The Association between Urinary Polycyclic Aromatic Hydrocarbons Metabolites and Type 2 Diabetes Mellitus
Abstract
:1. Introduction
2. Methods
2.1. Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Statistical Analysis Methods
3. Results
3.1. Potential Bias Due to Outcome Assessment
3.2. Potential Bias Due to the Exposure Assessment
3.3. Potential Bias Due to Confounder Adjustment
3.4. Publication Bias
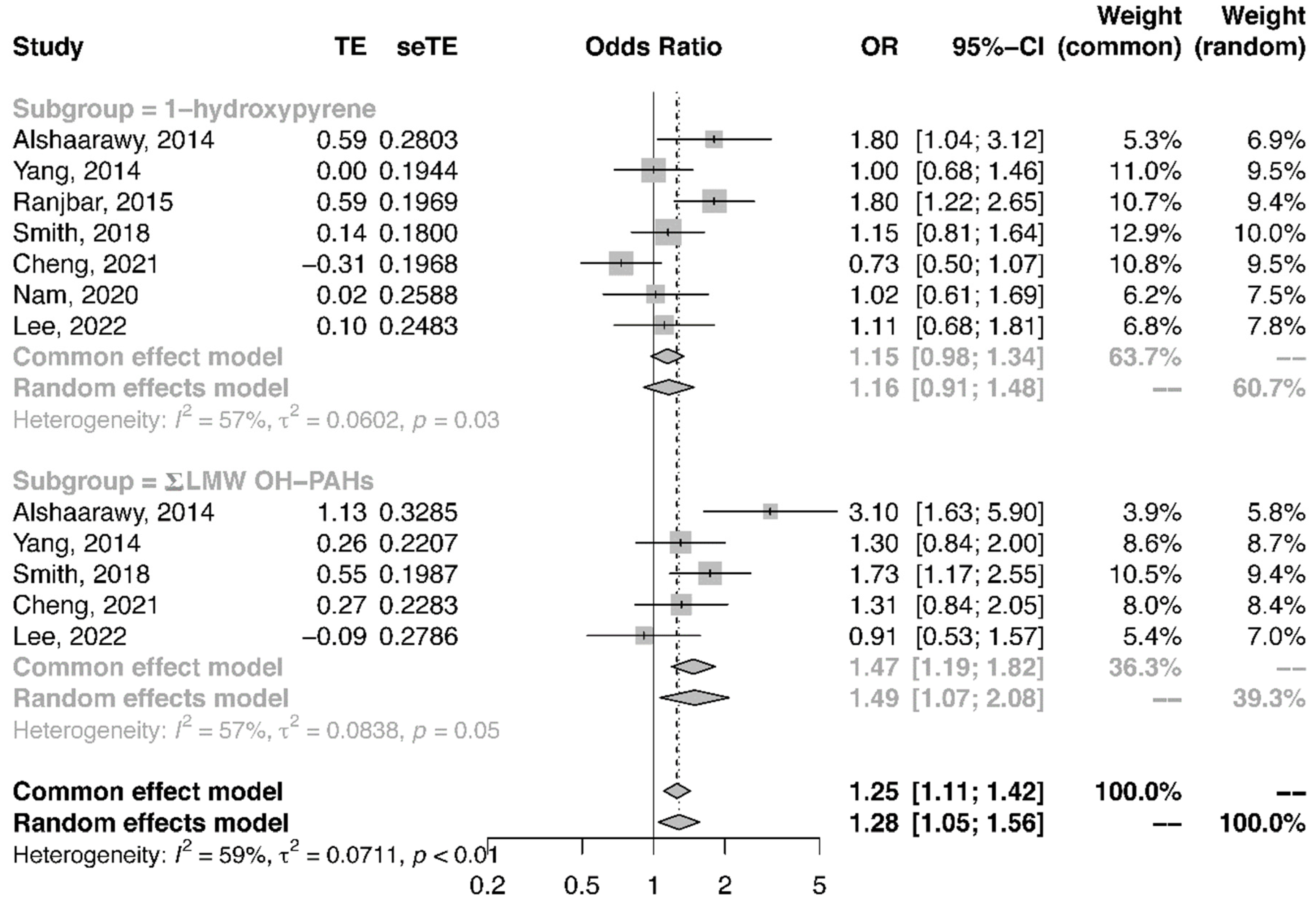
3.5. Meta-Analysis of the Association between Urinary PAH Metabolites and the Risk of T2DM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PAHs | polycyclic aromatic hydrocarbons |
| OH-PAHs | mono-hydroxylated PAHs |
| T2DM | type 2 diabetes |
| LMW | low molecule weight |
| HMW | high molecule weight |
| PM | particulate matter |
| 1-OHNa | 1-hydroxynaphthalene |
| 2-OHNa | 2-hydroxynaphthalene |
| 2-OHFlu | 2-hydroxyfluorene |
| 3-OHFlu | 3-hydroxyfluorene |
| 9-OHFlu | 9-hydroxyfluorene |
| 1-OHPh | 1-hydroxyphenanthrene |
| 2-OHPh | 2-hydroxyphenanthrene |
| 3-OHPh | 3-hydroxyphenanthrene |
| 4-OHPh | 4-hydroxyphenanthrene |
| 9-OHPh | 9-hydroxyphenanthrene |
| 1-OHP | 1-hydroxypyrene |
| BAP | benzo(a)pyrene |
| CIs | confidence intervals |
| OR | odds ratio |
| RR | relative risk |
| HR | hazard ratio |
| 8-OHdG | 8-hydroxydeoxyguanosine |
| SES | socioeconomic status |
References
- Liu, C.; Chen, R.; Sera, F.; Vicedo-Cabrera, A.M.; Guo, Y.; Tong, S.; Coelho, M.; Saldiva, P.H.N.; Lavigne, E.; Matus, P.; et al. Ambient Particulate Air Pollution and Daily Mortality in 652 Cities. N. Engl. J. Med. 2019, 381, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, S.; Wang, H.; Tao, S.; Kiyama, R. Biological impact of environmental polycyclic aromatic hydrocarbons (ePAHs) as endocrine disruptors. Environ. Pollut. 2016, 213, 809–824. [Google Scholar] [CrossRef]
- Gao, P.; da Silva, E.; Hou, L.; Denslow, N.D.; Xiang, P.; Ma, L.Q. Human exposure to polycyclic aromatic hydrocarbons: Metabolomics perspective. Environ. Int. 2018, 119, 466–477. [Google Scholar] [CrossRef]
- Srogi, K. Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: A review. Environ. Chem. Lett. 2007, 5, 169–195. [Google Scholar] [CrossRef] [Green Version]
- Khalil, A.; Villard, P.H.; Dao, M.A.; Burcelin, R.; Champion, S.; Fouchier, F.; Savouret, J.F.; Barra, Y.; Seree, E. Polycyclic aromatic hydrocarbons potentiate high-fat diet effects on intestinal inflammation. Toxicol. Lett. 2010, 196, 161–167. [Google Scholar] [CrossRef]
- Everett, C.J.; King, D.E.; Player, M.S.; Matheson, E.M.; Post, R.E.; Mainous, A.G., 3rd. Association of urinary polycyclic aromatic hydrocarbons and serum C-reactive protein. Environ. Res. 2010, 110, 79–82. [Google Scholar] [CrossRef]
- Choi, Y.H.; Kim, J.H.; Hong, Y.C. Sex-dependent and body weight-dependent associations between environmental PAHs exposure and insulin resistance: Korean urban elderly panel. J. Epidemiol. Community Health 2015, 69, 625–631. [Google Scholar] [CrossRef] [Green Version]
- Gato, W.E.; Hunter, D.A.; Whitby, S.L.; Mays, C.A.; Yau, W. Investigating susceptibility to diabetes using features of the adipose tissue in response to in utero polycyclic aromatic hydrocarbons exposure. Diabetes Metab. J. 2016, 40, 494–508. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Huang, K.; Zhang, X.; Zhang, W.; Guan, L.; Kuang, D.; Deng, Q.; Deng, H.; Zhang, X.; He, M.; et al. Women are more susceptible than men to oxidative stress and chromosome damage caused by polycyclic aromatic hydrocarbons exposure. Environ. Mol. Mutagen. 2014, 55, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Archibong, A.E.; Inyang, F.; Ramesh, A.; Greenwood, M.; Nayyar, T.; Kopsombut, P.; Hood, D.B.; Nyanda, A.M. Alteration of pregnancy related hormones and fetal survival in F-344 rats exposed by inhalation to benzo(a)pyrene. Reprod. Toxicol. 2002, 16, 801–808. [Google Scholar] [CrossRef]
- Makaji, E.; Raha, S.; Wade, M.G.; Holloway, A.C. Effect of environmental contaminants on Beta cell function. Int. J. Toxicol. 2011, 30, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Idowu, O.; Semple, K.T.; Ramadass, K.; O’Connor, W.; Hansbro, P.; Thavamani, P. Beyond the obvious: Environmental health implications of polar polycyclic aromatic hydrocarbons. Environ. Int. 2019, 123, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tao, S.; Peng, J.; Zhao, J.; Li, S.; Wu, N.; Wen, Y.; Xue, Q.; Yang, C.X.; Pan, X.F. High-sensitivity C-reactive protein and risk of type 2 diabetes: A nationwide cohort study and updated meta-analysis. Diabetes Metab. Res. Rev. 2021, 37, e3446. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, D.K.; Leem, J.H.; Kim, H.C.; Lee, J.Y.; Park, M.S.; Jung, D.Y.; Ko, J.K.; Ha, M.; Kim, Y.; Hong, Y.C.; et al. Impact of prenatal exposure to polycyclic aromatic hydrocarbons from maternal diet on birth outcomes: A birth cohort study in Korea. Public Health Nutr. 2016, 19, 2562–2571. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Qi, Q.; Workalemahu, T.; Hu, F.B.; Qi, L. Birth weight, genetic susceptibility, and adulthood risk of type 2 diabetes. Diabetes Care 2012, 35, 2479–2484. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Yan, K.; Zeng, D.; Lai, X.; Chen, X.; Fang, Q.; Guo, H.; Wu, T.; Zhang, X. Association of polycyclic aromatic hydrocarbons metabolites and risk of diabetes in coke oven workers. Environ. Pollut. 2017, 223, 305–310. [Google Scholar] [CrossRef]
- Khosravipour, M.; Khosravipour, H. The association between urinary metabolites of polycyclic aromatic hydrocarbons and diabetes: A systematic review and meta-analysis study. Chemosphere 2020, 247, 125680. [Google Scholar] [CrossRef]
- Stallings-Smith, S.; Mease, A.; Johnson, T.M.; Arikawa, A.Y. Exploring the association between polycyclic aromatic hydrocarbons and diabetes among adults in the United States. Environ. Res. 2018, 166, 588–594. [Google Scholar] [CrossRef]
- Alshaarawy, O.; Zhu, M.; Ducatman, A.M.; Conway, B.; Andrew, M.E. Urinary polycyclic aromatic hydrocarbon biomarkers and diabetes mellitus. Occup. Environ. Med. 2014, 71, 437–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranjbar, M.; Rotondi, M.A.; Ardern, C.I.; Kuk, J.L. Urinary Biomarkers of Polycyclic Aromatic Hydrocarbons Are Associated with Cardiometabolic Health Risk. PLoS ONE 2015, 10, e0137536. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Park, H.; Kim, M.J.; Kim, S.; Choi, S.; Park, J.; Cho, Y.H.; Hong, S.; Yoo, J.; Cheon, G.J.; et al. Exposure to polycyclic aromatic hydrocarbons and volatile organic compounds is associated with a risk of obesity and diabetes mellitus among Korean adults: Korean National Environmental Health Survey (KoNEHS) 2015–2017. Int. J. Hyg. Environ. Health 2022, 240, 113886. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhou, Y.; Sun, H.; Lai, H.; Liu, C.; Yan, K.; Yuan, J.; Wu, T.; Chen, W.; Zhang, X. Dose-response relationship between polycyclic aromatic hydrocarbon metabolites and risk of diabetes in the general Chinese population. Environ. Pollut. 2014, 195, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Zhou, Y.; Wang, B.; Mu, G.; Ma, J.; Zhou, M.; Wang, D.; Yang, M.; Cao, L.; Xie, L.; et al. IL-22: A potential mediator of associations between urinary polycyclic aromatic hydrocarbon metabolites with fasting plasma glucose and type 2 diabetes. J. Hazard. Mater. 2021, 401, 123278. [Google Scholar] [CrossRef]
- Viau, M.B. Urinary 1-hydroxypyrene as a biomarker of exposure to polycyclic aromatic hydrocarbons: Biological monitoring strategies and methodology for determining biological exposure indices for various work environments. Biomarkers 1999, 4, 159–187. [Google Scholar] [CrossRef]
- Liu, B.; Feng, W.; Wang, J.; Li, Y.; Han, X.; Hu, H.; Guo, H.; Zhang, X.; He, M. Association of urinary metals levels with type 2 diabetes risk in coke oven workers. Environ. Pollut. 2016, 210, 1–8. [Google Scholar] [CrossRef]
- Nam, Y.J.; Kim, S.H. Association of Urinary Polycyclic Aromatic Hydrocarbons and Diabetes in Korean Adults: Data from the Korean National Environmental Health Survey Cycle 2 (2012–2014). Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3993–4003. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, B.; Zhao, X.; Fu, Y.; Li, X.; Yang, A.; Li, Q.; Dong, J.; Nie, J.; Yang, J. The interaction effects of smoking and polycyclic aromatic hydrocarbons exposure on the prevalence of metabolic syndrome in coke oven workers. Chemosphere 2020, 247, 125880. [Google Scholar] [CrossRef]
- Barr, D.B.; Wilder, L.C.; Caudill, S.P.; Gonzalez, A.J.; Needham, L.L.; Pirkle, J.L. Urinary creatinine concentrations in the U.S. population: Implications for urinary biologic monitoring measurements. Environ. Health Perspect. 2005, 113, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wong, L.Y.; Meng, L.; Pittman, E.N.; Trinidad, D.A.; Hubbard, K.L.; Etheredge, A.; Del Valle-Pinero, A.Y.; Zamoiski, R.; van Bemmel, D.M.; et al. Urinary concentrations of monohydroxylated polycyclic aromatic hydrocarbons in adults from the U.S. Population Assessment of Tobacco and Health (PATH) Study Wave 1 (2013–2014). Environ. Int. 2019, 123, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Sun, H.; Zhou, Y.; Zhang, Y.; Yin, W.; Xu, T.; Cheng, J.; Chen, W.; Yuan, J. Environmental exposure to polycyclic aromatic hydrocarbons, kitchen ventilation, fractional exhaled nitric oxide, and risk of diabetes among Chinese females. Indoor Air 2018, 28, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Alshaarawy, O.; Zhu, M.; Ducatman, A.; Conway, B.; Andrew, M.E. Polycyclic aromatic hydrocarbon biomarkers and serum markers of inflammation. A positive association that is more evident in men. Environ. Res. 2013, 126, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Kan, H.D.; Kearney, G.D.; Xu, X.H. Associations between exposure to polycyclic aromatic hydrocarbons and glucose homeostasis as well as metabolic syndrome in nondiabetic adults. Sci. Total Environ. 2015, 505, 56–64. [Google Scholar] [CrossRef]
- Niu, X.; Ho, S.S.H.; Ho, K.F.; Huang, Y.; Sun, J.; Wang, Q.; Zhou, Y.; Zhao, Z.; Cao, J. Atmospheric levels and cytotoxicity of polycyclic aromatic hydrocarbons and oxygenated-PAHs in PM2.5 in the Beijing-Tianjin-Hebei region. Environ. Pollut. 2017, 231, 1075–1084. [Google Scholar] [CrossRef]
- Yang, B.; Deng, Q.; Zhang, W.; Feng, Y.; Dai, X.; Feng, W.; He, X.; Huang, S.; Zhang, X.; Li, X.; et al. Exposure to Polycyclic Aromatic Hydrocarbons, Plasma Cytokines, and Heart Rate Variability. Sci. Rep. 2016, 6, 19272. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.H.; Lee, Y.S.; Lee, D.H.; Kim, D.S. Polycyclic aromatic hydrocarbons are associated with insulin receptor substrate 2 methylation in adipose tissues of Korean women. Environ. Res. 2016, 150, 47–51. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, B.; Chen, Y.; Han, X.; Wei, X.; Zhu, Y.; Zhou, X.; Chen, J. Characterization of PAHs in size-fractionated submicron atmospheric particles and their association with the intracellular oxidative stress. Chemosphere 2017, 182, 1–7. [Google Scholar] [CrossRef]
- Ferguson, K.K.; McElrath, T.F.; Pace, G.G.; Weller, D.; Zeng, L.; Pennathur, S.; Cantonwine, D.E.; Meeker, J.D. Urinary Polycyclic Aromatic Hydrocarbon Metabolite Associations with Biomarkers of Inflammation, Angiogenesis, and Oxidative Stress in Pregnant Women. Environ. Sci. Technol. 2017, 51, 4652–4660. [Google Scholar] [CrossRef] [Green Version]
- Robertson, R.P. Oxidative stress and impaired insulin secretion in type 2 diabetes. Curr. Opin. Pharmacol. 2006, 6, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Magdalena, P.; Morimoto, S.; Ripoll, C.; Fuentes, E.; Nadal, A. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ. Health Perspect. 2006, 114, 106–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallah, M.A.; Changxing, L.; Mallah, M.A.; Noreen, S.; Liu, Y.; Saeed, M.; Xi, H.; Ahmed, B.; Feng, F.; Mirjat, A.A.; et al. Polycyclic aromatic hydrocarbon and its effects on human health: An overeview. Chemosphere 2022, 296, 133948. [Google Scholar] [CrossRef] [PubMed]
- Mallah, M.A.; Mallah, M.A.; Liu, Y.; Xi, H.; Wang, W.; Feng, F.; Zhang, Q. Relationship Between Polycyclic Aromatic Hydrocarbons and Cardiovascular Diseases: A Systematic Review. Front. Public Health 2021, 9, 763706. [Google Scholar] [CrossRef] [PubMed]
- Stading, R.; Gastelum, G.; Chu, C.; Jiang, W.; Moorthy, B. Molecular mechanisms of pulmonary carcinogenesis by polycyclic aromatic hydrocarbons (PAHs): Implications for human lung cancer. Semin. Cancer Biol. 2021, 76, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, B.; Chu, C.; Carlin, D.J. Polycyclic aromatic hydrocarbons: From metabolism to lung cancer. Toxicol. Sci. 2015, 145, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Barul, C.; Parent, M.E. Occupational exposure to polycyclic aromatic hydrocarbons and risk of prostate cancer. Environ. Health 2021, 20, 71. [Google Scholar] [CrossRef]
- Duan, W.; Meng, X.; Sun, Y.; Jia, C. Association between polycyclic aromatic hydrocarbons and osteoporosis: Data from NHANES, 2005–2014. Arch. Osteoporos. 2018, 13, 112. [Google Scholar] [CrossRef]
- Shiue, I. Are urinary polyaromatic hydrocarbons associated with adult hypertension, heart attack, and cancer? USA NHANES, 2011–2012. Environ. Sci. Pollut. Res. Int. 2015, 22, 16962–16968. [Google Scholar] [CrossRef]
- Xu, X.; Cook, R.L.; Ilacqua, V.A.; Kan, H.; Talbott, E.O.; Kearney, G. Studying associations between urinary metabolites of polycyclic aromatic hydrocarbons (PAHs) and cardiovascular diseases in the United States. Sci. Total Environ. 2010, 408, 4943–4948. [Google Scholar] [CrossRef]
- Clark, J.D., 3rd; Serdar, B.; Lee, D.J.; Arheart, K.; Wilkinson, J.D.; Fleming, L.E. Exposure to polycyclic aromatic hydrocarbons and serum inflammatory markers of cardiovascular disease. Environ. Res. 2012, 117, 132–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, L.; Wang, D.; Zhu, C.; Wang, B.; Cen, X.; Chen, A.; Zhou, H.; Ye, Z.; Tan, Q.; Nie, X.; et al. Polycyclic aromatic hydrocarbon exposure and atherosclerotic cardiovascular disease risk in urban adults: The mediating role of oxidatively damaged DNA. Environ. Pollut. 2020, 265, 114860. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Guo, W.; Zeng, D.; Ma, L.; Lai, X.; Fang, Q.; Guo, H.; Zhang, X. Heart rate variability mediates the association between polycyclic aromatic hydrocarbons exposure and atherosclerotic cardiovascular disease risk in coke oven workers. Chemosphere 2019, 228, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.W.; Kim, D.H.; Ryu, J.Y. Association between urinary polycyclic aromatic hydrocarbons and hypertension in the Korean population: Data from the Second Korean National Environmental Health Survey (2012–2014). Sci. Rep. 2020, 10, 17142. [Google Scholar] [CrossRef]
- Alhamdow, A.; Lindh, C.; Albin, M.; Gustavsson, P.; Tinnerberg, H.; Broberg, K. Early markers of cardiovascular disease are associated with occupational exposure to polycyclic aromatic hydrocarbons. Sci. Rep. 2017, 7, 9426. [Google Scholar] [CrossRef]
- Trasande, L.; Urbina, E.M.; Khoder, M.; Alghamdi, M.; Shabaj, I.; Alam, M.S.; Harrison, R.M.; Shamy, M. Polycyclic aromatic hydrocarbons, brachial artery distensibility and blood pressure among children residing near an oil refinery. Environ. Res. 2015, 136, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, L.; Buczynska, A.; Walgraeve, C.; Delcloo, A.; Potgieter-Vermaak, S.; Van Grieken, R.; Demeestere, K.; Dewulf, J.; Van Langenhove, H.; De Backer, H.; et al. Acute changes in pulse pressure in relation to constituents of particulate air pollution in elderly persons. Environ. Res. 2012, 117, 60–67. [Google Scholar] [CrossRef]
- Burstyn, I.; Kromhout, H.; Partanen, T.; Svane, O.; Langard, S.; Ahrens, W.; Kauppinen, T.; Stucker, I.; Shaham, J.; Heederik, D.; et al. Polycyclic aromatic hydrocarbons and fatal ischemic heart disease. Epidemiology 2005, 16, 744–750. [Google Scholar] [CrossRef]
- Feng, Y.; Sun, H.; Song, Y.; Bao, J.; Huang, X.; Ye, J.; Yuan, J.; Chen, W.; Christiani, D.C.; Wu, T.; et al. A community study of the effect of polycyclic aromatic hydrocarbon metabolites on heart rate variability based on the Framingham risk score. Occup. Environ. Med. 2014, 71, 338–345. [Google Scholar] [CrossRef]
- Li, N.; Mu, Y.; Liu, Z.; Deng, Y.; Guo, Y.; Zhang, X.; Li, X.; Yu, P.; Wang, Y.; Zhu, J. Assessment of interaction between maternal polycyclic aromatic hydrocarbons exposure and genetic polymorphisms on the risk of congenital heart diseases. Sci. Rep. 2018, 8, 3075. [Google Scholar] [CrossRef]
- Wang, L.; Hou, J.; Hu, C.; Zhou, Y.; Sun, H.; Zhang, J.; Li, T.; Gao, E.; Wang, G.; Chen, W.; et al. Mediating factors explaining the associations between polycyclic aromatic hydrocarbons exposure, low socioeconomic status and diabetes: A structural equation modeling approach. Sci. Total Environ. 2019, 648, 1476–1483. [Google Scholar] [CrossRef]

| Study ID | Location | Study Period (Years) | Population (n) | Age (Years) | Urinary PAH Metabolites | Measurement |
|---|---|---|---|---|---|---|
| General Population | ||||||
| Alshaarawy et al., 2014 [21] | USA | 2001–2002 2003–2004 2005–2006 | 3326 | 20–65 | 1-OHNa, 2-OHNa, 2-OHFlu, 3-OHFlu, 1-OHPh, 2-OHPh, 3-OHPh, and 1-OHP | Capillary gas chromatography combined with high-resolution mass spectrometry (GC-HRMS) |
| Yang et al., 2014 [24] | China | 2011 | 3092 | 18–90 | 1-OHP, 1-OHNa, 2-OHNa, 2-OHFlu, 9-OHFlu, 1-OHPh, 2-OHPh, 3-OHPh, 4-OHPh, 9-OHPh, 6-OHChr, and 3-OHBaP (6-OHChr and 3-OHBaP were below the limits of quantification) | Gas chromatographye mass spectrometry (GC/MS) |
| Ranjbar et al., 2015 [22] | USA | 2001–2008 | 4765 | ≥20 | 1-OHNa, 2-OHNa, 2-OHFlu, 3-OHFlu, 1-OHPh, 2-OHPh, 3-OHPh, and 1-OHP | Capillary gas chromatography combined with high-resolution mass spectrometry (GC-HRMS) |
| Smith et al., 2018 [20] | USA | 2005–2014 | 8664 | adults and children | 1-OHNa, 2-OHNa, 2-OHFlu, 3-OHFlu, 9-OHFlu, 1-OHPh, 2-OHPh, 3-OHPh, and 1-OHP | Gas and liquid chromatography-tandem mass spectrometry |
| Cheng et al., 2021 [25] | China | 2011.04~05, 2012.05 | 3031 | 18–80 | 1-OHNa, 2-OHNa, 2-OHFlu, 9-OHFlu, 1-OHPh, 2-OHPh, 3-OHPh, 4-OHPh, 9-OHPh, and 1-OHP. | Agilent 5975B/6890 N GCeMS System (Agilent, Santa Clara, CA, USA) |
| Nam et al., 2020 [28] | Korea | 2012–2014 | 6478 | ≥19 | 1-OHP, 2-OHNa, 1-OHPh, and 2-OHFlu | Gas chromatography-mass spectrometry (Clarus 680T, PerkinElmer, Waltham, MA, USA) |
| Lee et al., 2022 [23] | Korea | 2015–2017 | OH-PAHs except for 1-OHPhe (n = 3751), all OH-PAHs (n = 3754) | ≥19 | 1-OHP, 2-OHNa, 1-OHPh, and 2-OHFlu | Gas chromatography–mass spectrometry |
| Occupational workers (coke oven workers) | ||||||
| Yang et al., 2017 [18] | China | 2010–2014 | 1472 | 47.6 ± 7.1 for diabetic patients | 1-OHNa, 2-OHNa, 2-OHFlu, 9-OHFlu, 1-OHPh, 2-OHPh, 3-OHPh, 4-OHPh, 9-OHPh, 1-OHP, 6-OHChr, 3-OHBaP | Gas chromatography-mass spectrometry (GC-MS, Agilent, Santa Clara, CA, USA) |
| Zhang et al., 2020 [29] | China | 2017 | 682 | 31–48 | 1-OHNa, 2-OHNa, 2-OHFlu, 3-OHFlu, 1-OHPh, 2-OHPh, 9-OHPh, 1-OHP, 3-OHChr, 6-OHChr and, 9-OHBaP | High performance liquid chromatography mass spectrometry (HPLC-MS) |
| Study ID | Definition of T2DM | Adjusting Method for Urine Dilution | Adjusted Confounder | |||
| General Population | ||||||
| Alshaarawy et al., 2014 [21] | HbA1c level ≥ 6.5% (39 mmol/mol), a self-reported physician diagnosis of diabetes, or current use of oral hypoglycemic medication or insulin. | Urinary levels of OH-PAH (ng/L) were divided by urinary creatinine level (mg/dL) multiplied by 0.01, that is, (ng/L) ÷ (mg/dL × 0.01), and expressed as nanogram per gram of creatinine (ng/g creatinine) | age, sex, ethnicity, poverty–income ratio, alcohol drinking, BMI, total cholesterol and serum cotinine | |||
| Yang et al., 2014 [24] | FBG ≥ 7.0 mmol/L, self-reported physician-diagnosed diabetes, or taking oral hypoglycemic medication or insulin. | Valid urinary PAHs metabolites concentrations were calibrated by levels of urinary creatinine and calculated as nmol/mmol creatinine. | age, sex, BMI, smoking status, alcohol consumption, physical activity, education, family history of diabetes, total cholesterol, and triglycerides. | |||
| Ranjbar et al., 2015 [22] | FPG ≥ 7 mmol/L, HbA1c ≥ 6.5%, doctor diagnosed T2DM, taking diabetes medication, or were taking insulin. | Urinary creatinine was adjusted in logistic regressions model. | age, sex, poverty index ratio, ethnicity, BMI, smoking status and urinary creatinine | |||
| Smith et al., 2018 [20] | HbA1c ≥ 6.5%, self-reported diagnosis of diabetes by a physician, and/or self-reported insulin use. | Exposure variables were corrected for urinary creatinine in all analyses by dividing each of the PAHs (ng/L) by urinary creatinine (mg/dL) and multiplying by 0.01 to result in nanograms of PAHs per gram of creatinine (ng/g) | age, sex, race, poverty-income ratio, and serum cotinine. | |||
| Cheng et al., 2021 [25] | FPG ≥ 7.0 mmol/L, self-reported diagnosis of diabetes, using oral antidiabetic agents or insulin | The levels of urinary creatinine (Cr) were used to calibrate each valid urinary PAH metabolite concentrations. | age, sex, BMI, drug usage, smoking status, drinking status, physical activity, family income, city, and family history of diabetes | |||
| Nam et al., 2020 [28] | Self-report of physician-diagnosed diabetes mellitus or the use of oral hypoglycemics or insulin. | All urinary PAH levels were adjusted for the urinary creatinine levels. | sex, age, BMI, household income, alcohol consumption, physical activity, log-transformed urinary creatinine and cotinine, and menopausal status (in women) | |||
| Lee et al., 2022 [23] | Those who reported using DM medication were assumed to have T2DM. | Covariate-adjusted standardized chemical measure = Chemical concentration × the predicted Cr level/the measured Cr level; A conventional Cr adjustment was also applied | age, sex, BMI, cigarette smoking, alcohol drinking, education, and exercise | |||
| Occupational workers (coke oven workers) | ||||||
| Yang et al., 2017 [18] | Receiving diabetes medications, or FBG ≥ 7.0 mmol/L, or self-reported physician-diagnosed diabetes. | Valid urinary PAH metabolite concentrations were calibrated by levels of urinary creatinine and expressed as micrograms per millimole creatinine (mg/mmol creatinine). | working years, sex, BMI, smoking status, drinking status, physical activity, education, workshift, work sites, family history of diabetes, total cholesterol, and triglycerides | |||
| Zhang et al., 2020 [29] | High FBG levels as a component of MetS: FBG > 6.10 mmol/L or current use of medication to treat hyperglycaemia. | Valid urine concentrations of PAH metabolites were adjusted using urine gravity. | sex, age, smoking, drinking, cooking fumes, eating habits, BMI, and other PAH metabolites | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, A.; Xu, Q. The Association between Urinary Polycyclic Aromatic Hydrocarbons Metabolites and Type 2 Diabetes Mellitus. Int. J. Environ. Res. Public Health 2022, 19, 7605. https://doi.org/10.3390/ijerph19137605
Wang X, Li A, Xu Q. The Association between Urinary Polycyclic Aromatic Hydrocarbons Metabolites and Type 2 Diabetes Mellitus. International Journal of Environmental Research and Public Health. 2022; 19(13):7605. https://doi.org/10.3390/ijerph19137605
Chicago/Turabian StyleWang, Xue, Ang Li, and Qun Xu. 2022. "The Association between Urinary Polycyclic Aromatic Hydrocarbons Metabolites and Type 2 Diabetes Mellitus" International Journal of Environmental Research and Public Health 19, no. 13: 7605. https://doi.org/10.3390/ijerph19137605
APA StyleWang, X., Li, A., & Xu, Q. (2022). The Association between Urinary Polycyclic Aromatic Hydrocarbons Metabolites and Type 2 Diabetes Mellitus. International Journal of Environmental Research and Public Health, 19(13), 7605. https://doi.org/10.3390/ijerph19137605











