Recent Advances in Ionic Liquids and Ionic Liquid-Functionalized Graphene: Catalytic Application and Environmental Remediation
Abstract
:1. Introduction
2. Ionic Liquids
Characteristics of ILs
3. Application of ILs
3.1. The Restoration of Water Environments
3.2. Application in Catalytic Reactions
3.3. Application in Electrochemistry
4. IL-Functionalized Graphene
4.1. ILs Enhance the Dispersion of Graphene
4.2. ILs Improve the Conductivity of Graphene
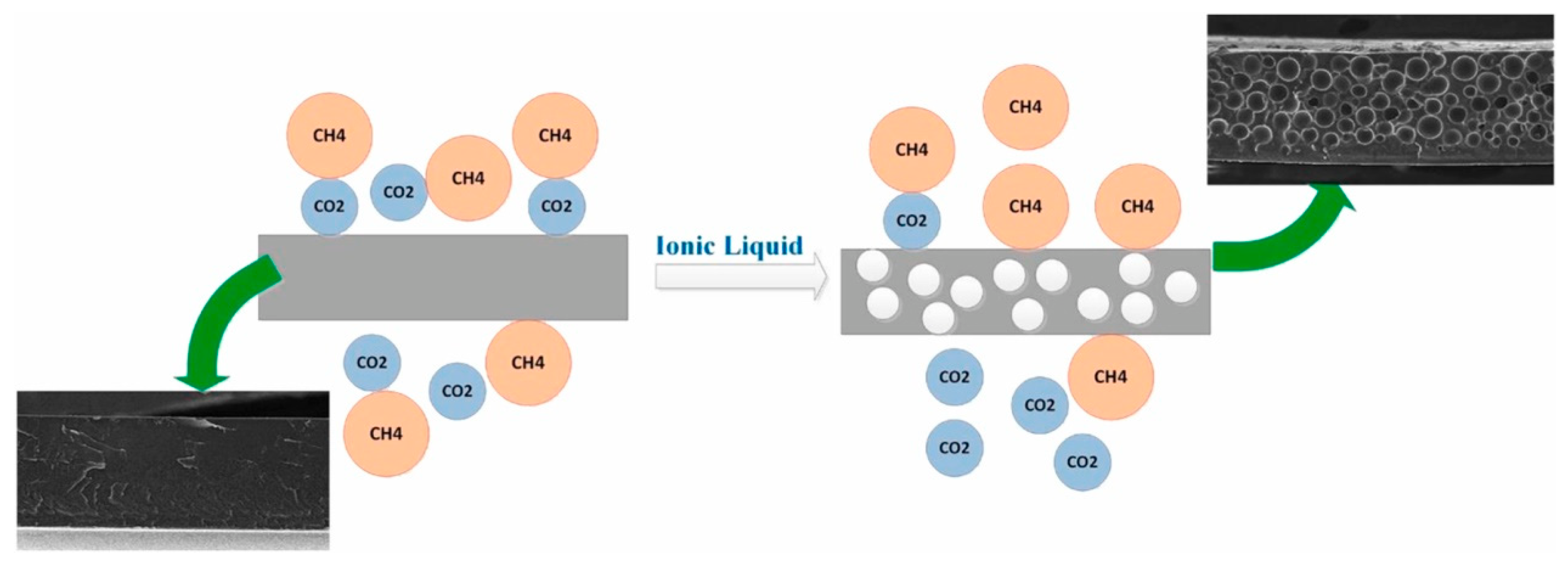
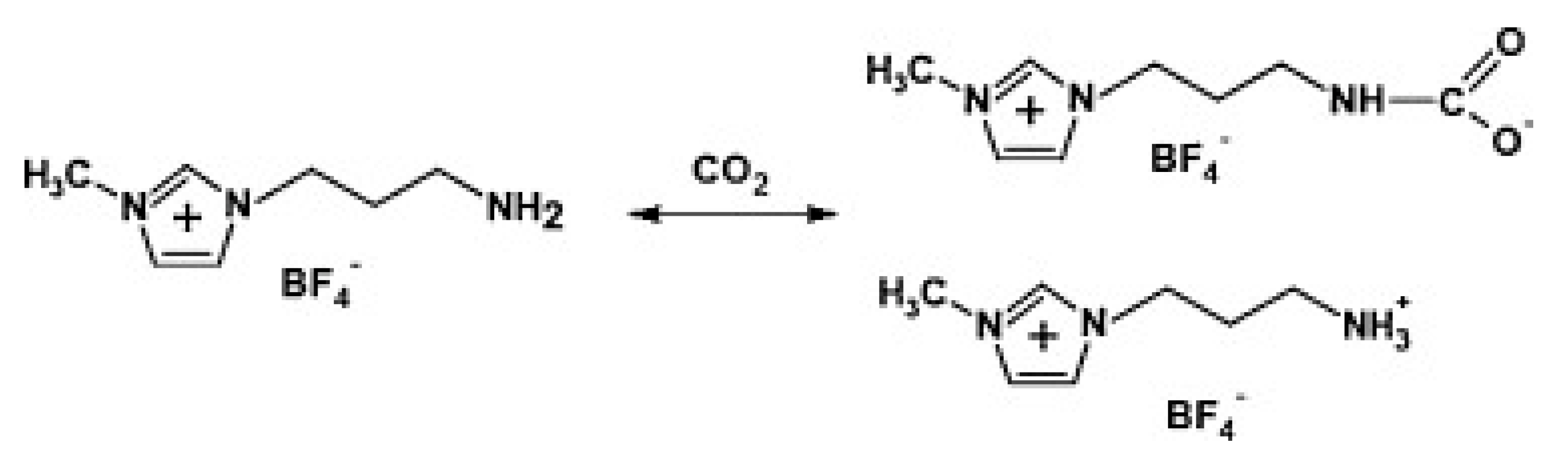
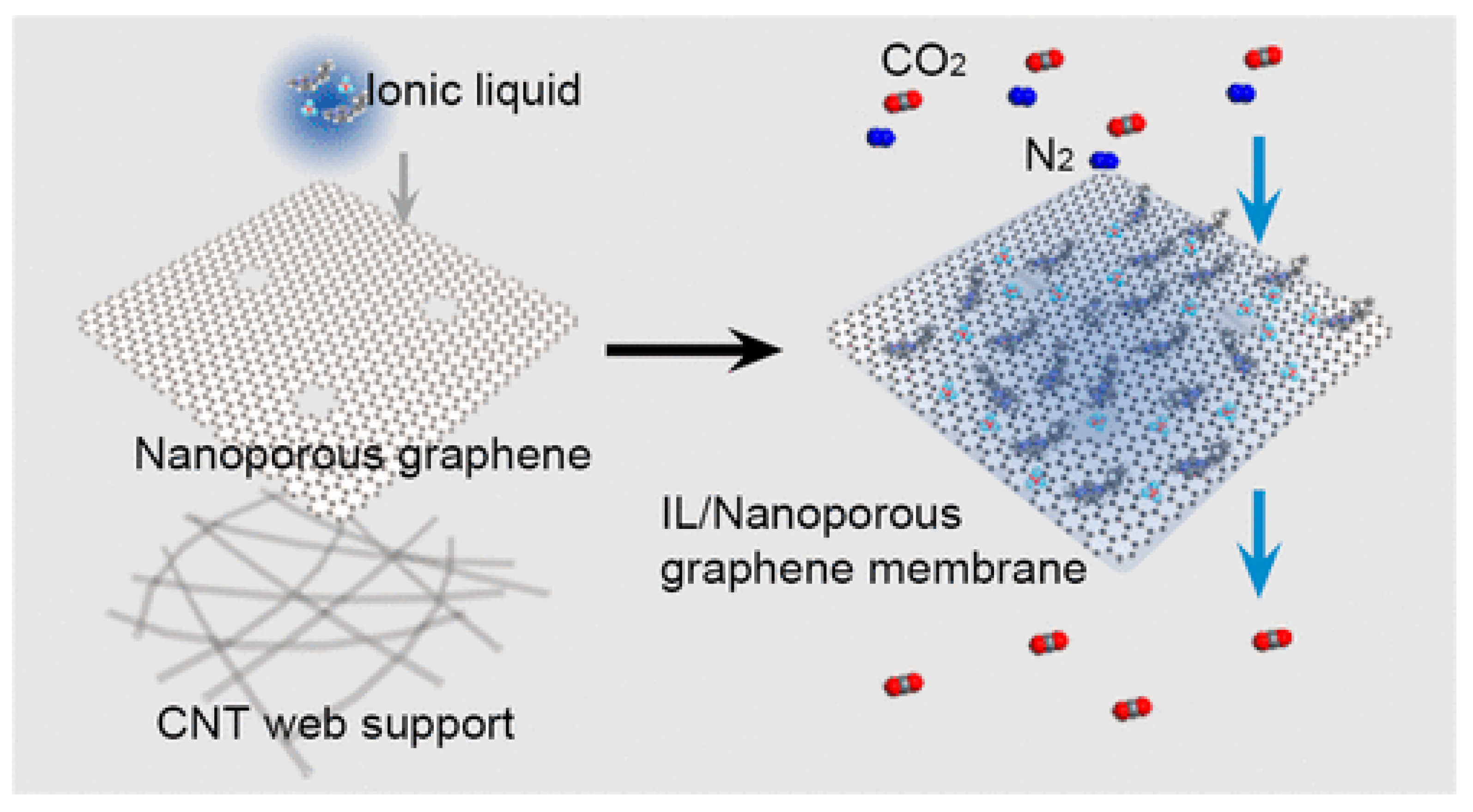
4.3. ILs Promote the Separation of CO2 from GO Composite Membrane Materials
4.4. ILs Enhance the Ability of Graphene Based Composites to Adsorb Heavy Metals
4.5. ILs Improve the Ability of Graphene-Based Electrodes to Degrade Pollutant PPCPs
4.5.1. Characteristics and Harmful Properties of PPCPs
4.5.2. Electrochemical Catalysis
4.5.3. Toxicity Discussion of ILs
Adsorption
Advanced Oxidation Processes (AOPs)
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sedghi, R.; Heidari, B.; Yassari, M. Novel molecularly imprinted polymer based on beta-cyclodextrin@graphene oxide: Synthesis and its application for selective diphenylamine determination. J. Colloid Interface Sci. 2017, 503, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Amde, M.; Liu, J.-F.; Pang, L. Environmental Application, Fate, Effects, and Concerns of Ionic Liquids: A Review. Environ. Sci. Technol. 2015, 49, 12611–12627. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, J.S.; Zaworotko, M.J. Air and water stable 1-ethyl-3-methylimidazolium based ionic liquids. J. Chem. Soc.-Chem. Commun. 1992, 13, 965–967. [Google Scholar] [CrossRef]
- Goutham, R.; Rohit, P.; Vigneshwar, S.S.; Swetha, A.; Arun, J.; Gopinath, K.P.; Pugazhendhi, A. Ionic liquids in wastewater treatment: A review on pollutant removal and degradation, recovery of ionic liquids, economics and future perspectives. J. Mol. Liq. 2022, 349, 118150. [Google Scholar] [CrossRef]
- Wei, G.T.; Yang, Z.S.; Chen, C.J. Room temperature ionic liquid as a novel medium for liquid/liquid extraction of metal ions. Anal. Chim. Acta 2003, 488, 183–192. [Google Scholar] [CrossRef]
- Solangi, N.H.; Anjum, A.; Tanjung, F.A.; Mazari, S.A.; Mubarak, N.M. A review of recent trends and emerging perspectives of ionic liquid membranes for CO2 separation. J. Environ. Chem. Eng. 2021, 9, 105860. [Google Scholar] [CrossRef]
- Kalidhasan, S.; Kumar, A.S.K.; Rajesh, V.; Rajesh, N. Enhanced adsorption of hexavalent chromium arising out of an admirable interaction between a synthetic polymer and an ionic liquid. Chem. Eng. J. 2013, 222, 454–463. [Google Scholar] [CrossRef]
- Nasrollahpour, A.; Moradi, S.; Moradi, S. Dispersive solid phase micro-extraction of mercury (II) from environmental water and vegetable samples with ionic liquid modified graphene oxide nanoparticles. J. Serb. Chem. Soc. 2017, 82, 551–565. [Google Scholar] [CrossRef] [Green Version]
- Bazrafshan, E.; Zarei, A.A.; Mohammadi, L.; Zafar, M.N.; Foroughi, M.; Aman, S.; Sabri, F.; Mahvi, A.H.; Barahuie, F.; Zafar, M. Efficient tetracycline removal from aqueous solutions using ionic liquid modified magnetic activated carbon (IL@mAC). J. Environ. Chem. Eng. 2021, 9, 106570. [Google Scholar] [CrossRef]
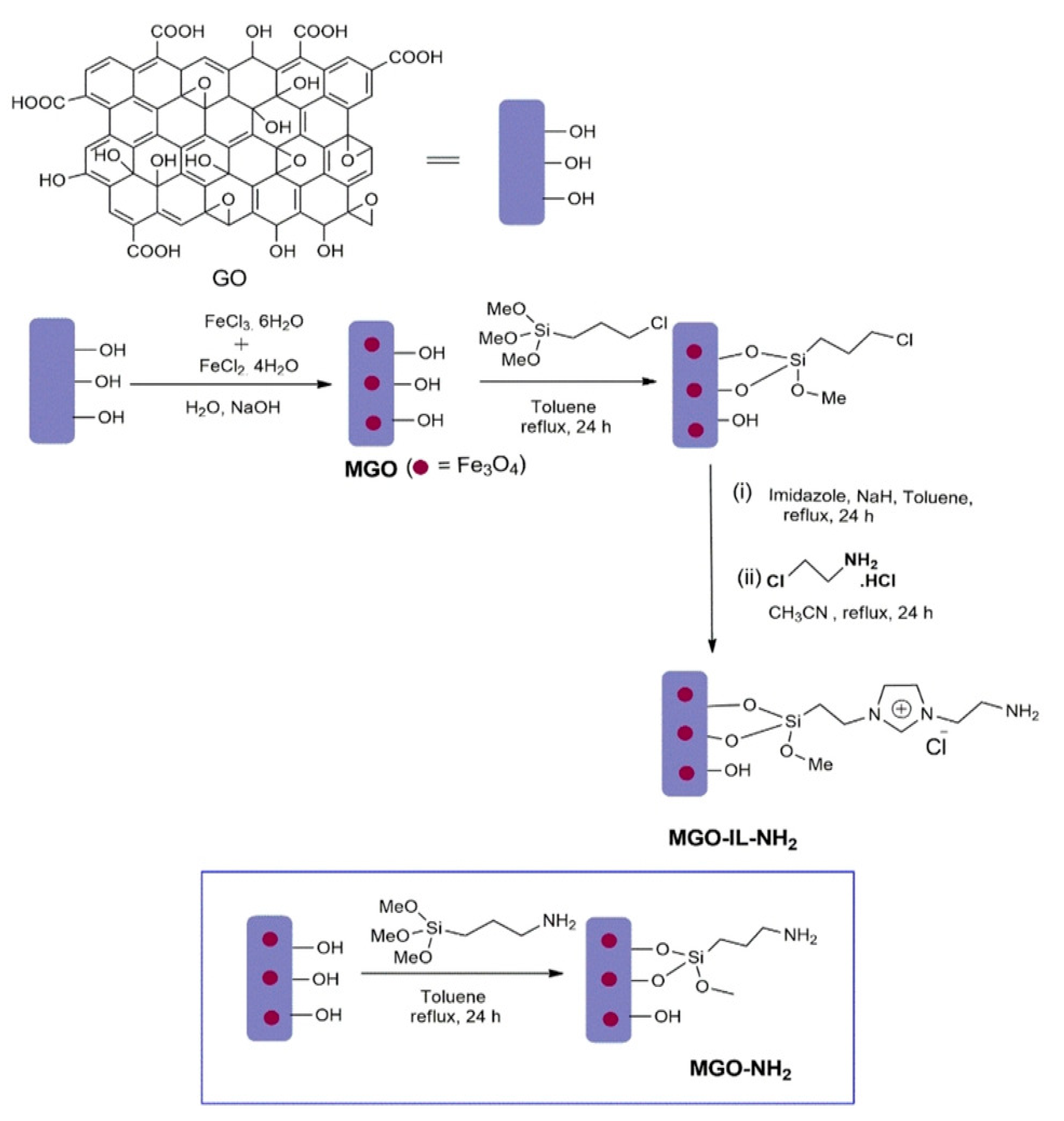
- Garkoti, C.; Shabir, J.; Mozumdar, S. Amine-Terminated Ionic Liquid Modified Magnetic Graphene Oxide (MGO-IL-NH2): A Highly Efficient and Reusable Nanocatalyst for the Synthesis of 3-Amino Alkylated Indoles. Chemistryselect 2020, 5, 4337–4346. [Google Scholar] [CrossRef]
- Turguła, A.; Graś, M.; Gabryelczyk, A.; Lota, G.; Pernak, J. Long-Chain Ionic Liquids Based on Monoquaternary DABCO Cations and TFSI Anions: Towards Stable Electrolytes for Electrochemical Capacitors. Chempluschem 2020, 85, 2679–2688. [Google Scholar] [CrossRef] [PubMed]
- Karimi, B.; Tavakolian, M.; Akbari, M.; Mansouri, F. Ionic Liquids in Asymmetric Synthesis: An Overall View from Reaction Media to Supported Ionic Liquid Catalysis. ChemCatChem 2018, 10, 3173–3205. [Google Scholar] [CrossRef]
- Dabiri, M.; Fazli, H.; Salarinejad, N.; Movahed, S.K. Pd nanoparticles supported on cubic shaped ZIF-based materials and their catalytic activates in organic reactions. Mater. Res. Bull. 2021, 133, 111015. [Google Scholar] [CrossRef]
- Tiong, Y.W.; Yap, C.L.; Gan, S.; Yap, W.S.P. Conversion of Biomass and Its Derivatives to Levulinic Acid and Levulinate Esters via Ionic Liquids. Ind. Eng. Chem. Res. 2018, 57, 4749–4766. [Google Scholar] [CrossRef]
- Mokhodoeva, O.B.; Maksimova, V.V.; Dzhenloda, R.K.; Shkinev, V.M. Magnetic Nanoparticles Modified by Ionic Liquids in Environmental Analysis. J. Anal. Chem. 2021, 76, 675–684. [Google Scholar] [CrossRef]
- Gan, C.; Liang, T.; Li, W.; Fan, X.; Zhu, M. Amine-terminated ionic liquid modified graphene oxide/copper nanocomposite toward efficient lubrication. Appl. Surf. Sci. 2019, 491, 105–115. [Google Scholar] [CrossRef]
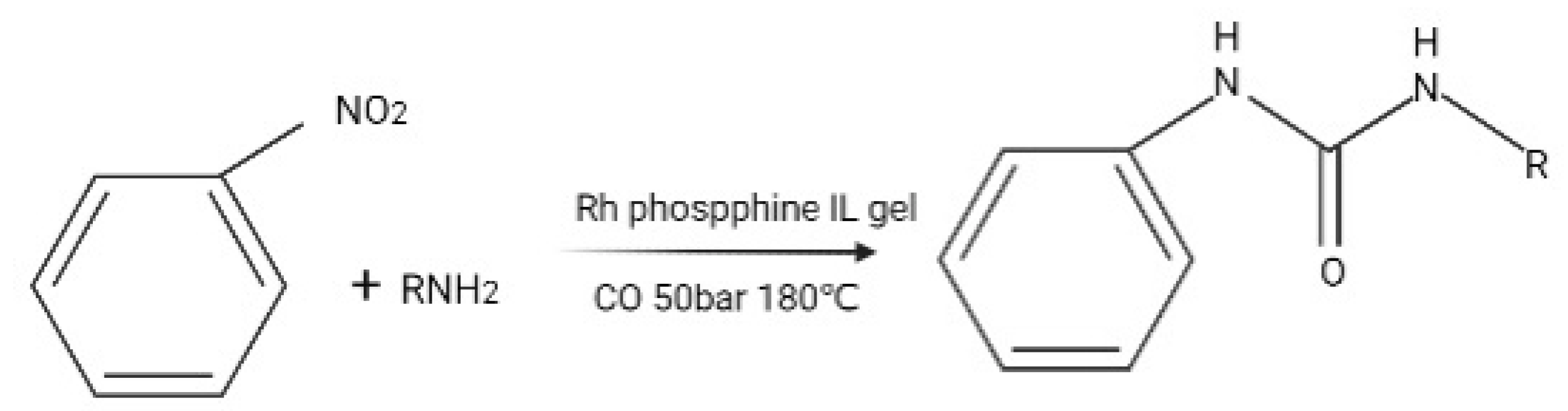
- Gaikwad, V.V.; Saptal, V.B.; Harada, K.; Sasaki, T.; Nishio-Hamane, D.; Bhanage, B.M. Ionic Liquid Immobilized on Graphene-Oxide-Containing Palladium Metal Ions as an Efficient Catalyst for the Alkoxy, Amino, and Phenoxy Carbonylation Reactions. Chemnanomat 2018, 4, 575–582. [Google Scholar] [CrossRef]
- Oluwole, A.O.; Omotola, E.O.; Olatunji, O.S. Pharmaceuticals and personal care products in water and wastewater: A review of treatment processes and use of photocatalyst immobilized on functionalized carbon in AOP degradation. BMC Chem. 2020, 14, 1–29. [Google Scholar]
- Can, E.; Uralcan, B.; Yildirim, R. Enhancing Charge Transfer in Photocatalytic Hydrogen Production over Dye-Sensitized Pt/TiO2 by Ionic Liquid Coating. ACS Appl. Energy Mater. 2021, 4, 10931–10939. [Google Scholar] [CrossRef]
- Mehnert, C.P.; Mozeleski, E.J.; Cook, R.A. Supported ionic liquid catalysis investigated for hydrogenation reactions. Chem. Commun. 2002, 24, 3010–3011. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, X.; Chen, S. A graphene oxide and ionic liquid assisted anion-immobilized polymer electrolyte with high ionic conductivity for dendrite-free lithium metal batteries. J. Power Sources 2020, 477, 228754. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Nie, Y.; Wang, C.; Ji, X.; Zhou, L.; Pan, F.; Zhang, S. Preparation of MWCNTs-Graphene-Cellulose Fiber with Ionic Liquids. ACS Sustain. Chem. Eng. 2019, 7, 20013–20021. [Google Scholar] [CrossRef]
- Wang, X.; Wu, M.; Li, H.; Wang, Q.; He, P.; Fang, Y. Simultaneous electrochemical determination of hydroquinone and catechol based on three-dimensional graphene/MWCNTs/BMIMPF 6 nanocomposite modified electrode. Sens. Actuators B Chem. 2014, 192, 452–458. [Google Scholar] [CrossRef]
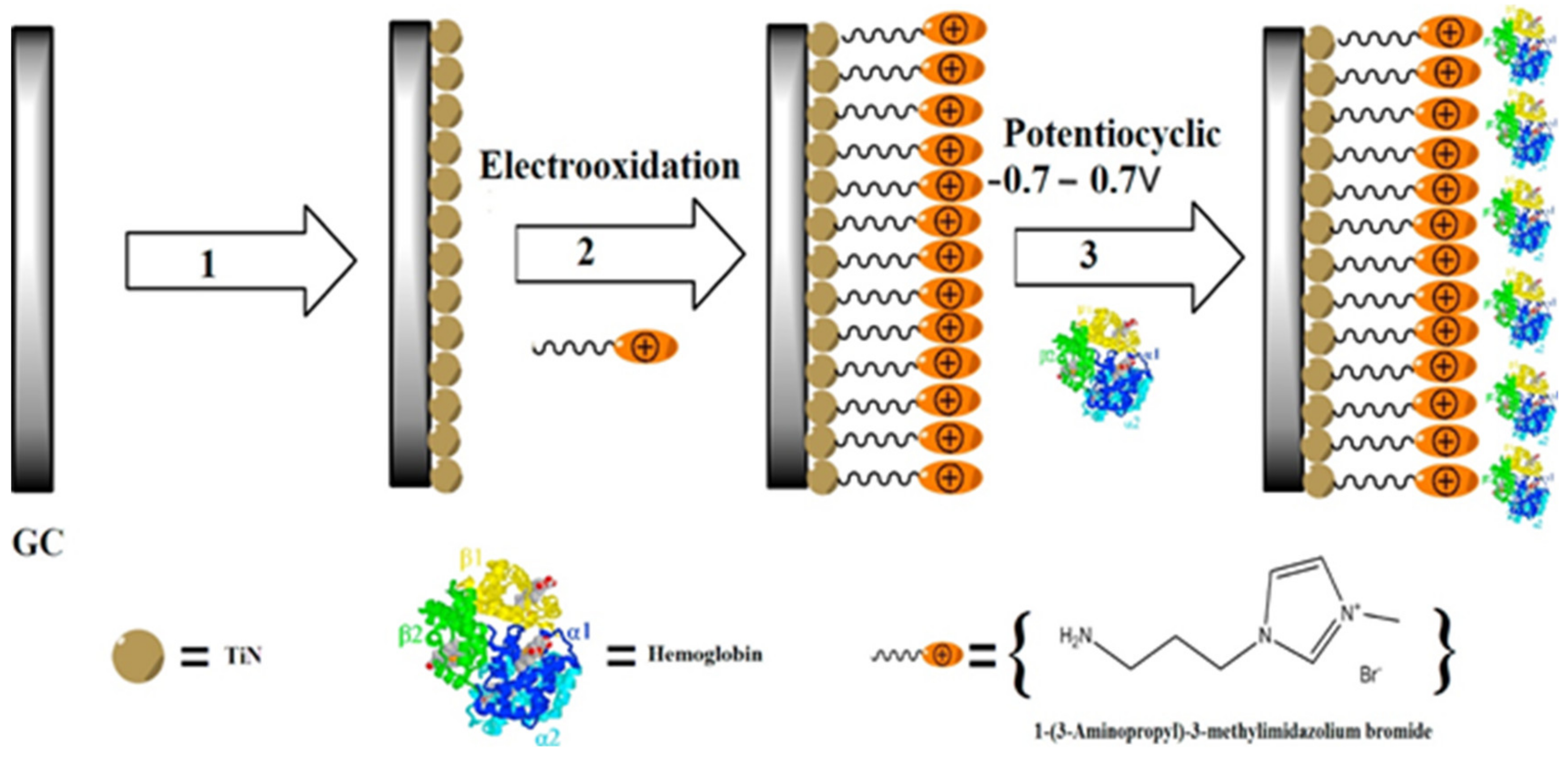
- Saadati, S.; Salimi, A.; Hallaj, R.; Rostami, A. Direct electron transfer and electrocatalytic properties of immobilized hemoglobin onto glassy carbon electrode modified with ionic-liquid/titanium-nitride nanoparticles: Application to nitrite detection. Sens. Actuators B Chem. 2014, 191, 625–633. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, Y.; Chen, D.; Liu, H.; Cui, P.; Yang, J. Ionic liquid-derived core-shell gold@palladium nanoparticles with tiny sizes for highly efficient electrooxidation of ethanol. Green Energy Environ. 2021, 6, 229–235. [Google Scholar] [CrossRef]
- Wang, X.; Zhi, L.; Muellen, K. Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett. 2008, 8, 323–327. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S. Preparation and electrochemical property of ionic liquid-attached graphene nanosheets for an application of supercapacitor electrode. Electrochim. Acta 2014, 119, 11–15. [Google Scholar] [CrossRef]
- Pu, J.; Wan, S.; Zhao, W.; Mo, Y.; Zhang, X.; Wang, L.; Xue, Q. Preparation and Tribological Study of Functionalized Graphene-IL Nanocomposite Ultrathin Lubrication Films on Si Substrates. J. Phys. Chem. C 2011, 115, 13275–13284. [Google Scholar] [CrossRef]
- Ji, Q.; Honma, I.; Paek, S.M.; Akada, M.; Hill, J.P.; Vinu, A.; Ariga, K. Layer-by-Layer Films of Graphene and Ionic Liquids for Highly Selective Gas Sensing. Angew. Chem. Int. Ed. 2010, 49, 9737–9739. [Google Scholar] [CrossRef]
- Yang, Y.K.; He, C.E.; Peng, R.G.; Baji, A.; Du, X.S.; Huang, Y.L.; Xie, X.L.; Mai, Y.W. Non-covalently modified graphene sheets by imidazolium ionic liquids for multifunctional polymer nanocomposites. J. Mater. Chem. 2012, 22, 5666–5675. [Google Scholar] [CrossRef]
- Xiao, W.; Sun, Z.; Chen, S.; Zhang, H.; Zhao, Y.; Huang, C.; Liu, Z. Ionic liquid-stabilized graphene and its use in immobilizing a metal nanocatalyst. RSC Adv. 2012, 2, 8189–8193. [Google Scholar] [CrossRef]
- Xu, J.; Xu, M.; Wu, J.; Wu, H.; Zhang, W.H.; Li, Y.X. Graphene oxide immobilized with ionic liquids: Facile preparation and efficient catalysis for solvent-free cycloaddition of CO2 to propylene carbonate. RSC Adv. 2015, 5, 72361–72368. [Google Scholar] [CrossRef]
- Zhang, B.; Ning, W.; Zhang, J.; Qiao, X.; Zhang, J.; He, J.; Liu, C.Y. Stable dispersions of reduced graphene oxide in ionic liquids. J. Mater. Chem. 2010, 20, 5401–5403. [Google Scholar] [CrossRef]
- McCrary, P.D.; Beasley, P.A.; Alaniz, S.A.; Griggs, C.S.; Frazier, R.M.; Rogers, R.D. Graphene and Graphene Oxide Can “Lubricate” Ionic Liquids based on Specific Surface Interactions Leading to Improved Low-Temperature Hypergolic Performance. Angew. Chem. Int. Ed. 2012, 51, 9784–9787. [Google Scholar] [CrossRef]
- Yang, H.; Shan, C.; Li, F.; Han, D.; Zhang, Q.; Niu, L. Covalent functionalization of polydisperse chemically-converted graphene sheets with amine-terminated ionic liquid. Chem. Commun. 2009, 26, 3880–3882. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, X.; Liu, Z.; Ma, Y.; Huang, Y.; Chen, Y. High-Efficiency Loading and Controlled Release of Doxorubicin Hydrochloride on Graphene Oxide. J. Phys. Chem. C 2008, 112, 17554–17558. [Google Scholar] [CrossRef]
- Bai, H.; Xu, Y.; Zhao, L.; Li, C.; Shi, G. Non-covalent functionalization of graphene sheets by sulfonated polyaniline. Chem. Commun. 2009, 13, 1667–1669. [Google Scholar] [CrossRef]
- Song, J.P.; Choi, S.H.; Chung, D.W.; Lee, S.J. Latex-Based Polystyrene Nanocomposites with Non-Covalently Modified Carbon Nanotubes. Polymers 2021, 13, 1168. [Google Scholar] [CrossRef]
- Plaquevent, J.C.; Levillain, J.; Guillen, F.; Malhiac, C.; Gaumont, A.C. Ionic liquids: New targets and media for alpha-amino acid and peptide chemistry. Chem. Rev. 2008, 108, 5035–5060. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Q.; Kuehner, D.; Xu, X.; Ivaska, A.; Niu, L. The synthesis of ionic-liquid-functionalized multiwalled carbon nanotubes decorated with highly dispersed Au nanoparticles and their use in oxygen reduction by electrocatalysis. Carbon 2008, 46, 1687–1692. [Google Scholar] [CrossRef]
- Kepley, L.J.; Crooks, R.M.; Ricco, A.J. Selective Surface Acoustic Wave-Based Organophosphonate Chemical Sensor Employing A Self-Assembled Composite Monolayer—A New Paradigm For Sensor Design. Anal. Chem. 1992, 64, 3191–3193. [Google Scholar] [CrossRef]
- Zhang, N.; Gao, N.; Fu, C.; Liu, D.; Li, S.; Jiang, L.; Zhou, H.; Kuang, Y. Hierarchical porous carbon spheres/graphene composite for supercapacitor with both aqueous solution and ionic liquid. Electrochim. Acta 2017, 235, 340–347. [Google Scholar] [CrossRef]
- Feng, L.; Wang, K.; Zhang, X.; Sun, X.; Li, C.; Ge, X.; Ma, Y. Flexible Solid-State Supercapacitors with Enhanced Performance from Hierarchically Graphene Nanocomposite Electrodes and Ionic Liquid Incorporated Gel Polymer Electrolyte. Adv. Funct. Mater. 2018, 28, 1704463. [Google Scholar] [CrossRef]
- Pereira, G.F.L.; Pereira, R.G.; Salanne, M.; Siqueira, L.J.A. Molecular Dynamics Simulations of Ether-Modified Phosphonium Ionic Liquid Confined in between Planar and Porous Graphene Electrode Models. J. Phys. Chem. C 2019, 123, 10816–10825. [Google Scholar] [CrossRef]
- Huang, K.J.; Niu, D.J.; Sun, J.Y.; Zhu, J.J. An electrochemical amperometric immunobiosensor for label-free detection of alpha-fetoprotein based on amine-functionalized graphene and gold nanoparticles modified carbon ionic liquid electrode. J. Electroanal. Chem. 2011, 656, 72–77. [Google Scholar] [CrossRef]
- Zhu, J.; Gu, Y.; Wu, J.; Zhu, X.; Xue, B.; Li, Y. Aqueous Grafting Ionic Liquid on Graphene Oxide for CO2 Cycloaddition. Catal. Lett. 2017, 147, 335–344. [Google Scholar] [CrossRef]
- Ying, W.; Cai, J.; Zhou, K.; Chen, D.; Ying, Y.; Guo, Y.; Kong, X.; Xu, Z.; Peng, X. Ionic Liquid Selectively Facilitates CO2 Transport through Graphene Oxide Membrane. ACS Nano 2018, 12, 5385–5393. [Google Scholar] [CrossRef]
- Guo, W.; Mahurin, S.M.; Unocic, R.R.; Luo, H.; Dai, S. Broadening the Gas Separation Utility of Monolayer Nanoporous Graphene Membranes by an Ionic Liquid Gating. Nano Lett. 2020, 20, 7995–8000. [Google Scholar] [CrossRef]
- Sanchez, L.M.G.; Meindersma, G.W.; De Haan, A.B. Kinetics of absorption of CO2 in amino-functionalized ionic liquids. Chem. Eng. J. 2011, 166, 1104–1115. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, X.; Li, J.; Huang, R.; Guo, L.; Zhang, X.; Fan, Y.; Xie, X.; Zeng, G. Enhanced removal of As(III) and As(V) from aqueous solution using ionic liquid-modified magnetic graphene oxide. Chemosphere 2019, 234, 196–203. [Google Scholar] [CrossRef]
- Etemadi, M.; Samadi, S.; Yazd, S.S.; Jafari, P.; Yousefi, N.; Aliabadi, M. Selective adsorption of Cr(VI) ions from aqueous solutions using Cr6+-imprinted Pebax/chitosan/GO/APTES nanofibrous adsorbent. Int. J. Biol. Macromol. 2017, 95, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahpour, A. Effective Removal of Hexavalent Chromium from Aqueous Solutions Using Ionic Liquid Modified Graphene Oxide Sorbent. Chem. Biochem. Eng. Q. 2017, 31, 325–334. [Google Scholar] [CrossRef]
- Nasrollahpour, A.; Moradi, S.E. Hexavalent chromium removal from water by ionic liquid modified metal-organic frameworks adsorbent. Microporous Mesoporous Mater. 2017, 243, 47–55. [Google Scholar] [CrossRef]
- Zambare, R.S.; Nemade, P.R. Ionic liquid-modified graphene oxide sponge for hexavalent chromium removal from water. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125657. [Google Scholar] [CrossRef]
- Lihua, C.; Liqian, C.; Fan, L. The adsorption performance of the ionic liquid functionalized graphene to Pb(Ⅱ) and Cd(Ⅱ). J. Nat. Sci. Heilongjiang Univ. 2015, 32, 645–653. [Google Scholar]
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 1999, 107, 907–938. [Google Scholar] [CrossRef]
- Ngoc Han, T.; Gin, K.Y.-H. Occurrence and removal of pharmaceuticals, hormones, personal care products, and endocrine disrupters in a full-scale water reclamation plant. Sci. Total Environ. 2017, 599, 1503–1516. [Google Scholar]
- Thelusmond, J.R.; Kawka, E.; Strathmann, T.J.; Cupples, A.M. Diclofenac, carbamazepine and triclocarban biodegradation in agricultural soils and the microorganisms and metabolic pathways affected. Sci. Total Environ. 2018, 640, 1393–1410. [Google Scholar] [CrossRef]
- Park, S.; Lee, W. Removal of selected pharmaceuticals and personal care products in reclaimed water during simulated managed aquifer recharge. Sci. Total Environ. 2018, 640, 671–677. [Google Scholar] [CrossRef]
- Aguilar-Pérez, K.M.; Avilés-Castrillo, J.I.; Ruiz-Pulido, G.; Medina, D.I.; Parra-Saldivar, R.; Iqbal, H.M. Nanoadsorbents in focus for the remediation of environmentally-related contaminants with rising toxicity concerns. Sci. Total Environ. 2021, 779, 146465. [Google Scholar] [CrossRef]
- Xiang, Y.; Wu, H.; Li, L.; Ren, M.; Qie, H.; Lin, A. A review of distribution and risk of pharmaceuticals and personal care products in the aquatic environment in China. Ecotoxicol. Environ. Saf. 2021, 213, 112044. [Google Scholar] [CrossRef] [PubMed]
- Norvill, Z.N.; Shilton, A.; Guieysse, B. Emerging contaminant degradation and removal in algal wastewater treatment ponds: Identifying the research gaps. J. Hazard. Mater. 2016, 313, 291–309. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.J. Recent advances in electro-oxidation technology for water treatment. J. Civ. Environ. Eng. 2022, 44, 104–118. [Google Scholar]
- Chiang, L.C.; Chang, J.E.; Wen, T.C. Indirect Oxidation Effect in Electrochemical Oxidation Treatment of Landfill Leachate. Water Res. 1995, 29, 671–678. [Google Scholar] [CrossRef]
- Rajeshwar, K.; Ibanez, J.G.; Swain, G.M. Electrochemistry and the Environment. J. Appl. Electrochem. 1994, 24, 1077–1091. [Google Scholar] [CrossRef]
- Motoc, S.; Manea, F.; Baciu, A.; Orha, C.; Pop, A. Electrochemical Method for Ease Determination of Sodium Diclofenac Trace Levels in Water Using Graphene-Multi-Walled Carbon Nanotubes Paste Electrode. Int. J. Environ. Res. Public Health 2022, 19, 29. [Google Scholar] [CrossRef]
- Masud, A.; Soria, N.G.C.; Aga, D.S.; Aich, N. Adsorption and advanced oxidation of diverse pharmaceuticals and personal care products (PPCPs) from water using highly efficient rGO-nZVI nanohybrids. Environ. Sci.-Water Res. Technol. 2020, 6, 2223–2238. [Google Scholar] [CrossRef]
- Teixeira, P.R.; Machado, T.R.; Machado, F.; Sodré, F.F.; Silva, J.G.; Neto, B.A.; Paterno, L.G. Au nanoparticle-poly(ionic liquid) nanocomposite electrode for the voltammetric detection of triclosan in lake water and toothpaste samples. Microchem. J. 2020, 152, 104421. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, F.; Zeng, B. Facile fabrication of a novel anisotropic gold nanoparticle-chitosan-ionic liquid/graphene modified electrode for the determination of theophylline and caffeine. Talanta 2014, 127, 116–122. [Google Scholar] [CrossRef]
- Qi, X.; Li, L.; Tan, T.; Chen, W.; Smith, R.L., Jr. Adsorption of 1-Butyl-3-Methylimidazolium Chloride Ionic Liquid by Functional Carbon Microspheres from Hydrothermal Carbonization of Cellulose. Environ. Sci. Technol. 2013, 47, 2792–2798. [Google Scholar] [CrossRef]
- Ngoc Lan, M.; Ahn, K.; Koo, Y.-M. Methods for recovery of ionic liquids-A review. Process Biochem. 2014, 49, 872–881. [Google Scholar]
- Bubalo, M.C.; Radošević, K.; Redovniković, I.R.; Halambek, J.; Srček, V.G. A brief overview of the potential environmental hazards of ionic liquids. Ecotoxicol. Environ. Saf. 2014, 99, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chen, L.; He, Y.Y.; Yan, Z.C.; Zheng, X.J. Degradation of imidazolium-based ionic liquids in aqueous solution using plasma electrolysis. J. Hazard. Mater. 2014, 265, 261–270. [Google Scholar] [CrossRef] [PubMed]

| PPCPs | Chemical Formula | Molecular Weight | Application | Toxicity |
|---|---|---|---|---|
| Diclofenac | C14H11Cl2NO2 | 296.15 | Non-steroidal anti-inflammatory drugs, anti-inflammatory, analgesic, antipyretic. | LD50 orally in mice: 390 mg/kg |
| Carbamazepine | C15H12N2O | 236.27 | Anticonvulsant, analgesic, anti-manic, antipsychotic. | LD50 orally in mice: 3750 mg/kg |
| Ibuprofen | C13H18O2 | 206.28 | Non-steroidal anti-inflammatory drugs, anti-inflammatory, analgesic, antipyretic. | LD50 orally in mice: 1255 mg/kg |
| Triclosan | C12H7Cl3O2 | 289.54 | Broad-spectrum antibacterial agent, used in daily chemicals. | LD50 orally in mice: 4000 mg/kg |
| Sulfamethoxazole | C10H11N3O3S | 253.28 | Broad-spectrum antibacterial agent, used in daily chemicals. | LD50 orally in mice: 3662 mg/kg |
| Tetracycline | C22H24N2O8 | 444.43 | Broad-spectrum antibiotics, bacteriostatic agents. | LD50 orally in mice: 807 mg/kg |
| Amoxicillin | C16H19N3O5S | 365.40 | Broad-spectrum semi-synthetic penicillin antibiotics. | LD50 orally in mice: 25 mg/kg |
| Paracetamol | C8H9NO2 | 151.16 | Broad-spectrum semi-synthetic penicillin antibiotics. | LD50 orally in mice: 338 mg/kg |
| Naproxen | C14H14O3 | 230.26 | Non-steroidal anti-inflammatory drugs, anti-inflammatory, analgesic, antipyretic. | LD50 orally in mice: 1234 mg/kg |
| Bisphenol A | C15H16O2 | 228.29 | Used as a PVC stabilizer, plastic antioxidant, UV absorber, fungicide | LC50 in rainbow trout: 3000−3500 mg/l |
| Ciprofloxacin | C17H18FN3O3 | 331.34 | Quinolone antibacterial, broad antibacterial spectrum, strong and bactericidal. | LD50 orally in rats: 2000 mg/kg |
| Caffeine | C8H10N4O2 | 194.19 | Astragalus alkaloid compound, central nervous system stimulant. | LD50 orally in mice: 127 mg/kg |
| Sulfamethazine | C12H14N4O2S | 278.33 | Broad spectrum bacteriostat. | LD50 i.p. in mice: 1776 mg/kg |
| Metoprolol | C15H25NO3 | 267.36 | Treating high blood pressure and angina. | LD50 orally in mice: 2193 mg/kg |
| β-Estradiol | C18H24O2 | 272.38 | Biochemical research, female hormone drugs | LD50 subcutaneous in rat: 300 mg/kg |
| Trimethoprim | C14H18N4O3 | 290.32 | Antibacterial potentiator, broad-spectrum antibacterial | LD50 orally in mice: 7000 mg/kg |
| Atenolol | C14H22N2O3 | 266.34 | Treating high blood pressure and angina. | LD50 orally in mice: 2000 mg/kg |
| Ofloxacin | C18H20FN3O4 | 361.37 | Quinolone antibacterial agent, broad-spectrum antibacterial. | LD50 orally in mice: 5290 mg/kg |
| Ranitidine | C18H20FN3O4 | 314.40 | Digestive system medication for the treatment of stomach acid, stomach ulcers. | LD50 orally in mice: 30 mg/kg |
| Norfloxacin | C16H18FN3O3 | 319.33 | Quinolone antibacterial agent, broad-spectrum antibacterial. | LD50 orally in mice: 4000 mg/kg |
| Title | Publication Date | Author |
|---|---|---|
| Electrochemical Method for Ease Determination of Sodium Diclofenac Trace Levels in Water Using Graphene—Multi-Walled Carbon Nanotubes Paste Electrode | 2022 | Sorina Motoc, Florica Manea, Anamaria Baciu, Corina Orha and Aniela Pop |
| Poly(ionic liquid)/graphene oxide-derived porous carbon materials as highly efcient electrocatalysts for hydrogen evolution reaction | 2022 | Chenming Liu, Honghong Song, Zhifeng Dai, Yubing Xiong |
| Composites of porous materials with ionic liquids: Synthesis, characterization, applications, and beyond | 2022 | Ozce Durak, Muhammad Zeeshan, Nitasha Habib, Hasan Can Gulbalkan, Ala Abdulalem Abdo Moqbel Alsuhile et al. |
| Functional Ionic Liquids Decorated Carbon Hybrid Nanomaterials for the Electrochemical Biosensors | 2021 | Pushpesh Ranjan, Shalu Yadav, Mohd Abubakar Sadique Raju Khan, Jamana Prasad Chaurasiaand Avanish Kumar Srivastava |
| Efficient tetracycline removal from aqueous solutions using ionic liquid modified magnetic activated carbon (IL@mAC) | 2021 | Edris Bazrafshan, Amin Allah Zarei, Leili Mohammadi et al. |
| Oxidized Graphene in Ionic Liquids for Assembling Chemically Modified Electrodes: A Structural and Electrochemical Characterization Study | 2021 | Valentini, F, Roscioli, D, Carbone, M, Conte, V, Floris, B, Palleschi, G, Flammini, R, Bauer, EM, Nasillo, G, Caponetti, E |
| Progress in the functional modification of graphene/graphene oxide: a review | 2020 | Wang Yu, Li Sisi, Yang Haiyan and Luo Jie |
| Specifying the Effects of Functionalization of Highly Reduced Graphene Oxide by an Ionic Liquid on Supercapacitive Features | 2020 | Mohammad Bagher Bakhshandeh and Elaheh Kowsari |
| Amine-Terminated Ionic Liquid Modified Magnetic Graphene Oxide (MGO-IL-NH2): A Highly Efficient and Reusable Nanocatalyst for the Synthesis of 3-Amino Alkylated Indoles | 2020 | Charu Garkoti, Javaid Shabir, and Subho Mozumdar |
| Adsorption and advanced oxidation of diverse pharmaceuticals and personal care products (PPCPs) from water using highly efficient rGO–nZVI nanohybrids | 2020 | Arvid Masud, Nita G. Chavez Soria, Diana S. Aga and Nirupam Aich |
| Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater | 2020 | S.F. Ahmed, M. Mofijur, Samiha Nuzhat et al. |
| Ionic liquid-modified reduced graphene oxide electrode material with favourable electrochemical properties | 2020 | Chang Dong, Yijia Yu, Xiaoling Zhang, Liyan Huang, Ying Wu, Jun Li and Zhengping Liu |
| Structural and electronic properties of graphene and its derivatives physisorbed by ionic liquids | 2020 | V.S. Anithaa, R. Shankar, S. Vijayakumar |
| Recent advances of ionic liquids in sample preparation | 2020 | Juanjuan Feng, Herman Maloko Loussala, Sen Han, Xiangping Ji, Chunying Li, Min Sun |
| Cellulose nanocrystals/graphene oxide composite for the adsorption andremoval of levofloxacin hydrochloride antibiotic from aqueous solution | 2020 | Junhong Tao, Jie Yang, Chengxiao Ma, Junfeng Li et al. |
| Imidazole polymerized ionic liquid as a precursor for an iron-nitrogen-doped carbon electrocatalyst used in the oxygen reduction reaction | 2020 | Bing Han, Shuping Yu, Zhongming Wang, Hong Zhu |
| Ionic liquid-modified composites for the adsorptive removal of emerging water contaminants: A review | 2019 | Ali Ayati, Sara Ranjbari, Bahareh Tanhaei, Mika Sillanpää |
| Amine-terminated ionic liquid modified graphene oxide/copper nanocomposite toward efficient lubrication | 2019 | Chaoliang Gana, Ting Lianga, Wen Lia, Xiaoqiang Fana, Minhao Zhu |
| New Directions in Using Ionic Liquids in Analytical Chemistry. 2: Electrochemical Methods | 2019 | I. V. Pletnev, S. V. Smirnova, and N. V. Shvedene |
| Ionic Liquid Immobilized on Graphene-Oxide-Containing Palladium Metal Ions as an Efficient Catalyst for the Alkoxy, Amino, and Phenoxy Carbonylation Reactions | 2018 | Vinayak V. Gaikwad, Vitthal B. Saptal, Kei Harada, Takehiko Sasaki, Daisuke NishioHamane, and Bhalchandra M. Bhanag |
| Ionic liquid and nanoparticle hybrid systems: Emerging applications | 2017 | Zhiqi He, Paschalis Alexandridis |
| Graphene oxide grafted hydroxyl-functionalized ionic liquid: A highly efficient catalyst for cycloaddition of CO2 with epoxides | 2016 | Wei-Hong Zhang, Pan-Pan He, Sheng Wu, Jie Xu, Yongxin Li, Gen Zhang, Xian-Yong Wei |
| Sono-assisted preparation of magnetic ferroferric oxide/graphene oxide nanoparticles and application on dye removal | 2015 | Guodong Jiang, Qing Chang, Fufu Yang, Xiaoyun Hu, Heqing Tang |
| Facile fabrication of a novel anisotropic gold nanoparticle–chitosan–ionic liquid/graphene modified electrode for the determination of theophylline and caffeine | 2014 | Guangming Yang, Faqiong Zhao, Baizhao Zeng |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Bai, S.; Zhang, Y.; Xu, D.; Wang, M. Recent Advances in Ionic Liquids and Ionic Liquid-Functionalized Graphene: Catalytic Application and Environmental Remediation. Int. J. Environ. Res. Public Health 2022, 19, 7584. https://doi.org/10.3390/ijerph19137584
Zhou H, Bai S, Zhang Y, Xu D, Wang M. Recent Advances in Ionic Liquids and Ionic Liquid-Functionalized Graphene: Catalytic Application and Environmental Remediation. International Journal of Environmental Research and Public Health. 2022; 19(13):7584. https://doi.org/10.3390/ijerph19137584
Chicago/Turabian StyleZhou, Han, Shaoyuan Bai, Yanan Zhang, Dandan Xu, and Mei Wang. 2022. "Recent Advances in Ionic Liquids and Ionic Liquid-Functionalized Graphene: Catalytic Application and Environmental Remediation" International Journal of Environmental Research and Public Health 19, no. 13: 7584. https://doi.org/10.3390/ijerph19137584
APA StyleZhou, H., Bai, S., Zhang, Y., Xu, D., & Wang, M. (2022). Recent Advances in Ionic Liquids and Ionic Liquid-Functionalized Graphene: Catalytic Application and Environmental Remediation. International Journal of Environmental Research and Public Health, 19(13), 7584. https://doi.org/10.3390/ijerph19137584







