The Phenotype of Bone Turnover in Patients with Fragility Hip Fracture: Experience in a Fracture Liaison Service Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
2.2. Laboratory Evaluation
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef]
- Cummings, S.R.; Melton, L.J. Epidemiology and outcomes of osteoporotic fractures. Lancet 2002, 359, 1761–1767. [Google Scholar] [CrossRef]
- Bhandari, M.; Swiontkowski, M. Management of acute hip fracture. N. Engl. J. Med. 2017, 377, 2053–2062. [Google Scholar] [CrossRef]
- Rapp, K.; Rothenbacher, D.; Magaziner, J.; Becker, C.; Benzinger, P.; König, H.H.; Jaensch, A.; Büchele, G. Risk of nursing home admission after femoral fracture compared with stroke, myocardial infarction, and pneumonia. J. Am. Med. Dir. Assoc. 2015, 16, 715.e7–715.e12. [Google Scholar] [CrossRef]
- Piscitelli, P.; Neglia, C.; Feola, M.; Rizzo, E.; Argentiero, A.; Ascolese, M.; Rivezzi, M.; Rao, C.; Miani, A.; Distante, A.; et al. Updated incidence and costs of hip fractures in elderly Italian population. Aging Clin. Exp. Res. 2020, 32, 2587–2593. [Google Scholar] [CrossRef] [PubMed]
- Papakitsou, E.; Paspati, I.; Rizou, S.; Lyritis, G.P. Bone metabolism subgroups identified as hip fracture patients via clustering. Hormones 2021, 20, 545–555. [Google Scholar] [CrossRef]
- Van Staa, T.P.; Leufkens, H.G.M.; Cooper, C. Does a fracture at one site predict later fractures at other sites? A British cohort study. Osteoporos. Int. 2002, 13, 624–629. [Google Scholar] [CrossRef] [Green Version]
- Kanis, J.A.; Johnell, O.; De Laet, C.E.D.H.; Johansson, H.; Odén, A.; Delmas, P.; Eisman, J.; Fujiwara, S.; Garnero, P.; Kroger, H.; et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone 2004, 35, 375–382. [Google Scholar] [CrossRef]
- Gonnelli, S.; Caffarelli, C.; Rossi, S.; Siviero, P.; Maggi, S.; Crepaldi, G.; Nuti, R. The Trochanteric Localization is a Mediator of Slower Short-Term Functional Recovery in Overweight and Obese Elderly Women with Recent Hip Fracture: The BREAK Study. Calcif. Tissue Int. 2015, 97, 560–567. [Google Scholar] [CrossRef]
- Ganda, K.; Puech, M.; Chen, J.S.; Speerin, R.; Bleasel, J.; Center, J.R.; Eisman, J.A.; March, L.; Seibel, M.J. Models of care for the secondary prevention of osteoporotic fractures: A systematic review and meta-analysis. Osteoporos. Int. 2013, 24, 393–406. [Google Scholar] [CrossRef]
- Pioli, G.; Bendini, C.; Pignedoli, P.; Giusti, A.; Marsh, D. Orthogeriatric co-management-managing frailty as well as fragility. Injury 2018, 49, 1398–1402. [Google Scholar] [CrossRef] [PubMed]
- Swart, K.; Van Vilsteren, M.; Van Hout, W.; Draak, E.; Van Der Zwaard, B.C.; Van Der Horst, H.E.; Hugtenburg, J.G.; Elders, P.J. Factors related to intennational of bisphosphonate treatment in patients with a high fracture risk in primary care: A qualitative study. BMC Fam. Pract. 2018, 19, 141. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Austin, S.R.; Wong, Y.N.; Uzzo, R.G.; Beck, J.R.; Egleston, B.L. Why summary comorbidity measures such as the Charlson comorbidity index and Elixhauser score work. Med. Care 2015, 53, e65–e72. [Google Scholar] [CrossRef] [Green Version]
- Giustina, A.; Adler, R.A.; Binkley, N.; Bollerslev, J.; Bouillon, R.; Dawson-Hughes, B.; Ebeling, P.R.; Feldman, D.; Formenti, A.M.; Lazaretti-Castro, M.; et al. Consensus statement from 2nd International Conference on Controversies in Vitamin D. Rev. Endocr. Metab. Disord. 2020, 21, 89–116. [Google Scholar] [CrossRef] [Green Version]
- Sempos, C.T.; Heijboer, A.C.; Bikle, D.D.; Bollerslev, J.; Bouillon, R.; Brannon, P.M.; DeLuca, H.F.; Jones, G.; Munns, C.F.; Bilezikian, J.P.; et al. Vitamin D assays and the definition of hypovitaminosis D: Results from the First International Conference on Controversies in Vitamin D. Br. J. Clin. Pharmacol. 2018, 84, 2194–2207. [Google Scholar] [CrossRef]
- Gonnelli, S.; Caffarelli, C.; Cappelli, S.; Rossi, S.; Giordano, N.; Nuti, R. Gender-specific associations of appendicular muscle mass with BMD in elderly Italian subjects. Calcif. Tissue Int. 2014, 95, 340–348. [Google Scholar] [CrossRef]
- Sakuma, M.; Endo, N.; Oinuma, T.; Hayami, T.; Endo, E.; Yazawa, T.; Watanabe, K.; Watanabe, S. Vitamin D and intact PTH status in patients with hip fracture. Osteoporos. Int. 2006, 17, 1608–1614. [Google Scholar] [CrossRef]
- Fisher, A.; Srikusalanukul, W.; Davis, M.; Smith, P. Hip fracture type: Important role of parathyroid hormone (PTH) response to hypovitaminosis D. Bone 2010, 47, 400–407. [Google Scholar] [CrossRef]
- Di Monaco, M.; Castiglioni, C.; Vallero, F.; Di Monaco, R.; Tappero, R. Parathyroid hormone response to severe Vitamin D deficiency is sex associated: An observational study of 571 hip fracture inpatients. J. Nutr. Health Aging 2013, 17, 180–184. [Google Scholar] [CrossRef]
- Alarcón, T.; González-Montalvo, J.I.; Hoyos, R.; Diez-Sebastián, J.; Otero, A.; Mauleon, J.L. Parathyroid hormone response to two levels of vitamin D deficiency is associated with high risk of medical problems during hospitalization in patients with hip fracture. J. Endocrinol. Investig. 2015, 38, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Dretakis, K.; Igoumenou, V.G. The role of parathyroid hormone (PTH) and Vitamin D in falls and hip fracture type. Aging Clin. Exp. Res. 2019, 31, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Gonnelli, S.; Caffarelli, C.; Iolascon, G.; Bertoldo, F.; Letizia Mauro, G.; Patti, A.; Nuti, R. Prescription of anti-osteoporosis medications after hospitalization for hip fracture: A multicentre Italian survey. Aging Clin. Exp. Res. 2017, 29, 1031–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seitz, S.; Koehne, T.; Ries, C.; De Novo Oliveira, A.; Barvencik, F.; Busse, B.; Eulenburg, C.; Schinke, T.; Püschel, K.; Rueger, J.M.; et al. Impaired bone mineralization accompanied by low Vitamin D and secondary hyperparathyroidism in patients with femoral neck fracture. Osteoporos. Int. 2013, 24, 641–649. [Google Scholar] [CrossRef]
- Fisher, A.; Fisher, L.; Srikusalanukul, W.; Smith, P.N. Usefulness of simple biomarkers at admission as independent indicators and predictors of in-hospital mortality in older hip fracture patients. Injury 2018, 49, 829–840. [Google Scholar] [CrossRef]
- Lips, P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: Consequences for bone loss and fractures and therapeutic implications. Endocr. Rev. 2001, 22, 477–501. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Can, U.; Staehelin, H.B.; Platz, A.; Henschkowski, J.; Michel, B.A.; Dawson-Hughes, B.; Theiler, R. Severe Vitamin D deficiency in swiss hip fracture patients. Bone 2008, 42, 597–602. [Google Scholar] [CrossRef]
- Montoya, M.J.; Giner, M.; Miranda, C.; Vázquez, M.A.; Caeiro, J.R.; Guede, D.; Pérez-Cano, R. Microstructural trabecular bone from patients with osteoporotic hip fracture or osteoarthritis: Its relationship with bone mineral density and bone remodelling markers. Maturitas 2014, 79, 299–305. [Google Scholar] [CrossRef]
- Ganhão, S.; Guerra, M.G.; Lucas, R.; Terroso, G.; Aguiar, F.; Costa, L.; Vaz, C. Predictors of Mortality and Refracture in Patients Older than 65 Years with a Proximal Femur Fracture. J. Clin. Rheumatol. 2022, 1, e49–e55. [Google Scholar] [CrossRef]
- Port, L.; Center, J.; Briffa, N.K.; Nguyen, T.; Cumming, R.; Eisman, J. Osteoporotic fracture: Missed opportunity for intervention. Osteoporos. Int. 2003, 14, 780–784. [Google Scholar] [CrossRef]
- Edwards, B.J.; Bunta, A.D.; Simonelli, C.; Bolander, M.; Fitzpatrick, L.A. Prior fractures are common in patients with subsequent hip fractures. Clin. Orthop. Relat. Res. 2007, 461, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Gonnelli, S.; Caffarelli, C.; Maggi, S.; Rossi, S.; Siviero, P.; Gandolini, G.; Cisari, C.; Rossini, M.; Iolascon, G.; Letizia Mauro, G.; et al. The assessment of vertebral fractures in elderly women with recent hip fractures: The BREAK Study. Osteoporos. Int. 2013, 24, 1151–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosa, H.M.; Saavedra, S.P.; Grupo de trabajo en osteoporosis de la Sociedad Española de Medicina Interna (SEMI). Prevalence of vertebral fractures in hip fracture patients. Rev. Clin. Esp. 2007, 207, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Mautalen, C.A.; Vega, E.M.; Einhorn, T.A. Are the aetiologies of cervical and trochanteric hip fractures different? Bone 1996, 18, 133S–137S. [Google Scholar] [CrossRef]



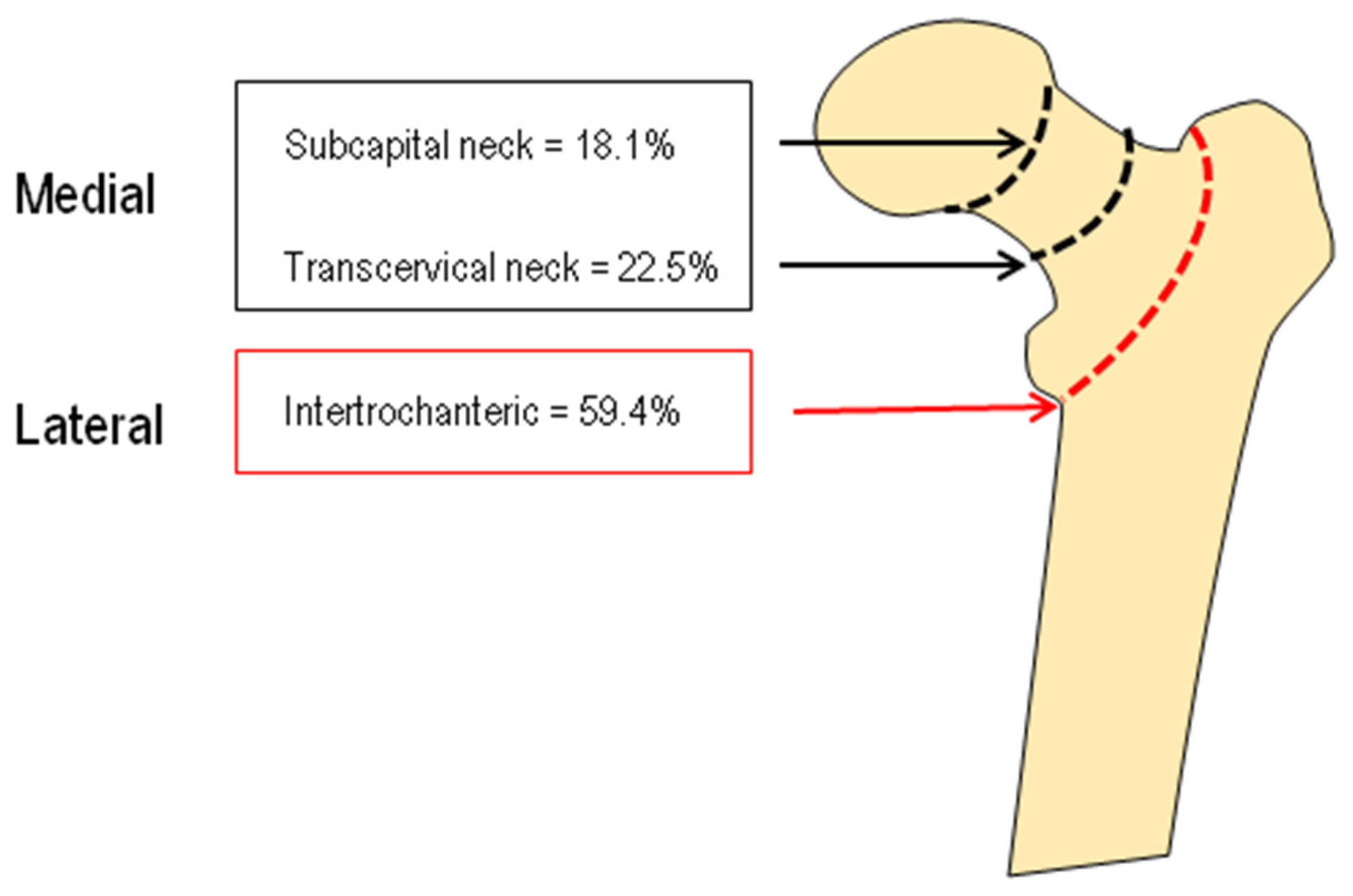

| Female (n = 289) | Male (n = 74) | |
|---|---|---|
| Age (years) | 84.5 ± 9.2 | 82.4 ± 9.5 |
| BMI (Kg/m2) | 24.1 ± 4.9 | 24.6 ± 4.0 |
| Creatinine (mg/dL) | 0.91 ± 0.32 | 1.08 ± 0.46 * |
| Calcium (mg/dL) | 8.24 ± 0.58 | 8.24 ± 0.51 |
| Phosphate (mg/dL) | 3.00 ± 0.79 | 2.90 ± 0.92 |
| Albumin (g/dL) | 3.15 ± 0.31 | 3.08 ± 0.30 |
| ALP (UI/L) | 85.61 ± 44.86 | 75.84 ± 28.66 |
| 25OHD (ng/mL) | 15.45 ± 14.85 | 12.86 ± 9.10 |
| 1,25(OH)D2 (pg/mL) | 36.68 ± 23.77 | 31.39 ± 22.30 |
| PTH (pg/mL) | 78.61 ± 57.01 | 75.02 ± 47.56 |
| B-ALP (µg/L) | 12.11 ± 7.52 | 9.15 ± 4.31 |
| β-CTX (ng/L) | 1.062 ± 1.018 | 0.988 ± 0.675 |
| Hip Fracture (n = 363) | Controls (n = 194) | |
|---|---|---|
| Sex (F/M) | 289/74 | 140/54 |
| Age (years) | 84.0 ± 9.2 | 72.8 ± 2.6 ** |
| BMI (Kg/m2) | 24.1 ± 4.9 | 25.9 ± 2.9 * |
| Creatinine (mg/dL) | 0.92 ± 0.32 | 0.95 ± 0.20 * |
| Calcium (mg/dL) | 8.24 ± 0.56 | 9.24 ± 0.52 ** |
| Phosphate (mg/dL) | 2.98 ± 0.82 | 3.31 ± 0.60 ** |
| ALP (UI/L) | 83.62 ± 42.19 | 75.84 ± 28.66 |
| 25OHD (ng/mL) | 14.90 ± 13.88 | 24.7 ± 9.10 ** |
| PTH (pg/mL) | 77.8 ± 55.01 | 23.99 ± 13.60 ** |
| B-ALP (µg/L) | 11.6 ± 7.10 | 11.80 ± 5.43 |
| β-CTX (ng/L) | 1.250 ± 0.500 | 0.616 ± 0.296 ** |
| Charlson Comorbidity Index | Previous Fragility Fracture | |
|---|---|---|
| 25OHD (ng/mL) | −0.042 | −0.115 |
| 1.25(OH)D2 (pg/mL) | −0.066 | −0.090 |
| PTH (pg/mL) | 0.178 ** | 0.271 * |
| B-ALP (µg/L) | 0.079 | −0.022 |
| β-CTX (ng/L) | 0.177 ** | 0.389 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caffarelli, C.; Mondanelli, N.; Crainz, E.; Giannotti, S.; Frediani, B.; Gonnelli, S. The Phenotype of Bone Turnover in Patients with Fragility Hip Fracture: Experience in a Fracture Liaison Service Population. Int. J. Environ. Res. Public Health 2022, 19, 7362. https://doi.org/10.3390/ijerph19127362
Caffarelli C, Mondanelli N, Crainz E, Giannotti S, Frediani B, Gonnelli S. The Phenotype of Bone Turnover in Patients with Fragility Hip Fracture: Experience in a Fracture Liaison Service Population. International Journal of Environmental Research and Public Health. 2022; 19(12):7362. https://doi.org/10.3390/ijerph19127362
Chicago/Turabian StyleCaffarelli, Carla, Nicola Mondanelli, Eduardo Crainz, Stefano Giannotti, Bruno Frediani, and Stefano Gonnelli. 2022. "The Phenotype of Bone Turnover in Patients with Fragility Hip Fracture: Experience in a Fracture Liaison Service Population" International Journal of Environmental Research and Public Health 19, no. 12: 7362. https://doi.org/10.3390/ijerph19127362






