Sleep Problems and 6-Sulfatoxymelatonin as Possible Predictors of Symptom Severity, Adaptive and Maladaptive Behavior in Children with Autism Spectrum Disorder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample
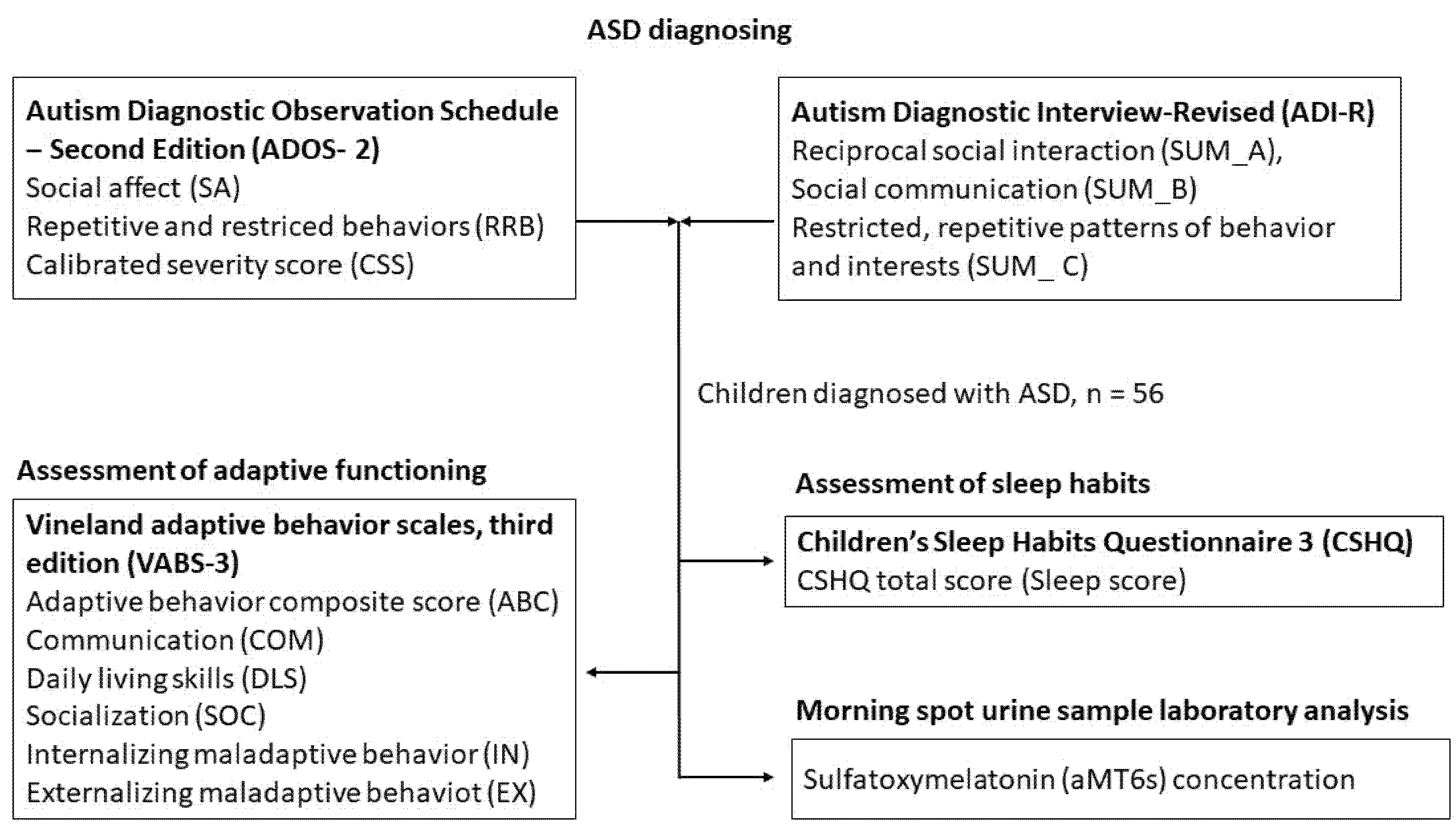
2.2. Diagnostic Evaluation of ASD
2.3. Assessment of Adaptive Functioning
2.4. Assessment of Sleep Habits
2.5. Urine Collection
2.6. Statistical Analysis
3. Results
3.1. Explorative Correlation Analysis
3.2. aMT6s/Creatinine as a Predictor of ASD Symptom Severity
3.3. CSHQ Sleep Score as a Predictor of Adaptive Functioning
3.4. CSHQ Sleep Score as a Predictor of Maladaptive Functioning
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- American Psychiatric Association (Ed.) Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 978-0-89042-554-1. [Google Scholar]
- Lee, H.J.; Park, H.R. An Integrated Literature Review on the Adaptive Behavior of Individuals with Asperger Syndrome. Remedial Spec. Educ. 2007, 28, 132–139. [Google Scholar] [CrossRef]
- Frye, R.E.; Rossignol, D.A. Identification and Treatment of Pathophysiological Comorbidities of Autism Spectrum Disorder to Achieve Optimal Outcomes. Clin. Med. Insights Pediatr. 2016, 10, 43–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, D.; Belli, A.; Ferri, R.; Bruni, O. Sleeping without Prescription: Management of Sleep Disorders in Children with Autism with Non-Pharmacological Interventions and Over-the-Counter Treatments. Brain Sci. 2020, 10, 441. [Google Scholar] [CrossRef] [PubMed]
- Sikora, D.M.; Johnson, K.; Clemons, T.; Katz, T. The Relationship between Sleep Problems and Daytime Behavior in Children of Different Ages with Autism Spectrum Disorders. Pediatrics 2012, 130 (Suppl. 2), S83–S90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregory, A.M.; Sadeh, A. Annual Research Review: Sleep Problems in Childhood Psychiatric Disorders—A Review of the Latest Science. J. Child Psychol. Psychiatry 2016, 57, 296–317. [Google Scholar] [CrossRef]
- Cohen, S.; Conduit, R.; Lockley, S.W.; Rajaratnam, S.M.; Cornish, K.M. The Relationship between Sleep and Behavior in Autism Spectrum Disorder (ASD): A Review. J. Neurodev. Disord. 2014, 6, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollway, J.A.; Aman, M.G.; Butter, E. Correlates and Risk Markers for Sleep Disturbance in Participants of the Autism Treatment Network. J. Autism Dev. Disord. 2013, 43, 2830–2843. [Google Scholar] [CrossRef]
- Schreck, K.A.; Mulick, J.A.; Smith, A.F. Sleep Problems as Possible Predictors of Intensified Symptoms of Autism. Res. Dev. Disabil. 2004, 25, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Cho, S.C.; Cho, I.H.; Kim, B.N.; Kim, J.W.; Shin, M.S.; Chung, U.S.; Park, T.W.; Son, J.W.; Yoo, H.J. Sleep Problems and Their Correlates and Comorbid Psychopathology of Children with Autism Spectrum Disorders. Res. Autism Spectr. Disord. 2012, 6, 1068–1072. [Google Scholar] [CrossRef]
- Tudor, M.E.; Hoffman, C.D.; Sweeney, D.P. Children with Autism: Sleep Problems and Symptom Severity. Focus Autism Other Dev. Disabl. 2012, 27, 254–262. [Google Scholar] [CrossRef]
- Mazurek, M.O.; Sohl, K. Sleep and Behavioral Problems in Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2016, 46, 1906–1915. [Google Scholar] [CrossRef]
- Krakowiak, P.; Goodlin-Jones, B.; Hertz-Picciotto, I.; Croen, L.A.; Hansen, R.L. Sleep Problems in Children with Autism Spectrum Disorders, Developmental Delays, and Typical Development: A Population-Based Study. J. Sleep Res. 2008, 17, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Leader, G.; Dooley, E.; Whelan, S.; Gilroy, S.P.; Chen, J.L.; Farren Barton, A.; Coyne, R.; Mannion, A. Attention-Deficit/Hyperactivity Disorder Symptoms, Gastrointestinal Symptoms, Sleep Problems, Challenging Behavior, Adaptive Behavior, and Quality of Life in Children and Adolescents with Autism Spectrum Disorder. Dev. Neurorehabil. 2022, 25, 217–228. [Google Scholar] [CrossRef]
- Taylor, M.A.; Schreck, K.A.; Mulick, J.A. Sleep Disruption as a Correlate to Cognitive and Adaptive Behavior Problems in Autism Spectrum Disorders. Res. Dev. Disabil. 2012, 33, 1408–1417. [Google Scholar] [CrossRef]
- Tordjman, S.; Najjar, I.; Bellissant, E.; Anderson, G.M.; Barburoth, M.; Cohen, D.; Jaafari, N.; Schischmanoff, O.; Fagard, R.; Lagdas, E.; et al. Advances in the Research of Melatonin in Autism Spectrum Disorders: Literature Review and New Perspectives. Int. J. Mol. Sci. 2013, 14, 20508–20542. [Google Scholar] [CrossRef] [Green Version]
- Geoffray, M.-M.; Nicolas, A.; Speranza, M.; Georgieff, N. Are Circadian Rhythms New Pathways to Understand Autism Spectrum Disorder? J. Physiol. 2016, 110, 434–438. [Google Scholar] [CrossRef]
- Leu, R.M.; Beyderman, L.; Botzolakis, E.J.; Surdyka, K.; Wang, L.; Malow, B.A. Relation of Melatonin to Sleep Architecture in Children with Autism. J. Autism Dev. Disord. 2011, 41, 427–433. [Google Scholar] [CrossRef]
- da Silveira Cruz-Machado, S.; Guissoni Campos, L.M.; Fadini, C.C.; Anderson, G.; Markus, R.P.; Pinato, L. Disrupted Nocturnal Melatonin in Autism: Association with Tumor Necrosis Factor and Sleep Disturbances. J. Pineal Res. 2021, 70, e12715. [Google Scholar] [CrossRef]
- Tordjman, S.; Anderson, G.M.; Pichard, N.; Charbuy, H.; Touitou, Y. Nocturnal Excretion of 6-Sulphatoxymelatonin in Children and Adolescents with Autistic Disorder. Biol. Psychiatry 2005, 57, 134–138. [Google Scholar] [CrossRef]
- Tordjman, S.; Anderson, G.M.; Bellissant, E.; Botbol, M.; Charbuy, H.; Camus, F.; Graignic, R.; Kermarrec, S.; Fougerou, C.; Cohen, D.; et al. Day and Nighttime Excretion of 6-Sulphatoxymelatonin in Adolescents and Young Adults with Autistic Disorder. Psychoneuroendocrinology 2012, 37, 1990–1997. [Google Scholar] [CrossRef]
- Lord, C.; Risi, S.; Lambrecht, L.; Cook, E.H.; Leventhal, B.L.; DiLavore, P.C.; Pickles, A.; Rutter, M. The Autism Diagnostic Observation Schedule—Generic: A Standard Measure of Social and Communication Deficits Associated with the Spectrum of Autism. J. Autism Dev. Disord. 2000, 30, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Gotham, K.; Pickles, A.; Lord, C. Standardizing ADOS Scores for a Measure of Severity in Autism Spectrum Disorders. J. Autism Dev. Disord. 2009, 39, 693–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lord, C.; Rutter, M.; Le Couteur, A. Autism Diagnostic Interview-Revised: A Revised Version of a Diagnostic Interview for Caregivers of Individuals with Possible Pervasive Developmental Disorders. J. Autism Dev. Disord. 1994, 24, 659–685. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, S.S.; Saulnier, C.A.; Cicchetti, D.V.; Doll, E.A. Vineland-3: Vineland Adaptive Behavior Scales; PsychCorp: Bloomington, MN, USA, 2016. [Google Scholar]
- Owens, J.A.; Spirito, A.; McGuinn, M. The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric Properties of a Survey Instrument for School-Aged Children. Sleep 2000, 23, 1043–1051. [Google Scholar] [CrossRef]
- Tang, K.; Toh, Q.; Teo, B. Normalisation of Urinary Biomarkers to Creatinine for Clinical Practice and Research—When and Why. Singap. Med. J. 2015, 56, 7–10. [Google Scholar] [CrossRef] [Green Version]
- Definition. Available online: https://www.aaidd.org/intellectual-disability/definition (accessed on 22 February 2022).
- Toth, K.; Dawson, G.; Meltzoff, A.N.; Greenson, J.; Fein, D. Early Social, Imitation, Play, and Language Abilities of Young Non-Autistic Siblings of Children with Autism. J. Autism Dev. Disord. 2007, 37, 145–157. [Google Scholar] [CrossRef] [Green Version]
- Zwaigenbaum, L.; Bryson, S.E.; Szatmari, P.; Brian, J.; Smith, I.M.; Roberts, W.; Vaillancourt, T.; Roncadin, C. Sex Differences in Children with Autism Spectrum Disorder Identified within a High-Risk Infant Cohort. J. Autism Dev. Disord. 2012, 42, 2585–2596. [Google Scholar] [CrossRef]
- Shephard, E.; Milosavljevic, B.; Pasco, G.; Jones, E.J.H.; Gliga, T.; Happé, F.; Johnson, M.H.; Charman, T. BASIS Team Mid-Childhood Outcomes of Infant Siblings at Familial High-Risk of Autism Spectrum Disorder. Autism Res. 2017, 10, 546–557. [Google Scholar] [CrossRef] [Green Version]
- Balboni, G.; Mumbardó-Adam, C.; Coscarelli, A. Influence of Adaptive Behaviour on the Quality of Life of Adults with Intellectual and Developmental Disabilities. J. Appl. Res. Intellect. Disabil. 2020, 33, 584–594. [Google Scholar] [CrossRef]
- Balboni, G.; Bacherini, A.; Rebecchini, G.; Cagiano, R.; Mancini, A.; Tancredi, R.; Igliozzi, R.; Muratori, F. Individual and Environmental Factors Affecting Adaptive Behavior of Toddlers with Autism Spectrum Disorder: Role of Parents’ Socio-Cultural Level. J. Autism Dev. Disord. 2021, 51, 3469–3482. [Google Scholar] [CrossRef]
- Farley, M.A.; McMahon, W.M.; Fombonne, E.; Jenson, W.R.; Miller, J.; Gardner, M.; Block, H.; Pingree, C.B.; Ritvo, E.R.; Ritvo, R.A.; et al. Twenty-Year Outcome for Individuals with Autism and Average or near-Average Cognitive Abilities. Autism Res. 2009, 2, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Klin, A.; Saulnier, C.A.; Sparrow, S.S.; Cicchetti, D.V.; Volkmar, F.R.; Lord, C. Social and Communication Abilities and Disabilities in Higher Functioning Individuals with Autism Spectrum Disorders: The Vineland and the ADOS. J. Autism Dev. Disord. 2007, 37, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, S.S. Vineland Adaptive Behavior Scales. In Encyclopedia of Clinical Neuropsychology; Kreutzer, J.S., DeLuca, J., Caplan, B., Eds.; Springer: New York, NY, USA, 2011; pp. 2618–2621. ISBN 978-0-387-79948-3. [Google Scholar]
- Tomanik, S.S.; Pearson, D.A.; Loveland, K.A.; Lane, D.M.; Bryant Shaw, J. Improving the Reliability of Autism Diagnoses: Examining the Utility of Adaptive Behavior. J. Autism Dev. Disord. 2007, 37, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Paynter, J.M.; Gilmore, L. Vineland Adaptive Behavior Scales: II Profile of Young Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2016, 46, 64–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, S.; Fulcher, B.D.; Rajaratnam, S.M.W.; Conduit, R.; Sullivan, J.P.; Hilaire, M.A.S.; Phillips, A.J.; Loddenkemper, T.; Kothare, S.V.; McConnell, K.; et al. Behaviorally-Determined Sleep Phenotypes Are Robustly Associated with Adaptive Functioning in Individuals with Low Functioning Autism. Sci. Rep. 2017, 7, 14228. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.; Fulcher, B.D.; Rajaratnam, S.M.W.; Conduit, R.; Sullivan, J.P.; Hilaire, M.A.S.; Phillips, A.J.K.; Loddenkemper, T.; Kothare, S.V.; McConnell, K.; et al. Sleep Patterns Predictive of Daytime Challenging Behavior in Individuals with Low-Functioning Autism. Autism Res. 2018, 11, 391–403. [Google Scholar] [CrossRef]
- Veatch, O.J.; Sutcliffe, J.S.; Warren, Z.E.; Keenan, B.T.; Potter, M.H.; Malow, B.A. Shorter Sleep Duration Is Associated with Social Impairment and Comorbidities in ASD. Autism Res. 2017, 10, 1221–1238. [Google Scholar] [CrossRef]
- Malhi, P.; Kaur, A.; Singhi, P.; Sankhyan, N. Sleep Dysfunction and Behavioral Daytime Problems in Children with Autism Spectrum Disorders: A Comparative Study. Indian J. Pediatr. 2019, 86, 12–17. [Google Scholar] [CrossRef]
- Lambert, A.; Tessier, S.; Rochette, A.-C.; Scherzer, P.; Mottron, L.; Godbout, R. Poor Sleep Affects Daytime Functioning in Typically Developing and Autistic Children Not Complaining of Sleep Problems: A Questionnaire-Based and Polysomnographic Study. Res. Autism Spectr. Disord. 2016, 23, 94–106. [Google Scholar] [CrossRef]
- Adams, H.L.; Matson, J.L.; Cervantes, P.E.; Goldin, R.L. The Relationship between Autism Symptom Severity and Sleep Problems: Should Bidirectionality Be Considered? Res. Autism Spectr. Disord. 2014, 3, 193–199. [Google Scholar] [CrossRef]
- Guénolé, F.; Godbout, R.; Nicolas, A.; Franco, P.; Claustrat, B.; Baleyte, J.-M. Melatonin for Disordered Sleep in Individuals with Autism Spectrum Disorders: Systematic Review and Discussion. Sleep Med. Rev. 2011, 15, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.C.; Corkum, P.; Smith, I.M. Case Study: A Case-Series Evaluation of a Behavioral Sleep Intervention for Three Children with Autism and Primary Insomnia. J. Pediatr. Psychol. 2011, 36, 47–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deliens, G.; Peigneux, P. Sleep–Behaviour Relationship in Children with Autism Spectrum Disorder: Methodological Pitfalls and Insights from Cognition and Sensory Processing. Dev. Med. Child Neurol. 2019, 61, 1368–1376. [Google Scholar] [CrossRef] [Green Version]
- Hollway, J.A.; Aman, M.G. Sleep Correlates of Pervasive Developmental Disorders: A Review of the Literature. Res. Dev. Disabil. 2011, 32, 1399–1421. [Google Scholar] [CrossRef] [PubMed]
- Carmassi, C.; Palagini, L.; Caruso, D.; Masci, I.; Nobili, L.; Vita, A.; Dell’Osso, L. Systematic Review of Sleep Disturbances and Circadian Sleep Desynchronization in Autism Spectrum Disorder: Toward an Integrative Model of a Self-Reinforcing Loop. Front. Psychiatry 2019, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J.; El-Sheikh, M. Reciprocal Relations between Children’s Sleep and Their Adjustment over Time. Dev. Psychol. 2014, 50, 1137–1147. [Google Scholar] [CrossRef] [Green Version]
- Johnson, C.R.; Smith, T.; DeMand, A.; Lecavalier, L.; Evans, V.; Gurka, M.; Swiezy, N.; Bearss, K.; Scahill, L. Exploring Sleep Quality of Young Children with Autism Spectrum Disorder and Disruptive Behaviors. Sleep Med. 2018, 44, 61–66. [Google Scholar] [CrossRef]
- Choi, B.C.K.; Pak, A.W.P. A Catalog of Biases in Questionnaires. Prev. Chronic Dis. 2005, 2, A13. [Google Scholar]
- Rossignol, D.A.; Frye, R.E. Melatonin in Autism Spectrum Disorders: A Systematic Review and Meta-Analysis: Review. Dev. Med. Child Neurol. 2011, 53, 783–792. [Google Scholar] [CrossRef]
- Graham, C.; Cook, M.R.; Kavet, R.; Sastre, A.; Smith, D.K. Prediction of Nocturnal Plasma Melatonin from Morning Urinary Measures. J. Pineal Res. 1998, 24, 230–238. [Google Scholar] [CrossRef]
- Babinska, K.; Siklenkova, L.; Stebelova, K.; Waczulikova, I.; Celusakova, H.; Vidosovicova, M.; Bartakovicova, K.; Szapuova, Z.; Kemenyova, P. Urinary Levels of 6-Sulphatoxymelatonin and Their Associations with Sleep Disorders and Behavioural Impairments in Children with Autism Spectrum Disorder. Bratisl. Lek. Listy 2019, 120, 849–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, E.K.; Richdale, A.L.; Hazi, A.; Prendergast, L.A. Assessing the Dim Light Melatonin Onset in Adults with Autism Spectrum Disorder and No Comorbid Intellectual Disability. J. Autism Dev. Disord. 2017, 47, 2120–2137. [Google Scholar] [CrossRef]
- Abdulamir, H.A.; Abdul-Rasheed, O.F.; Abdulghani, E.A. Low Oxytocin and Melatonin Levels and Their Possible Role in the Diagnosis and Prognosis in Iraqi Autistic Children. Saudi Med. J. 2016, 37, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagnon, K.; Godbout, R. Melatonin and Comorbidities in Children with Autism Spectrum Disorder. Curr. Dev. Disord. Rep. 2018, 5, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Huang, S.; Zou, J.; Wang, Q.; Naveed, M.; Bao, H.; Wang, W.; Fukunaga, K.; Han, F. Autism Spectrum Disorder (ASD): Disturbance of the Melatonin System and Its Implications. Biomed. Pharmacother. 2020, 130, 110496. [Google Scholar] [CrossRef] [PubMed]
- Villagonzalo, K.-A.; Dodd, S.; Dean, O.; Gray, K.; Tonge, B.; Berk, M. Oxidative Pathways as a Drug Target for the Treatment of Autism. Expert Opin. Targets 2010, 14, 1301–1310. [Google Scholar] [CrossRef]
- Frustaci, A.; Neri, M.; Cesario, A.; Adams, J.B.; Domenici, E.; Dalla Bernardina, B.; Bonassi, S. Oxidative Stress-Related Biomarkers in Autism: Systematic Review and Meta-Analyses. Free Radic. Biol. Med. 2012, 52, 2128–2141. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, L.; Anckarsäter, H.; Zettergren, A.; Westberg, L.; Walum, H.; Lundström, S.; Larsson, H.; Lichtenstein, P.; Melke, J. Association between ASMT and Autistic-like Traits in Children from a Swedish Nationwide Cohort. Psychiatr. Genet. 2014, 24, 21–27. [Google Scholar] [CrossRef]
- Pagan, C.; Delorme, R.; Callebert, J.; Goubran-Botros, H.; Amsellem, F.; Drouot, X.; Boudebesse, C.; Le Dudal, K.; Ngo-Nguyen, N.; Laouamri, H.; et al. The Serotonin-N-Acetylserotonin-Melatonin Pathway as a Biomarker for Autism Spectrum Disorders. Transl. Psychiatry 2014, 4, e479. [Google Scholar] [CrossRef] [PubMed]
- Braam, W.; Ehrhart, F.; Maas, A.P.H.M.; Smits, M.G.; Curfs, L. Low Maternal Melatonin Level Increases Autism Spectrum Disorder Risk in Children. Res. Dev. Disabil. 2018, 82, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Kanne, S.M.; Gerber, A.J.; Quirmbach, L.M.; Sparrow, S.S.; Cicchetti, D.V.; Saulnier, C.A. The Role of Adaptive Behavior in Autism Spectrum Disorders: Implications for Functional Outcome. J. Autism Dev. Disord. 2011, 41, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Nevill, R.E.; Hedley, D.; Uljarević, M.; Butter, E.; Mulick, J.A. Adaptive Behavior Profiles in Young Children with Autism Spectrum Disorder Diagnosed under DSM-5 Criteria. Res. Autism Spectr. Disord. 2017, 43, 53–66. [Google Scholar] [CrossRef]
- Pathak, M.; Bennett, A.; Shui, A.M. Correlates of Adaptive Behavior Profiles in a Large Cohort of Children with Autism: The Autism Speaks Autism Treatment Network Registry Data. Autism 2019, 23, 87–99. [Google Scholar] [CrossRef]
- Hus, V.; Lord, C. Effects of Child Characteristics on the Autism Diagnostic Interview-Revised: Implications for Use of Scores as a Measure of ASD Severity. J. Autism Dev. Disord. 2013, 43, 371–381. [Google Scholar] [CrossRef] [Green Version]
- Rana, M.; Kothare, S.; DeBassio, W. The Assessment and Treatment of Sleep Abnormalities in Children and Adolescents with Autism Spectrum Disorder: A Review. J. Can. Acad. Child Adolesc. Psychiatry 2021, 30, 25–35. [Google Scholar] [PubMed]
- Cuomo, B.M.; Vaz, S.; Lee, E.A.L.; Thompson, C.; Rogerson, J.M.; Falkmer, T. Effectiveness of Sleep-Based Interventions for Children with Autism Spectrum Disorder: A Meta-Synthesis. Pharmacotherapy 2017, 37, 555–578. [Google Scholar] [CrossRef] [PubMed]

| Mean | SD | Median | Minimum | Maximum | |
|---|---|---|---|---|---|
| age (years) | 5.3 | 2.4 | 4.8 | 2.8 | 13.3 |
| aMT6s (ng/mL) | 173.7 | 114.4 | 158.6 | 11.0 | 590.7 |
| creatinine (mg/mL) | 0.9 | 0.3 | 0.9 | 0.1 | 1.4 |
| aMT6s/creatinine (ng/mg) | 197.3 | 130.5 | 157.9 | 14.1 | 739.0 |
| CSHQ Sleep score | 44.5 | 6.3 | 45.0 | 33.0 | 58.0 |
| ADI-R | |||||
| SUM_A (%) | 0.4 | 0.2 | 0.4 | 0.1 | 0.9 |
| SUM_B (%) | 0.5 | 0.2 | 0.5 | 0.1 | 0.9 |
| SUM_C (%) | 0.3 | 0.2 | 0.3 | 0.1 | 0.8 |
| ADOS-2 | |||||
| SA | 7.1 | 1.5 | 7.0 | 5.0 | 10.0 |
| RRB | 8.7 | 1.3 | 9.0 | 4.0 | 10.0 |
| CSS | 7.9 | 1.4 | 8.0 | 6.0 | 10.0 |
| Adaptive behavior | |||||
| ABC | 70.6 | 10.5 | 70.0 | 51.0 | 98.0 |
| COM | 67.0 | 18.2 | 67.0 | 24.0 | 113.0 |
| DLS | 76.4 | 10.3 | 76.0 | 54.0 | 98.0 |
| SOC | 68.3 | 14.9 | 66.0 | 38.0 | 98.0 |
| IN | 18.1 | 2.5 | 19.0 | 12.0 | 22.0 |
| EX | 18.5 | 2.4 | 18.0 | 11.0 | 22.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartakovicova, K.; Kemenyova, P.; Belica, I.; Janik Szapuova, Z.; Stebelova, K.; Waczulikova, I.; Ostatnikova, D.; Babinska, K. Sleep Problems and 6-Sulfatoxymelatonin as Possible Predictors of Symptom Severity, Adaptive and Maladaptive Behavior in Children with Autism Spectrum Disorder. Int. J. Environ. Res. Public Health 2022, 19, 7594. https://doi.org/10.3390/ijerph19137594
Bartakovicova K, Kemenyova P, Belica I, Janik Szapuova Z, Stebelova K, Waczulikova I, Ostatnikova D, Babinska K. Sleep Problems and 6-Sulfatoxymelatonin as Possible Predictors of Symptom Severity, Adaptive and Maladaptive Behavior in Children with Autism Spectrum Disorder. International Journal of Environmental Research and Public Health. 2022; 19(13):7594. https://doi.org/10.3390/ijerph19137594
Chicago/Turabian StyleBartakovicova, Kristina, Petra Kemenyova, Ivan Belica, Zofia Janik Szapuova, Katarina Stebelova, Iveta Waczulikova, Daniela Ostatnikova, and Katarina Babinska. 2022. "Sleep Problems and 6-Sulfatoxymelatonin as Possible Predictors of Symptom Severity, Adaptive and Maladaptive Behavior in Children with Autism Spectrum Disorder" International Journal of Environmental Research and Public Health 19, no. 13: 7594. https://doi.org/10.3390/ijerph19137594







