Screening of Serum Biomarkers of Coal Workers’ Pneumoconiosis by Metabolomics Combined with Machine Learning Strategy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Serum Sample Collection
2.3. Serum Metabolomics
2.4. Biomarker Screening by Machine Learning Strategy
2.5. Statistical Analysis
3. Results
3.1. The Characteristics of Subjects
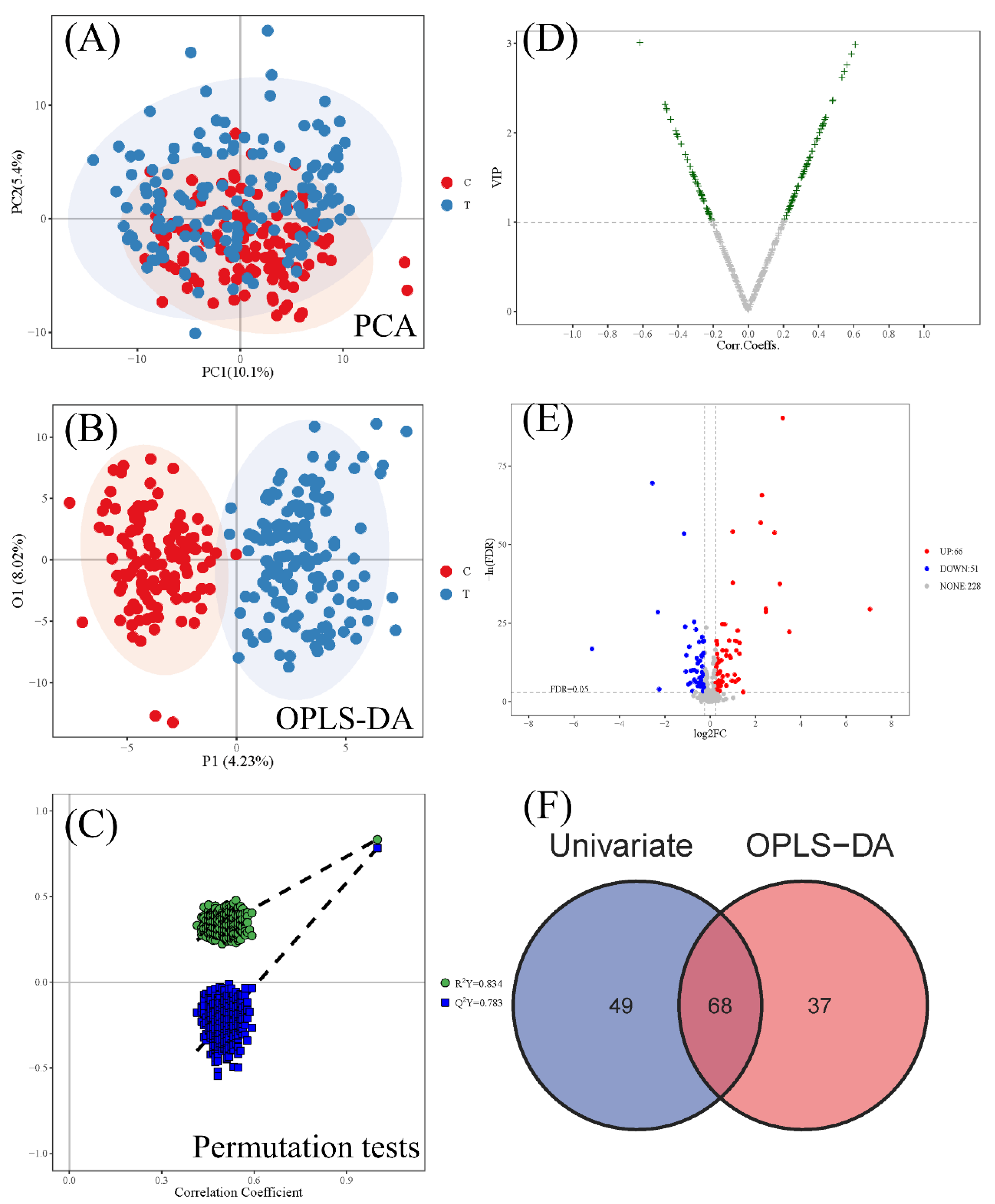
3.2. Differential Metabolites between the CWP Case Group and Control Group
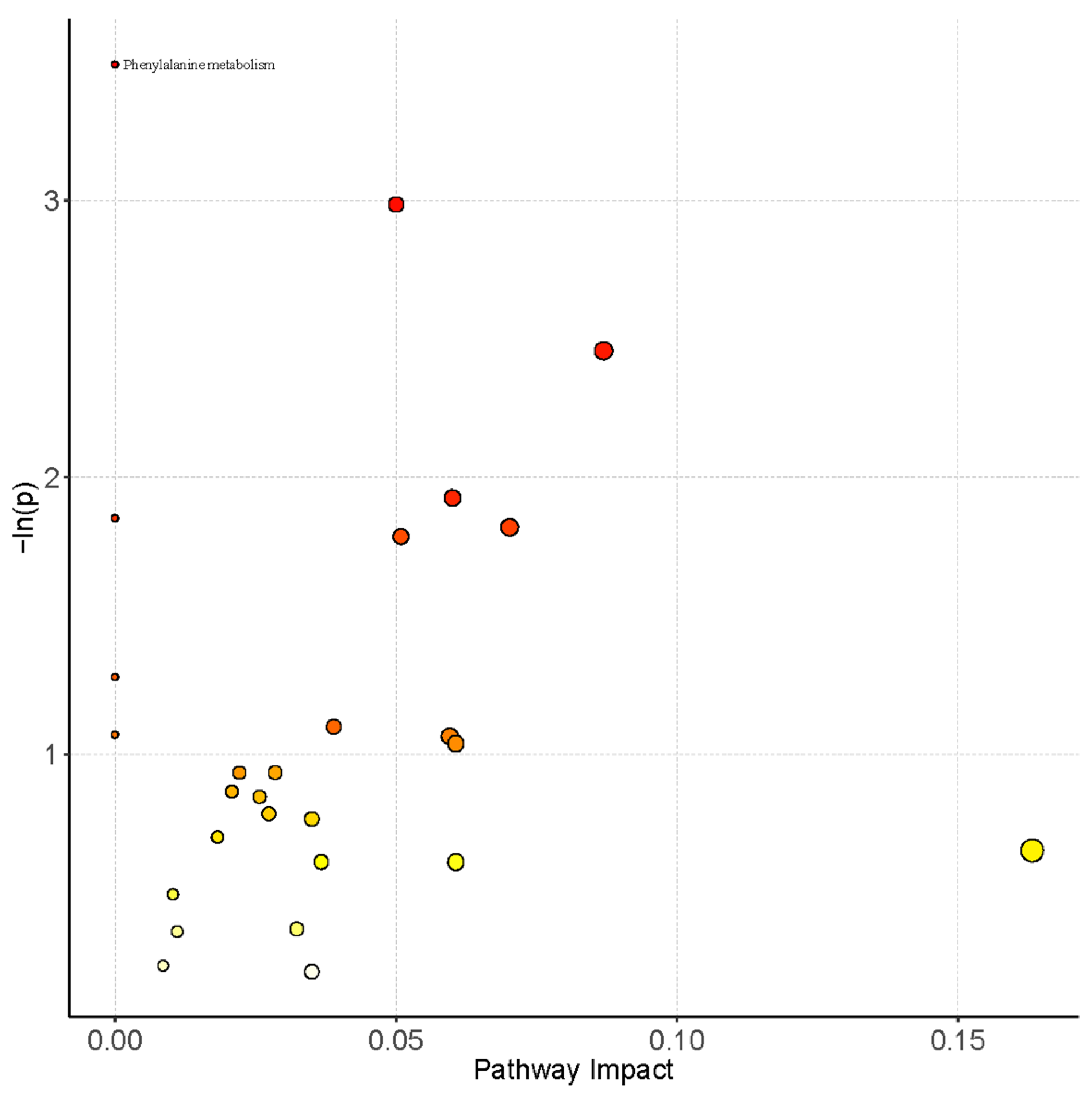
3.3. Pathway Analysis of Serum Metabolomics
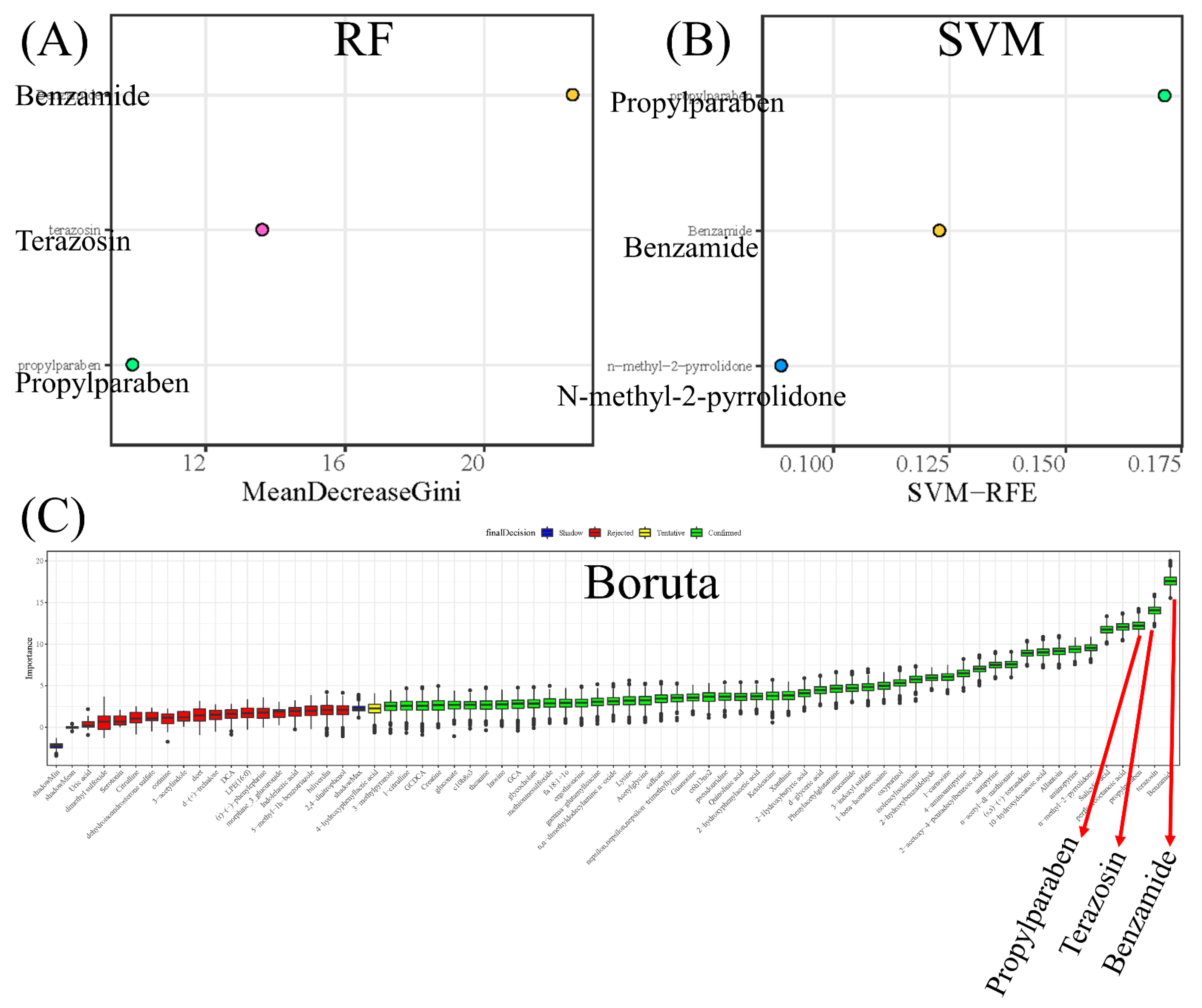
3.4. Screening Potential Biomarkers of CWP
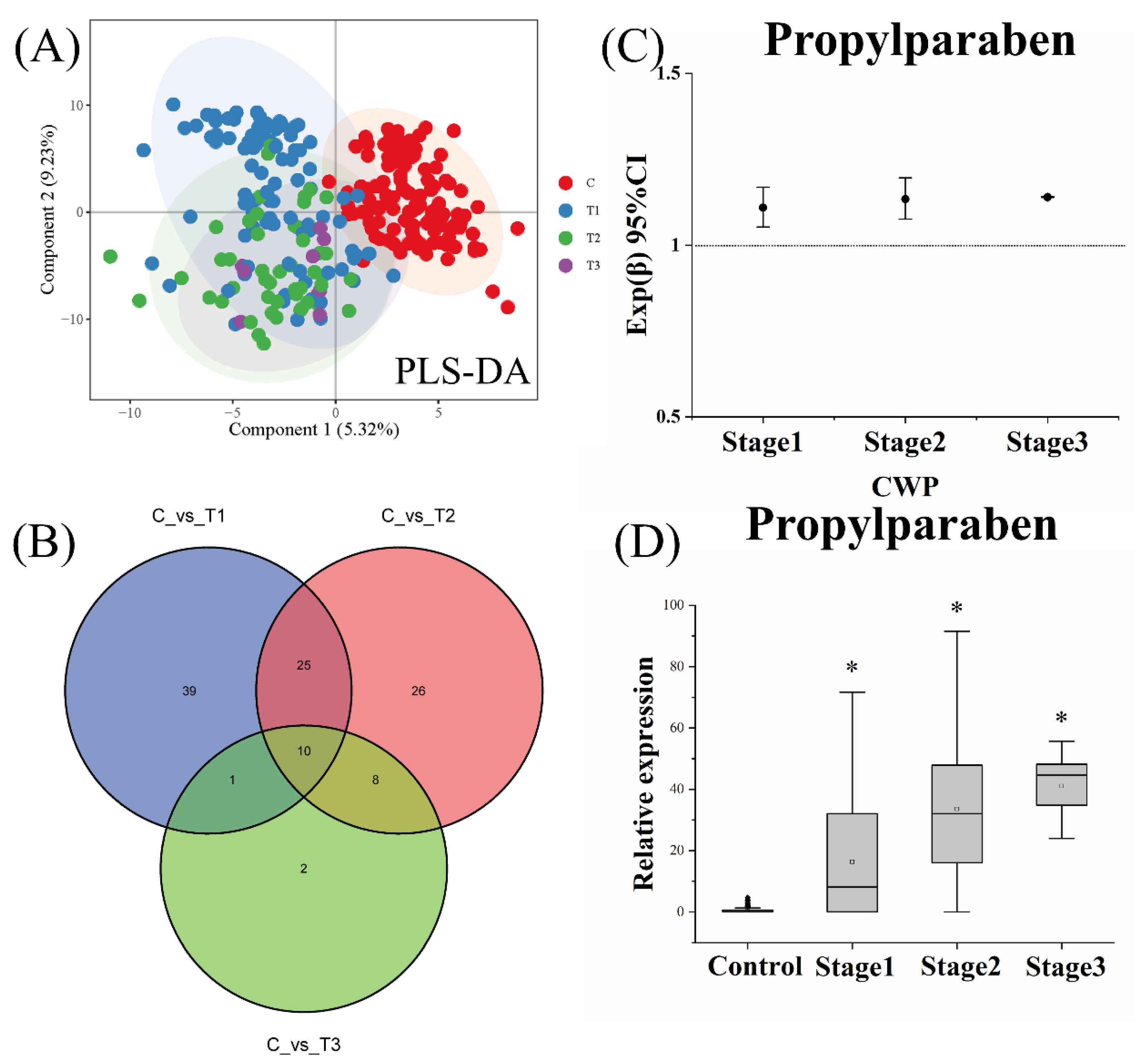
3.5. Effect of CWP Stage on the Biomarker Screening
3.6. Diagnostic Analysis of Potential Biomarkers for CWP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, P.; Xing, X.; Xi, S.; Jing, H.; Yuan, J.; Fu, Z.; Zhao, H. Trends in global, regional and national incidence of pneumoconiosis caused by different aetiologies: An analysis from the Global Burden of Disease Study 2017. Occup. Environ. Med. 2020, 77, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, T.Y.; Chiu, Y.H.; Cen, H.; Lin, Y.N. Efficiency assessment of coal energy and non-coal energy under bound dynamic DDF DEA. Environ. Sci. Pollut. Res. Int. 2021, 28, 20093–20110. [Google Scholar] [CrossRef] [PubMed]
- Lancet. Improving occupational health in China. Lancet 2019, 394, 443. [Google Scholar] [CrossRef]
- Morgan, J. Black lung is still a threat. Lancet Respir. Med. 2018, 6, 745–746. [Google Scholar] [CrossRef]
- Furuya, S.; Chimed-Ochir, O.; Takahashi, K.; David, A.; Takala, J. Global Asbestos Disaster. Int. J. Environ. Res. Public Health 2018, 15, 1000. [Google Scholar] [CrossRef] [Green Version]
- Leung, C.C.; Yu, I.T.; Chen, W. Silicosis. Lancet 2012, 379, 2008–2018. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, X.T. Dose-response relationship analysis between cumulative coal dust exposure and pneumoconiosis risk. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2020, 38, 433–437. [Google Scholar] [CrossRef]
- Liu, T.; Liu, S. The impacts of coal dust on miners’ health: A review. Environ. Res. 2020, 190, 109849. [Google Scholar] [CrossRef]
- Wang, X.; Hu, W.; Zhang, S.; Kang, N.; Wang, H.; Dong, Y.; Bian, H.; Meng, Y. Occupational Dust Hazards and Risk Assessment of Coal-Fired Thermal Power Plants of Different Capacities—China, 2017–2019. China CDC Wkly. 2021, 3, 901–905. [Google Scholar] [CrossRef]
- Almberg, K.S.; Friedman, L.S.; Rose, C.S.; Go, L.H.T.; Cohen, R.A. Progression of coal workers’ pneumoconiosis absent further exposure. Occup. Environ. Med. 2020, 77, 748–751. [Google Scholar] [CrossRef]
- Paglia, G.; Astarita, G. Metabolomics and lipidomics using traveling-wave ion mobility mass spectrometry. Nat. Protoc. 2017, 12, 797–813. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, C.; Chen, L.; Rabinowitz, J.D. Metabolomics and Isotope Tracing. Cell 2018, 173, 822–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goecks, J.; Jalili, V.; Heiser, L.M.; Gray, J.W. How Machine Learning Will Transform Biomedicine. Cell 2020, 181, 92–101. [Google Scholar] [CrossRef]
- Huang, L.; Wang, L.; Hu, X.; Chen, S.; Tao, Y.; Su, H.; Yang, J.; Xu, W.; Vedarethinam, V.; Wu, S.; et al. Machine learning of serum metabolic patterns encodes early-stage lung adenocarcinoma. Nat. Commun. 2020, 11, 3556. [Google Scholar] [CrossRef]
- Das, P.; Roychowdhury, A.; Das, S.; Roychoudhury, S.; Tripathy, S. sigFeature: Novel Significant Feature Selection Method for Classification of Gene Expression Data Using Support Vector Machine and t Statistic. Front. Genet. 2020, 11, 247. [Google Scholar] [CrossRef]
- Yin, P.; Xu, G. Metabolomics Toward Biomarker Discovery. Methods Mol. Biol. 2017, 1619, 467–475. [Google Scholar] [CrossRef]
- Kumar, A.; Misra, B.B. Challenges and Opportunities in Cancer Metabolomics. Proteomics 2019, 19, e1900042. [Google Scholar] [CrossRef]
- Chen, X.; Yu, D. Metabolomics study of oral cancers. Metab. Off. J. Metab. Soc. 2019, 15, 22. [Google Scholar] [CrossRef]
- McGarrah, R.W.; Crown, S.B.; Zhang, G.F.; Shah, S.H.; Newgard, C.B. Cardiovascular Metabolomics. Circ. Res. 2018, 122, 1238–1258. [Google Scholar] [CrossRef]
- Boone, S.; Mook-Kanamori, D.; Rosendaal, F.; den Heijer, M.; Lamb, H.; de Roos, A.; le Cessie, S.; Willems van Dijk, K.; de Mutsert, R. Metabolomics: A search for biomarkers of visceral fat and liver fat content. Metab. Off. J. Metab. Soc. 2019, 15, 139. [Google Scholar] [CrossRef] [Green Version]
- Bowerman, K.L.; Rehman, S.F.; Vaughan, A.; Lachner, N.; Budden, K.F.; Kim, R.Y.; Wood, D.L.A.; Gellatly, S.L.; Shukla, S.D.; Wood, L.G.; et al. Disease-associated gut microbiome and metabolome changes in patients with chronic obstructive pulmonary disease. Nat. Commun. 2020, 11, 5886. [Google Scholar] [CrossRef]
- Zhou, W.; Sailani, M.R.; Contrepois, K.; Zhou, Y.; Ahadi, S.; Leopold, S.R.; Zhang, M.J.; Rao, V.; Avina, M.; Mishra, T.; et al. Longitudinal multi-omics of host-microbe dynamics in prediabetes. Nature 2019, 569, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Ristori, M.V.; Mortera, S.L.; Marzano, V.; Guerrera, S.; Vernocchi, P.; Ianiro, G.; Gardini, S.; Torre, G.; Valeri, G.; Vicari, S.; et al. Proteomics and Metabolomics Approaches towards a Functional Insight onto AUTISM Spectrum Disorders: Phenotype Stratification and Biomarker Discovery. Int. J. Mol. Sci. 2020, 21, 6274. [Google Scholar] [CrossRef] [PubMed]
- Soni, M.G.; Carabin, I.G.; Burdock, G.A. Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food Chem. Toxicol. 2005, 43, 985–1015. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Chen, L.; Kannan, K. Occurrence of parabens in foodstuffs from China and its implications for human dietary exposure. Environ. Int. 2013, 57–58, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Liu, F.; Kannan, K. Occurrence of and dietary exposure to parabens in foodstuffs from the United States. Environ. Sci. Technol. 2013, 47, 3918–3925. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Kannan, K. A survey of phthalates and parabens in personal care products from the United States and its implications for human exposure. Environ. Sci. Technol. 2013, 47, 14442–14449. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, L.; Kannan, K. Phthalates and parabens in personal care products from China: Concentrations and human exposure. Arch. Environ. Contam. Toxicol. 2014, 66, 113–119. [Google Scholar] [CrossRef]
- Dodge, L.E.; Kelley, K.E.; Williams, P.L.; Williams, M.A.; Hernández-Díaz, S.; Missmer, S.A.; Hauser, R. Medications as a source of paraben exposure. Reprod. Toxicol. 2015, 52, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Gao, L.; Shi, Y.; Wang, Y.; Liu, J.; Cai, Y. Spatial distribution, temporal variation and risks of parabens and their chlorinated derivatives in urban surface water in Beijing, China. Sci. Total Environ. 2016, 539, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liao, C.; Liu, F.; Wu, Q.; Guo, Y.; Moon, H.B.; Nakata, H.; Kannan, K. Occurrence and human exposure of p-hydroxybenzoic acid esters (parabens), bisphenol A diglycidyl ether (BADGE), and their hydrolysis products in indoor dust from the United States and three East Asian countries. Environ. Sci. Technol. 2012, 46, 11584–11593. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, R.; Jáuregui, O.; Queipo-Ortuño, M.I.; Andrés-Lacueva, C. Characterization of the Human Exposome by a Comprehensive and Quantitative Large-Scale Multianalyte Metabolomics Platform. Anal. Chem. 2020, 92, 13767–13775. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Taketani, M.; Shii, M.; Hosokawa, M.; Chiba, K. Substrate specificity of carboxylesterase isozymes and their contribution to hydrolase activity in human liver and small intestine. Drug Metab. Dispos. 2006, 34, 1734–1741. [Google Scholar] [CrossRef] [Green Version]
- Fujino, C.; Watanabe, Y.; Uramaru, N.; Kitamura, S. Transesterification of a series of 12 parabens by liver and small-intestinal microsomes of rats and humans. Food Chem. Toxicol. 2014, 64, 361–368. [Google Scholar] [CrossRef]
- Ye, X.; Tao, L.J.; Needham, L.L.; Calafat, A.M. Automated on-line column-switching HPLC-MS/MS method for measuring environmental phenols and parabens in serum. Talanta 2008, 76, 865–871. [Google Scholar] [CrossRef]
- Darbre, P.D.; Aljarrah, A.; Miller, W.R.; Coldham, N.G.; Sauer, M.J.; Pope, G.S. Concentrations of parabens in human breast tumours. J. Appl. Toxicol. JAT 2004, 24, 5–13. [Google Scholar] [CrossRef]
- Jiménez-Díaz, I.; Vela-Soria, F.; Zafra-Gómez, A.; Navalón, A.; Ballesteros, O.; Navea, N.; Fernández, M.F.; Olea, N.; Vílchez, J.L. A new liquid chromatography-tandem mass spectrometry method for determination of parabens in human placental tissue samples. Talanta 2011, 84, 702–709. [Google Scholar] [CrossRef]
- Ye, X.; Bishop, A.M.; Reidy, J.A.; Needham, L.L.; Calafat, A.M. Parabens as urinary biomarkers of exposure in humans. Environ. Health Perspect. 2006, 114, 1843–1846. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.L.; Wang, L.; Guo, Y.; Liu, L.Y.; Qi, H.; Zhu, N.Z.; Gao, C.J.; Li, Y.F.; Kannan, K. Urinary concentrations of parabens in Chinese young adults: Implications for human exposure. Arch. Environ. Contam. Toxicol. 2013, 65, 611–618. [Google Scholar] [CrossRef]
- Lite, C.; Guru, A.; Juliet, M.; Arockiaraj, J. Embryonic exposure to butylparaben and propylparaben induced developmental toxicity and triggered anxiety-like neurobehavioral response associated with oxidative stress and apoptosis in the head of zebrafish larvae. Environ. Toxicol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.M.P.; Freire, P.F.; Daimiel, L.; Martínez-Botas, J.; Sánchez, C.M.; Lasunción, M.; Peropadre, A.; Hazen, M.J. The antioxidant butylated hydroxyanisole potentiates the toxic effects of propylparaben in cultured mammalian cells. Food Chem. Toxicol. 2014, 72, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Gulumian, M.; Borm, P.J.; Vallyathan, V.; Castranova, V.; Donaldson, K.; Nelson, G.; Murray, J. Mechanistically identified suitable biomarkers of exposure, effect, and susceptibility for silicosis and coal-worker’s pneumoconiosis: A comprehensive review. J. Toxicol. Environ. Health Part B Crit. Rev. 2006, 9, 357–395. [Google Scholar] [CrossRef]
- Peruzzi, C.P.; Brucker, N.; Bubols, G.; Cestonaro, L.; Moreira, R.; Domingues, D.; Arbo, M.; Neto, P.O.; Knorst, M.M.; Garcia, S.C. Occupational exposure to crystalline silica and peripheral biomarkers: An update. J. Appl. Toxicol. JAT 2022, 42, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Qi, X.; Luo, Y.; Li, X.; Shu, T.; Li, B.; Song, M.; Liu, Y.; Wei, D.; Chen, J.; et al. Multi-omics study of silicosis reveals the potential therapeutic targets PGD(2) and TXA(2). Theranostics 2021, 11, 2381–2394. [Google Scholar] [CrossRef]
- Jacob, M.; Lopata, A.L.; Dasouki, M.; Rahman, A.M.A. Metabolomics toward personalized medicine. Mass Spectrom. Rev. 2019, 38, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wang, X.; Sun, B.; Xia, T.; Hu, S. Predictive Metabolomic Signatures for Safety Assessment of Metal Oxide Nanoparticles. ACS Nano 2019, 13, 13065–13082. [Google Scholar] [CrossRef] [PubMed]

| Control Group (n = 120) | CWP Case Group (n = 150) | p | |
|---|---|---|---|
| Age (years) | 56.63 ± 3.03 | 69.02 ± 9.07 | <0.001 * |
| Gender | |||
| Male | 120 (100%) | 150 (100%) | |
| Female | 0 | 0 | |
| Smoking n (%) | <0.001 * | ||
| Yes | 63 (52.5) | 125 (83.3) | |
| No | 57 (47.5) | 25 (16.7) | |
| Dinking n (%) | 0.934 | ||
| Yes | 69 (57.5) | 87 (58.0) | |
| No | 51 (42.5) | 63 (42.0) | |
| Chronic disease n (%) | <0.001 * | ||
| Yes | 51 (42.5) | 106 (70.7) | |
| No | 69 (57.5) | 44 (29.3) | |
| Pneumoconiosis stage n (%) | |||
| 1 | 94 (62.7) | ||
| 2 | 47 (31.3) | ||
| 3 | 9 (6.0) | ||
| Working age (years) | 24.70 ± 8.48 | ||
| Complication n (%) | |||
| Tuberculosis | 19 (12.7) | ||
| COPD | 32 (21.3) | ||
| Chronic bronchitis | 56 (37.3) | ||
| Two complications | 4 (2.7) | ||
| No complication | 39 (26.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Shi, J.; Zhang, Y.; Zhang, J.; Li, S.; Guan, L.; Jia, G. Screening of Serum Biomarkers of Coal Workers’ Pneumoconiosis by Metabolomics Combined with Machine Learning Strategy. Int. J. Environ. Res. Public Health 2022, 19, 7051. https://doi.org/10.3390/ijerph19127051
Chen Z, Shi J, Zhang Y, Zhang J, Li S, Guan L, Jia G. Screening of Serum Biomarkers of Coal Workers’ Pneumoconiosis by Metabolomics Combined with Machine Learning Strategy. International Journal of Environmental Research and Public Health. 2022; 19(12):7051. https://doi.org/10.3390/ijerph19127051
Chicago/Turabian StyleChen, Zhangjian, Jiaqi Shi, Yi Zhang, Jiahe Zhang, Shuqiang Li, Li Guan, and Guang Jia. 2022. "Screening of Serum Biomarkers of Coal Workers’ Pneumoconiosis by Metabolomics Combined with Machine Learning Strategy" International Journal of Environmental Research and Public Health 19, no. 12: 7051. https://doi.org/10.3390/ijerph19127051






