Examining the Factors That Affect the Diagnosis of Patients with Positive Fecal Occult Blood Test Results
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
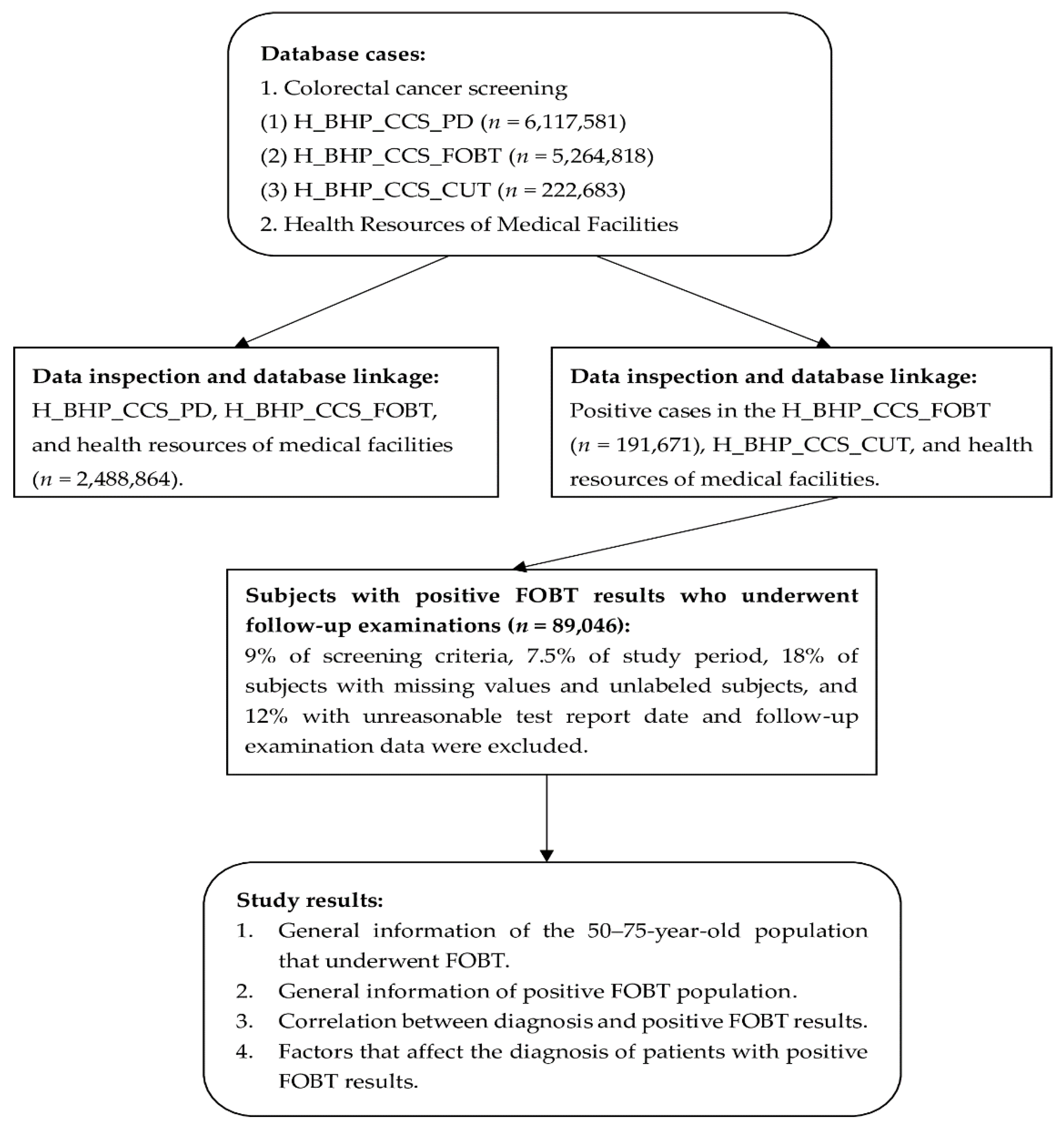
2.2. Study Materials and Subjects
2.3. Database Analysis
2.3.1. Data Inspection
2.3.2. Descriptive Statistics
2.3.3. Inferential Statistics
3. Results
3.1. General Information of the 50–75 Years Old Population That Underwent Their First FOBT
3.2. General Information of the Positive FOBT Population
3.3. Correlation between Diagnosis and Positive FOBT Results
3.4. Factors That Affect the Diagnosis of Patients with Positive FOBT Results
4. Discussion
4.1. Discussion on the General Information of FOBT Population and Positive FOBT Population
4.2. Correlation between Diagnosis and Positive FOBT Results
4.3. Factors That Affect the Diagnosis of Cases with Positive FOBT Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Cancer Observatory (GCO). Cancer Today. 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sheet.pdf (accessed on 5 November 2021).
- Global Cancer Observatory (GCO). Cancer Today. 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact-sheet.pdf (accessed on 5 November 2021).
- Global Cancer Observatory (GCO). Cancer Today. 2020. Available online: https://gco.iarc.fr/today/home (accessed on 5 November 2021).
- Global Cancer Observatory (GCO). Cancer Tomorrow. 2020. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/isotype?group_cancers=1&multiple_cancers=1&cancers=8_9&single_unit=50000&years=2025 (accessed on 5 November 2021).
- Global Cancer Observatory (GCO). Cancer Tomorrow. 2020. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/isotype?types=1&single_unit=50000&group_cancers=1&multiple_cancers=1&cancers=8_9&years=2025 (accessed on 5 November 2021).
- Ministry of Health and Welfare, R.O.C. (Taiwan). Death Statistics. 2020. Available online: https://dep.mohw.gov.tw/DOS/lp-4472-113.html (accessed on 6 November 2021).
- Health Promotion Administration, Ministry of Health and Welfare, R.O.C. (Taiwan). Cancer Registration Annual Report. 2018. Available online: https://www.hpa.gov.tw/Pages/List.aspx?nodeid=269 (accessed on 6 November 2021).
- Chiu, H.M.; Chen, S.L.; Yen, A.M.; Chiu, S.Y.H.; Fann, J.C.Y.; Lee, Y.C.; Pan, S.L.; Wu, M.S.; Liao, C.S.; Chen, H.H.; et al. Effectiveness of fecal immunochemical testing in reducing colorectal cancer mortality from the One Million Taiwanese Screening Program. Cancer 2015, 121, 3221–3229. [Google Scholar] [CrossRef] [PubMed]
- Joachim, C.; Macni, J.; Drame, M.; Pomier, A.; Escarmant, P.; Veronique-Baudin, J.; Vinh-Hung, V. Overall survival of colorectal cancer by stage at diagnosis: Data from the Martinique Cancer Registry. Medicine 2019, 98, e16941. [Google Scholar] [CrossRef] [PubMed]
- Azulay, R.; Valinsky, L.; Hershkowitz, F.; Magnezi, R. Repeated automated mobile text messaging reminders for follow-up of positive fecal occult blood tests: Randomized controlled trial. JMIR mHealth uHealth 2019, 7, e11114. [Google Scholar] [CrossRef] [PubMed]
- Ou, C.H.; Yu, F.J.; Hsu, W.H.; Wang, S.W.; Wu, D.C.; Hu, H.M. Current implementation status of colorectal cancer screening and monitoring in Taiwan. Taiwan Med. J. 2012, 55, 17–20. [Google Scholar]
- Tseng, C.C.; Lee, C.L.; Wu, C.H. Screening and surveillance of colorectal neoplasm-review and update of guidelines. J. Intern. Med. Taiwan 2009, 20, 506–513. [Google Scholar] [CrossRef]
- Lows & Regulations Database of the Republic of China. Cancer Control Act. 2018. Available online: https://law.moj.gov.tw/LawClass/LawAll.aspx?pcode=L0070008 (accessed on 6 November 2021).
- Wang, Y.W.; Chen, H.H.; Wu, M.S.; Chiu, H.M. Taiwanese Nationwide Colorectal Cancer Screening Program. Current status and future challenge of population-based organized colorectal cancer screening: Lesson from the first decade of Taiwanese program. J. Formos. Med. Assoc. 2018, 117, 358–364. [Google Scholar] [CrossRef]
- Young, G.P.; Rabeneck, L.; Winawer, S.J. The global paradigm shift in screening for colorectal cancer. Gastroenterology 2019, 156, 843.e2–851.e2. [Google Scholar] [CrossRef]
- Douma, L.N.; Uiters, E.; Timmermans, D.R.M. Why are the public so positive about colorectal cancer screening? BMC Public Health 2018, 18, 1212. [Google Scholar] [CrossRef]
- Hansen, A.T.; Hoffmann-Lücke, E.; Nielsen, B.K.; Reinholdt, B.; Hindersson, P.; Heidemann, K.; Hornung, N. Delayed sample arrival at the laboratory does not lead to more false negatives in the Danish population screening for colorectal cancer. Scand. J. Clin. Lab. Investig. 2017, 77, 685–688. [Google Scholar] [CrossRef]
- Saito, H.; Soma, Y.; Koeda, J.; Kawaguchi, H.; Sobue, T.; Aisawa, T.; Yoshida, Y. Reduction in risk of mortality from colorectal cancer by fecal occult blood screening with immunochemical hemagglutination test. A case-control study. Int. J. Cancer 1995, 61, 465–469. [Google Scholar] [CrossRef]
- Chiu, H.M. Essential Knowledge on for Bowel Activity: Colorectal Cancer is 90% Treatable When Detected Early! Effective Interventions to Prevent Colorectal Cancer; Commonwealth Publishing Group: Taipei, Taiwan, 2017. [Google Scholar]
- Flugelman, A.A.; Stein, N.; Segol, O.; Lavi, I.; Keinan-Boker, L. Delayed colonoscopy following a positive fecal test result and cancer mortality. JNCI Cancer Spectr. 2019, 3, pkz024. [Google Scholar] [CrossRef] [PubMed]
- Barouni, M.; Larizadeh, M.H.; Sabermahani, A.; Ghaderi, H. Markov’s modeling for screening strategies for colorectal cancer. Asian Pac. J. Cancer Prev. 2012, 13, 5125–5129. [Google Scholar] [CrossRef][Green Version]
- Morrison, D.S.; Parr, C.L.; Lam, T.H.; Ueshima, H.; Kim, H.C.; Jee, S.H.; Murakami, Y.; Giles, G.; Fang, X.; Barzi, F.; et al. Behavioural and metabolic risk factors for mortality from colon and rectum cancer: Analysis of data from the Asia-pacific cohort studies collaboration. Asian Pac. J. Cancer Prev. 2013, 14, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Zhiqiang, F.; Jie, C.; Yuqiang, N.; Chenghua, G.; Hong, W.; Zheng, S.; Wanglin, L.; Yongjian, Z.; Liping, D.; Lizhong, Z.; et al. Analysis of population-based colorectal cancer screening in Guangzhou, 2011–2015. Cancer Med. 2019, 8, 2496–2502. [Google Scholar] [CrossRef] [PubMed]
- Amlani, B.; Radaelli, F.; Bhandari, P. A survey on colonoscopy shows poor understanding of its protective value and widespread misconceptions across Europe. PLoS ONE 2020, 15, e0233490. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Gupta, S.; Skinner, C.S.; Ahn, C.; Santini, N.O.; Agrawal, D.; Mayorga, C.A.; Murphy, A.; Tiro, J.A.; McCallister, K.; et al. Effect of colonoscopy outreach vs fecal immunochemical test outreach on colorectal cancer screening completion: A randomized clinical trial. JAMA 2017, 318, 806–815. [Google Scholar] [CrossRef]
- Hsieh, M.H.; Kung, P.T.; Kuo, W.Y.; Ke, T.W.; Tsai, W.C. Recurrence, death risk, and related factors in patients with stage 0 colorectal cancer: A nationwide population-based study. Medicine 2020, 99, e21688. [Google Scholar] [CrossRef]
- Takahashi, Y.; Sugimachi, K.; Yamamoto, K.; Niida, A.; Shimamura, T.; Sato, T.; Watanabe, M.; Tanaka, J.; Kudo, S.; Sugihara, K.; et al. Japanese genome-wide association study identifies a significant colorectal cancer susceptibility locus at chromosome 10p14. Cancer Sci. 2017, 108, 2239–2247. [Google Scholar] [CrossRef]
- Tanskanen, T.; van den Berg, L.; Välimäki, N.; Aavikko, M.; Ness-Jensen, E.; Hveem, K.; Wettergren, Y.; Lindskog, E.B.; Tõnisson, N.; Metspalu, A.; et al. Genome-wide association study and meta-analysis in northern European populations replicate multiple colorectal cancer risk loci. Int. J. Cancer 2018, 142, 540–546. [Google Scholar] [CrossRef]
- Ahmed, M. Colon cancer: A clinician’s perspective in 2019. Gastroenterol. Res. 2020, 13, 1–10. [Google Scholar] [CrossRef]
- Wang, H.; Wang, P.; Liu, X.; Li, L.; Xiao, X.; Liu, P.; Zhang, D.; Li, Y.; Xu, G.; Tu, M.; et al. Factors predicting the colorectal adenoma detection rate in colonoscopic screening of a Chinese population: A prospective study. Medicine 2019, 98, e15103. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.R.A.; Leong, T.W.; Andu, D.F.O.; Hat, H.; Mustapha, N.R.N. Evaluation of a colorectal carcinoma screening program in Kota Setar and Kuala Muda districts, Malaysia. Asian Pac. J. Cancer Prev. 2016, 17, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Akere, A.; Oke, T.O.; Otegbayo, J.A. Colonoscopy at a tertiary healthcare facility in Southwest Nigeria: Spectrum of indications and colonic abnormalities. Ann. Afr. Med. 2016, 15, 109–113. [Google Scholar] [CrossRef]
- Tan, Y.J.; Wendy, T.; Chieng, J.Y. Detection rate of colonic polyp among patients who had undergone colonoscopy at gastroenterology unit of Serdang Hospital, Malaysia. Med. J. Malays. 2019, 74, 20–24. [Google Scholar]
- Eide, T.J. Risk of colorectal cancer in adenoma-bearing individuals within a defined population. Int. J. Cancer 1986, 38, 173–176. [Google Scholar] [CrossRef]
- Health and Welfare Data Science Center, Ministry of Health and Welfare, R.O.C. (Taiwan). Colorectal Cancer Screening (Health-55: H_BHP_CCS). Available online: https://dep.mohw.gov.tw/DOS/lp-3147-113-3-20.html (accessed on 10 November 2021).
- Health and Welfare Data Science Center, Ministry of Health and Welfare, R.O.C. (Taiwan). Health Resources of Medical Facilities (H_OST_RESMF). Available online: https://dep.mohw.gov.tw/DOS/lp-3147-113.html (accessed on 10 November 2021).
- Department of Household Registration, MOI, Ministry of the Interior, R.O.C. (Taiwan). Annual Counties, Cities and National Statistics. Available online: https://www.ris.gov.tw/app/portal/346 (accessed on 10 November 2021).
- Ministry of Health and Welfare, R.O.C. (Taiwan). Annual Report of Medical Care Institution’s Status & Hospital Utilization 2010. Available online: https://dep.mohw.gov.tw/DOS/np-1865-113.html (accessed on 10 November 2021).
- Ministry of Health and Welfare, R.O.C. (Taiwan). Annual Report of Medical Care Institution’s Status & Hospital Utilization 2011. Available online: https://dep.mohw.gov.tw/DOS/np-1865-113.html (accessed on 10 November 2021).
- Ministry of Health and Welfare, R.O.C. (Taiwan). Annual Report of Medical Care Institution’s Status & Hospital Utilization 2012. Available online: https://dep.mohw.gov.tw/DOS/np-1865-113.html (accessed on 10 November 2021).
- Ministry of Health and Welfare, R.O.C. (Taiwan). Annual Report of Medical Care Institution’s Status & Hospital Utilization 2013. Available online: https://dep.mohw.gov.tw/DOS/np-1865-113.html (accessed on 10 November 2021).
- Ministry of Health and Welfare, R.O.C. (Taiwan). Annual Report of Medical Care Institution’s Status & Hospital Utilization 2020. Available online: https://dep.mohw.gov.tw/DOS/np-1865-113.html (accessed on 10 November 2021).
- National Development Council, R.O.C. (Taiwan). Urban and Regional Development Statistics 2013. Available online: https://www.ndc.gov.tw/en/News_Content.aspx?n=AC1FC98C56BFAE2D&sms=F7FE3808BEC335F3&s=E6CE6ED9A99DB123 (accessed on 10 November 2021).
- Morris, S.; Baio, G.; Kendall, E.; von Wagner, C.; Wardle, J.; Atkin, W.; Halloran, S.P.; Handley, G.; Logan, R.F.; Obichere, A.; et al. Socioeconomic variation in uptake of colonoscopy following a positive faecal occult blood test result: A retrospective analysis of the NHS Bowel Cancer Screening Programme. Br. J. Cancer 2012, 107, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.S.; Lee, H.Y.; Jun, J.K.; Shin, A.; Park, E.C. Adherence to follow-up after a positive fecal occult blood test in an organized colorectal cancer screening program in Korea, 2004–2008. J. Gastroenterol. Hepatol. 2012, 27, 1070–1077. [Google Scholar] [CrossRef]
- Singh, H.; Kadiyala, H.; Bhagwath, G.; Shethia, A.; El-Serag, H.; Walder, A.; Velez, M.E.; Petersen, L.A. Using a multifaceted approach to improve the follow-up of positive fecal occult blood test results. Am. J. Gastroenterol. 2009, 104, 942–952. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oluloro, A.; Petrik, A.F.; Turner, A.; Kapka, T.; Rivelli, J.; Carney, P.A.; Saha, S.; Coronado, G.D. Timeliness of colonoscopy after abnormal fecal test results in a safety net practice. J. Commun. Health 2016, 41, 864–870. [Google Scholar] [CrossRef]
- Major, D.; Bryant, H.; Delaney, M.; Fekete, S.; Gentile, L.; Harrison, M.; Mai, V.; Nicholson, E.; Taylor, Y.; on behalf of the Colorectal Cancer Screening Monitoring Program Performance Working Group and the Canadian Partnership Against Cancer. Colorectal cancer screening in Canada: Results from the first round of screening for five provincial programs. Curr. Oncol. 2013, 20, 252–257. [Google Scholar] [CrossRef]
- Jensen, C.D.; Corley, D.A.; Quinn, V.P.; Doubeni, C.A.; Zauber, A.G.; Lee, J.K.; Zhao, W.K.; Marks, A.M.; Schottinger, J.E.; Ghai, N.R.; et al. Fecal immunochemical test program performance over 4 rounds of annual screening: A retrospective cohort study. Ann. Intern. Med. 2016, 164, 456–463. [Google Scholar] [CrossRef]
- Paszat, L.; Rabeneck, L.; Kiefer, L.; Mai, V.; Ritvo, P.; Sullivan, T. Endoscopic follow-up of positive fecal occult blood testing in the Ontario FOBT Project. Can. J. Gastroenterol. 2007, 21, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Leddin, D.; Armstrong, D.; Borgaonkar, M.; Bridges, R.J.; Fallone, C.A.; Telford, J.J.; Chen, Y.; Colacino, P.; Sinclair, P. The 2012 SAGE wait times program: Survey of access to gastroenterology in Canada. Can. J. Gastroenterol. 2013, 27, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, D.A.; Williams, J.L.; Holub, J.L.; Morris, C.D.; Logan, J.R.; Eisen, G.M.; Carney, P. Race, ethnicity, and sex affect risk for polyps >9 mm in average-risk individuals. Gastroenterology 2014, 147, 351–358. [Google Scholar] [CrossRef]
- Zauber, A.G.; Winawer, S.J.; O’Brien, M.J.; Lansdorp-Vogelaar, I.; van Ballegooijen, M.; Hankey, B.F.; Shi, W.; Bond, J.H.; Schapiro, M.; Panish, J.F.; et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N. Engl. J. Med. 2012, 366, 687–696. [Google Scholar] [CrossRef]
- Corley, D.A.; Jensen, C.D.; Quinn, V.P.; Doubeni, C.A.; Zauber, A.G.; Lee, J.K.; Schottinger, J.E.; Marks, A.R.; Zhao, W.K.; Ghai, N.R.; et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA 2017, 317, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- Levin, B.; Lieberman, D.A.; McFarland, B.; Smith, R.A.; Brooks, D.; Andrews, K.S.; Dash, C.; Giardiello, F.M.; Glick, S.; Levin, T.R.; et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 2008, 134, 1570–1595. [Google Scholar] [CrossRef] [PubMed]
- Partin, M.R.; Burgess, D.J.; Burgess, J.F., Jr.; Gravely, A.; Haggstrom, D.; Lillie, S.E.; Nugent, S.; Powell, A.A.; Shaukat, A.; Walter, L.C.; et al. Organizational predictors of colonoscopy follow-up for positive fecal occult blood test results: An observational study. Cancer Epidemiol. Biomark. Prev. 2015, 24, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Chubak, J.; Garcia, M.P.; Burnett-Hartman, A.N.; Zheng, Y.; Corley, D.A.; Halm, E.A.; Singal, A.G.; Klabunde, C.N.; Doubeni, C.A.; Kamineni, A.; et al. Time to colonoscopy after positive fecal blood test in four US health care systems. Cancer Epidemiol. Biomark. Prev. 2016, 25, 344–350. [Google Scholar] [CrossRef]
- Ministry of Health and Welfare, R.O.C. (Taiwan). Tracking Rate of Positive Cancer Screening Cases. 2020. Available online: https://dep.mohw.gov.tw/dos/cp-4491-48829-113.html (accessed on 10 November 2021).
- Leslie, A.; Carey, F.A.; Pratt, N.R.; Steele, R.J. The colorectal adenoma-carcinoma sequence. Br. J. Surg. 2002, 89, 845–860. [Google Scholar] [CrossRef]
- Stewart, S.L.; Wike, J.M.; Kato, I.; Lewis, D.R.; Michaud, F. A population-based study of colorectal cancer histology in the United States, 1998–2001. Cancer 2006, 107, 1128–1141. [Google Scholar] [CrossRef] [PubMed]
- Asadzadeh Aghdaei, H.; Nazemalhosseini Mojarad, E.; Ashtari, S.; Pourhoseingholi, M.A.; Chaleshi, V.; Anaraki, F.; Haghazali, M.; Zali, M.R. Polyp detection rate and pathological features in patients undergoing a comprehensive colonoscopy screening. World J. Gastrointest. Pathophysiol. 2017, 8, 3–10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, K.; Fathan, M.I.; Patel, K.; Zhang, T.; Zhong, C.; Bansal, A.; Rastogi, A.; Wang, J.S.; Wang, G. Colonoscopy polyp detection and classification: Dataset creation and comparative evaluations. PLoS ONE 2021, 16, e0255809. [Google Scholar] [CrossRef] [PubMed]
- Henley, S.J.; King, J.B.; German, R.R.; Richardson, L.C.; Plescia, M.; Centers for Disease Control and Prevention (CDC). Surveillance of screening-detected cancers (colon and rectum, breast, and cervix)—United States, 2004–2006. MMWR Surveill. Summ. 2010, 59, 1–25. [Google Scholar] [PubMed]
| Variables | Categories | n | (%) | Mean ± SD |
|---|---|---|---|---|
| Total number of cases | 2,488,864 | 100 | ||
| FOBT response present | ||||
| Negative | 2,297,193 | 92.3 | ||
| Positive | 191,671 | 7.7 | ||
| Gender | ||||
| Male | 1,076,162 | 43.2 | ||
| Female | 1,412,702 | 56.8 | ||
| Region of the medical institution where screening was performed | ||||
| Counties on outlying islands | 7668 | 0.3 | ||
| Northern Taiwan | 1,135,051 | 45.6 | ||
| Central Taiwan | 560,391 | 22.5 | ||
| Southern Taiwan | 718,091 | 28.9 | ||
| Eastern Taiwan | 67,663 | 2.7 | ||
| Age at screening | 58.9 ± 6.3 | |||
| 50 years | 192,265 | 7.7 | ||
| 51–55 years | 679,984 | 27.3 | ||
| 56–60 years | 660,560 | 26.5 | ||
| 61–65 years | 527,245 | 21.2 | ||
| 66–70 years | 331,548 | 13.3 | ||
| 71–75 years | 97,262 | 3.9 | ||
| Screening site | ||||
| Community or workplace screening site | 490,129 | 19.7 | ||
| Outpatient | 1,935,212 | 77.8 | ||
| Inpatient | 27,315 | 1.1 | ||
| Others | 36,208 | 1.5 | ||
| Screening medical unit | ||||
| Outpatient | 2,436,071 | 97.9 | ||
| Inpatient | 52,793 | 2.1 | ||
| Family history of colorectal cancer | ||||
| No | 2,269,198 | 91.2 | ||
| Yes | 150,898 | 6.1 | ||
| Does not know | 68,768 | 2.8 |
| Variables | Categories | n | (%) | Mean ± SD |
|---|---|---|---|---|
| Total number of cases | 89,046 | 100 | ||
| Gender | Male | 48,342 | 54.3 | |
| Female | 40,704 | 45.7 | ||
| Region of follow-up examination medical institution | Counties on outlying islands | 260 | 0.3 | |
| Northern Taiwan | 38,326 | 43.0 | ||
| Central Taiwan | 19,859 | 22.3 | ||
| Southern Taiwan | 28,337 | 31.8 | ||
| Eastern Taiwan | 2264 | 2.5 | ||
| Age of patients with positive response | 50 years | 5603 | 6.3 | 60.04 ± 6.488 |
| 51–55 years | 20,178 | 22.7 | ||
| 56–60 years | 22,356 | 25.1 | ||
| 61–65 years | 21,085 | 23.7 | ||
| 66–70 years | 14,682 | 16.5 | ||
| 71–75 years | 5142 | 5.8 | ||
| Screening site | Community or workplace screening site | 15,428 | 17.3 | |
| Outpatient | 71,334 | 80.1 | ||
| Inpatient | 1271 | 1.4 | ||
| Others | 1013 | 1.1 | ||
| Category of follow-up examination medical unit | Outpatient | 79,104 | 88.8 | |
| Inpatient | 9942 | 11.2 | ||
| Family history of colorectal cancer | No | 80,841 | 90.8 | |
| Yes | 6104 | 6.9 | ||
| Does not know | 2101 | 2.4 | ||
| Follow-up examination methods | Colonoscopy | 83,854 | 94.2 | |
| Double contrast barium enema plus flexible sigmoidoscopy | 880 | 1.0 | ||
| Others | 4312 | 4.8 | ||
| Diagnosis | Normal | 9055 | 10.2 | |
| Hemorrhoids | 25,369 | 28.5 | ||
| Ulcerative colitis | 470 | 0.5 | ||
| Polyps | 46,488 | 52.2 | ||
| CRC | 3796 | 4.3 | ||
| Others | 3868 | 4.3 | ||
| Follow-up time | ≤30 days | 49,365 | 55.4 | 36.37 ± 25.799 |
| 31–60 days | 26,067 | 29.3 | ||
| 61–90 days | 8424 | 9.5 | ||
| ≥91 days | 5190 | 5.8 |
| Variables | Categories | Diagnosis | p-Value | ||
|---|---|---|---|---|---|
| Normal n = 9055 (%) | Non-CRC n = 76,195 (%) | CRC n = 3796 (%) | |||
| Gender | Male | 3997 (44.1) | 42,126 (55.3) | 2219 (58.5) | |
| Female | 5058 (55.9) | 34,069 (44.7) | 1577 (41.5) | <0.001 | |
| Region of follow-up examination medical institution | Counties on outlying islands | 11 (0.1) | 240 (0.3) | 9 (0.2) | |
| Northern Taiwan | 3892 (43.0) | 32,856 (43.1) | 1578 (41.6) | ||
| Central Taiwan | 2153 (23.8) | 16,865 (22.1) | 841 (22.2) | ||
| Southern Taiwan | 2885 (31.9) | 24,155 (31.7) | 1297 (34.2) | ||
| Eastern Taiwan | 114 (1.3) | 2079 (2.7) | 71 (1.9) | <0.001 | |
| Age of patients with positive response | 50 years | 676 (7.5) | 4753 (6.2) | 174 (4.6) | |
| 51–55 years | 2271 (25.1) | 17,267 (22.7) | 640 (16.9) | ||
| 56–60 years | 2225 (24.6) | 19,208 (25.2) | 923 (24.3) | ||
| 61–65 years | 2039 (22.5) | 18,047 (23.7) | 999 (26.3) | ||
| 66–70 years | 1382 (15.3) | 12,527 (16.4) | 773 (20.4) | ||
| 71–75 years | 462 (5.1) | 4393 (5.8) | 287 (7.6) | <0.001 | |
| Screening site | Community or workplace screening | 1574 (17.4) | 13,249 (17.4) | 605 (15.9) | |
| Outpatient | 7289 (80.5) | 60,939 (80.0) | 3106 (81.8) | ||
| Inpatient | 132 (1.5) | 1097 (1.4) | 42 (1.1) | ||
| Others | 60 (0.7) | 910 (1.2) | 43 (1.1) | <0.001 | |
| Category of follow-up examination medical unit | Outpatient | 8471 (93.6) | 67,244 (88.3) | 3389 (89.3) | |
| Inpatient | 584 (6.4) | 8951 (11.7) | 407 (10.7) | <0.001 | |
| Family history of colorectal cancer | No | 8303 (91.7) | 69,165 (90.8) | 3373 (88.9) | |
| Yes | 550 (6.1) | 5236 (6.9) | 318 (8.4) | ||
| Does not know | 202 (2.2) | 1794 (2.4) | 105 (2.8) | <0.001 | |
| Follow-up examination methods | Colonoscopy | 7215 (79.7) | 73,029 (95.8) | 3610 (95.1) | |
| Double contrast barium enema plus flexible sigmoidoscopy | 137 (1.5) | 697 (0.9) | 46 (1.2) | ||
| Others | 1703 (18.8) | 2469 (3.2) | 140 (3.7) | <0.001 | |
| Follow-up time | ≤30 days | 4872 (53.8) | 42,241 (55.4) | 2252 (59.3) | |
| 31–60 days | 2851 (31.5) | 22,187 (29.1) | 1029 (27.1) | ||
| 61–90 days | 808 (8.9) | 7292 (9.6) | 324 (8.5) | ||
| ≥91 days | 524 (5.8) | 4475 (5.9) | 191 (5.0) | <0.001 | |
| Variables | Categories | Diagnosis | |||||
|---|---|---|---|---|---|---|---|
| Non-CRC vs. Normal | CRC vs. Normal | ||||||
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | ||
| Gender | Female | Reference group | |||||
| Male | 1.550 | 1.481–1.621 | <0.001 | 1.735 | 1.606–1.876 | <0.001 | |
| Region of follow-up examination medical institution | Eastern Taiwan | Reference group | |||||
| Counties on outlying islands | 1.170 | 0.619–2.211 | 0.630 | 1.342 | 0.528–3.413 | 0.536 | |
| Northern Taiwan | 0.489 | 0.402–0.594 | <0.001 | 0.679 | 0.500–0.921 | 0.013 | |
| Central Taiwan | 0.411 | 0.337–0.501 | <0.001 | 0.607 | 0.445–0.828 | 0.002 | |
| Southern Taiwan | 0.482 | 0.396–0.586 | <0.001 | 0.760 | 0.559–1.032 | 0.079 | |
| Age of patients with positive response | 71–75 years | Reference group | |||||
| 50 years | 0.722 | 0.634–0.821 | <0.001 | 0.414 | 0.330–0.519 | <0.001 | |
| 51–55 years | 0.787 | 0.705–0.878 | <0.001 | 0.457 | 0.383–0.544 | <0.001 | |
| 56–60 years | 0.897 | 0.803–1.001 | 0.051 | 0.673 | 0.568–0.798 | <0.001 | |
| 61–65 years | 0.941 | 0.843–1.051 | 0.281 | 0.811 | 0.685–0.962 | 0.016 | |
| 66–70 years | 0.977 | 0.871–1.096 | 0.694 | 0.944 | 0.792–1.125 | 0.520 | |
| Screening site | Others | Reference group | |||||
| Community or workplace screening site | 0.620 | 0.473–0.813 | 0.001 | 0.613 | 0.409–0.921 | 0.018 | |
| Outpatient | 0.591 | 0.453–0.771 | <0.001 | 0.635 | 0.427–0.945 | 0.025 | |
| Inpatient | 0.662 | 0.478–0.917 | 0.013 | 0.545 | 0.321–0.925 | 0.025 | |
| Category of follow-up examination medical unit | Inpatient | Reference group | |||||
| Outpatient | 0.497 | 0.455–0.543 | <0.001 | 0.565 | 0.493–0.647 | <0.001 | |
| Family history of colorectal cancer | Dose not know | Reference group | |||||
| No | 1.124 | 0.965–1.310 | 0.134 | 0.984 | 0.770–1.258 | 0.900 | |
| Yes | 1.276 | 1.070–1.523 | 0.007 | 1.411 | 1.066–1.868 | 0.016 | |
| Follow-up examination method | Others | Reference group | |||||
| Colonoscopy | 7.006 | 6.550–7.494 | <0.001 | 6.249 | 5.232–7.465 | <0.001 | |
| Double contrast barium enema plus flexible sigmoidoscopy | 3.590 | 2.954–4.363 | <0.001 | 4.141 | 2.838–6.043 | <0.001 | |
| Follow-up time | - | 0.999 | 0.999–1.000 | 0.178 | 0.997 | 0.995–0.998 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.-W.; Li, Y.-C. Examining the Factors That Affect the Diagnosis of Patients with Positive Fecal Occult Blood Test Results. Int. J. Environ. Res. Public Health 2022, 19, 7569. https://doi.org/10.3390/ijerph19137569
Cheng Y-W, Li Y-C. Examining the Factors That Affect the Diagnosis of Patients with Positive Fecal Occult Blood Test Results. International Journal of Environmental Research and Public Health. 2022; 19(13):7569. https://doi.org/10.3390/ijerph19137569
Chicago/Turabian StyleCheng, Yin-Wen, and Ying-Chun Li. 2022. "Examining the Factors That Affect the Diagnosis of Patients with Positive Fecal Occult Blood Test Results" International Journal of Environmental Research and Public Health 19, no. 13: 7569. https://doi.org/10.3390/ijerph19137569
APA StyleCheng, Y.-W., & Li, Y.-C. (2022). Examining the Factors That Affect the Diagnosis of Patients with Positive Fecal Occult Blood Test Results. International Journal of Environmental Research and Public Health, 19(13), 7569. https://doi.org/10.3390/ijerph19137569






