gao, W.J.; Yuan, C.R. Self-management programme for cancer patients: A literature review. Int. Nurs. Rev. 2011, 58, 288–295. [Google Scholar] [CrossRef]
- Chen, H.M.; Tsai, C.M.; Wu, Y.C.; Lin, K.C.; Lin, C.C. Randomised controlled trial on the effectiveness of home-based walking exercise on anxiety, depression and cancer-related symptoms in patients with lung cancer. Br. J. Cancer 2015, 112, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.M.; Tsai, C.M.; Wu, Y.C.; Lin, K.C.; Lin, C.C. Effect of walking on circadian rhythms and sleep quality of patients with lung cancer: A randomised controlled trial. Br. J. Cancer 2016, 115, 1304–1312. [Google Scholar] [CrossRef] [Green Version]
- Edbrooke, L.; Aranda, S.; Granger, C.L.; McDonald, C.F.; Krishnasamy, M.; Mileshkin, L.; Clark, R.A.; Gordon, I.; Irving, L.; Denehy, L. Multidisciplinary home-based rehabilitation in inoperable lung cancer: A randomised controlled trial. Thorax 2019, 74, 787–796. [Google Scholar] [CrossRef]
- Edbrooke, L.; Aranda, S.; Granger, C.L.; McDonald, C.F.; Krishnasamy, M.; Mileshkin, L.; Irving, L.; Braat, S.; Clark, R.A.; Gordon, I.; et al. Benefits of home-based multidisciplinary exercise and supportive care in inoperable non-small cell lung cancer—Protocol for a phase II randomised controlled trial. BMC Cancer 2017, 17, 663. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Qiu, T.; Pei, L.; Zhang, Y.; Xu, L.; Cui, Y.; Liang, N.; Li, S.; Chen, W.; Huang, Y. Two-week multimodal prehabilitation program improves perioperative functional capability in patients undergoing thoracoscopic lobectomy for lung cancer: A randomized controlled trial. Anesth. Analg. 2020, 131, 840–849. [Google Scholar] [CrossRef]
- Mills, M.E.; Murray, L.J.; Johnston, B.T.; Cardwell, C.; Donnelly, M. Does a Patient-Held Quality-of-Life Diary Benefit Patients With Inoperable Lung Cancer? J. Clin. Oncol. 2009, 27, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Schofield, P.; Ugalde, A.; Gough, K.; Reece, J.; Krishnasamy, M.; Carey, M.; Ball, D.; Aranda, S. A tailored, supportive care intervention using systematic assessment designed for people with inoperable lung cancer: A randomised controlled trial. Psycho-Oncology 2013, 22, 2315–2453. [Google Scholar] [CrossRef]
- Wangnum, K.; Thanarojanawanich, T.; Chinwatanachai, K.; Jamprasert, L.; Maleehuan, O.; Janthakun, V. Impact of the multidisciplinary education program in self-care on fatigue in lung cancer patients receiving chemotherapy. J. Med. Assoc. Thail. Chotmaihet Thangphaet 2013, 96, 1601–1608. [Google Scholar]
- Yount, S.E.; Rothrock, N.; Bass, M.; Beaumont, J.L.; Pach, D.; Lad, T.; Patel, J.; Corona, M.; Weiland, R.; Del Ciello, K.; et al. A randomized trial of weekly symptom telemonitoring in advanced lung cancer. J. Pain Symptom Manag. 2014, 47, 973–989. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Wang, S.Z.; Chen, H.L.; Yuan, A.Z. Tai Chi Exercise for Cancer-Related Fatigue in Patients With Lung Cancer Undergoing Chemotherapy: A Randomized Controlled Trial. J. Pain Symptom Manag. 2016, 51, 504–511. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Han, S.; Chu, H.; Wang, M.; Chen, S. Influence of self-management exercise intervention on the cancer related fatigue severity and self-management efficacy of patients with non-small cell lung cancer after operation. JPMA J. Pak. Med. Assoc. 2020, 70, 88–93. [Google Scholar]
- Ganti, A.K.; Gerber, D.E. Lung Cancer (Oxford American Oncology Library); Oxford University Press: New York, NY, USA, 2013. [Google Scholar]
- Beckmann, K.R.; Bennett, A.; Young, G.P.; Cole, S.R.; Joshi, R.; Adams, J.; Singhal, N.; Karapetis, C.; Wattchow, D.; Roder, D. Sociodemographic disparities in survival from colorectal cancer in South Australia: A population-wide data linkage study. BMC Health Serv. Res. 2016, 16, 24. [Google Scholar] [CrossRef] [Green Version]
- McIntyre, A.; Ganti, A.K. Lung cancer—A global perspective. J. Surg. Oncol. 2017, 115, 550–554. [Google Scholar] [CrossRef]
- Willers, C.; Iderberg, H.; Axelsen, M.; Dahlström, T.; Julin, B.; Leksell, J.; Lindberg, A.; Lindgren, P.; Looström Muth, K.; Svensson, A.M.; et al. Sociodemographic determinants and health outcome variation in individuals with type 1 diabetes mellitus: A register-based study. PLoS ONE 2018, 13, e0199170. [Google Scholar] [CrossRef]
- Chen, H.L.; Liu, K.; You, Q.S. Effects of couple based coping intervention on self-efficacy and quality of life in patients with resected lung cancer. Patient Educ. Couns. 2017, 100, 2297–2302. [Google Scholar] [CrossRef]
- Porter, L.S.; Keefe, F.J.; Garst, J.; Baucom, D.H.; McBride, C.M.; McKee, D.C.; Sutton, L.; Carson, K.; Knowles, V.; Rumble, M.; et al. Caregiver-assisted coping skills training for lung cancer: Results of a randomized clinical trial. J. Pain Symptom Manag. 2011, 41, 1–13. [Google Scholar] [CrossRef]
- Jones, S.R.; Carley, S.; Harrison, M. An introduction to power and sample size estimation. Emerg. Med. J. 2003, 20, 453. [Google Scholar] [CrossRef]
- Hackshaw, A. Small studies: Strengths and limitations. Eur. Respir. J. 2008, 32, 1141–1143. [Google Scholar] [CrossRef]
- Hróbjartsson, A.; Emanuelsson, F.; Skou Thomsen, A.S.; Hilden, J.; Brorson, S. Bias due to lack of patient blinding in clinical trials. A systematic review of trials randomizing patients to blind and nonblind sub-studies. Int. J. Epidemiol. 2014, 43, 1272–1283. [Google Scholar] [CrossRef] [Green Version]
- Stout, N.L.; Baima, J.; Swisher, A.K.; Winters-Stone, K.M.; Welsh, J. A Systematic Review of Exercise Systematic Reviews in the Cancer Literature (2005–2017). PM&R 2017, 9, S347–S384. [Google Scholar]
- Tomlinson, D.; Diorio, C.; Beyene, J.; Sung, L. Effect of Exercise on Cancer-Related Fatigue: A Meta-analysis. Am. J. Phys. Med. Rehabil. 2014, 93, 675–686. [Google Scholar] [CrossRef]
- Cavalheri, V.; Granger, C.L. Exercise training as part of lung cancer therapy. Respirology 2020, 25, 80–87. [Google Scholar] [CrossRef]
- Peddle-McIntyre, C.J.; Singh, F.; Thomas, R.; Newton, R.U.; Galvão, D.A.; Cavalheri, V. Exercise training for advanced lung cancer. Cochrane Database Syst. Rev. 2019, 2, CD012685. [Google Scholar] [CrossRef]
- Patel, J.D. Lung Cancer: A Biologically Different Disease in Women? Women’s Health 2009, 5, 685–691. [Google Scholar] [CrossRef]
- Cavalli-Björkman, N.; Qvortrup, C.; Sebjørnsen, S.; Pfeiffer, P.; Wentzel-Larsen, T.; Glimelius, B.; Sorbye, H. Lower treatment intensity and poorer survival in metastatic colorectal cancer patients who live alone. Br. J. Cancer 2012, 107, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Carnio, S.; di Stefano, R.F.; Novello, S. Fatigue in lung cancer patients: Symptom burden and management of challenges. Lung Cancer 2016, 7, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global epidemiology of lung cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef] [Green Version]
- Williamson, T.J.; Kwon, D.M.; Riley, K.E.; Shen, M.J.; Hamann, H.A.; Ostroff, J.S. Lung Cancer Stigma: Does Smoking History Matter? Ann. Behav. Med. 2020, 54, 535–540. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Takada, M.; Kubo, A.; Matsumura, A.; Fukai, S.; Tamura, A.; Saito, R.; Maruyama, Y.; Kawahara, M.; Ignatius Ou, S.H. Performance Status and Smoking Status Are Independent Favorable Prognostic Factors for Survival in Non-small Cell Lung Cancer: A Comprehensive Analysis of 26,957 Patients with NSCLC. J. Thorac. Oncol. 2010, 5, 620–630. [Google Scholar] [CrossRef] [Green Version]
- Shenoy, P.; Harugeri, A. Elderly patients’ participation in clinical trials. Perspect. Clin. Res. 2015, 6, 184–189. [Google Scholar] [CrossRef]
- Gomez, S.L.; Hurley, S.; Canchola, A.J.; Keegan, T.H.; Cheng, I.; Murphy, J.D.; Clarke, C.A.; Glaser, S.L.; Martínez, M.E. Effects of marital status and economic resources on survival after cancer: A population-based study. Cancer 2016, 122, 1618–1625. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).