Microplastics Affect the Inflammation Pathway in Human Gingival Fibroblasts: A Study in the Adriatic Sea
Abstract
:1. Introduction
2. Materials and Methods
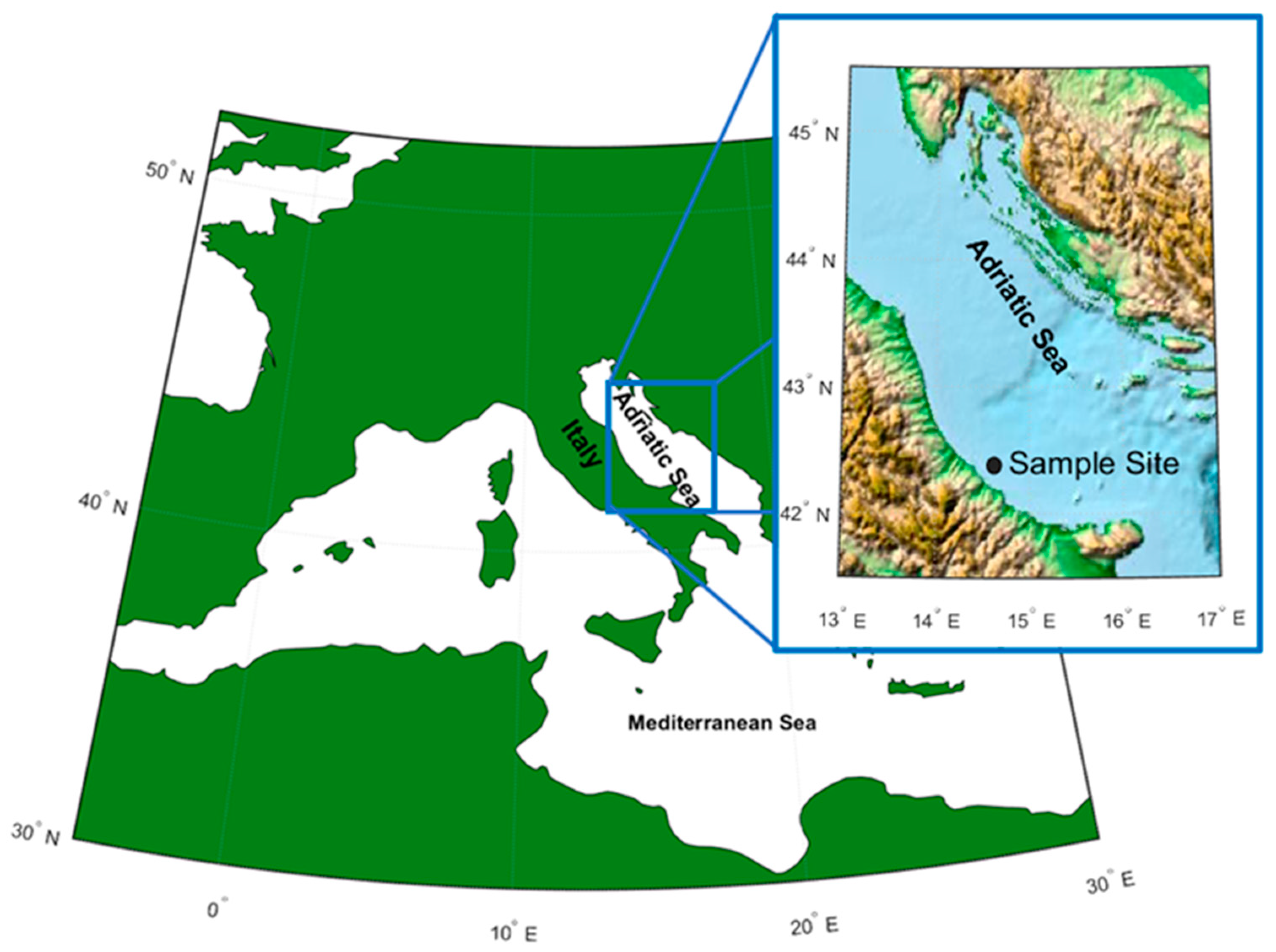
2.1. Sampling Procedures and Estimation of Microplastics (MPs) Recovery
2.2. Cell Culture Establishment
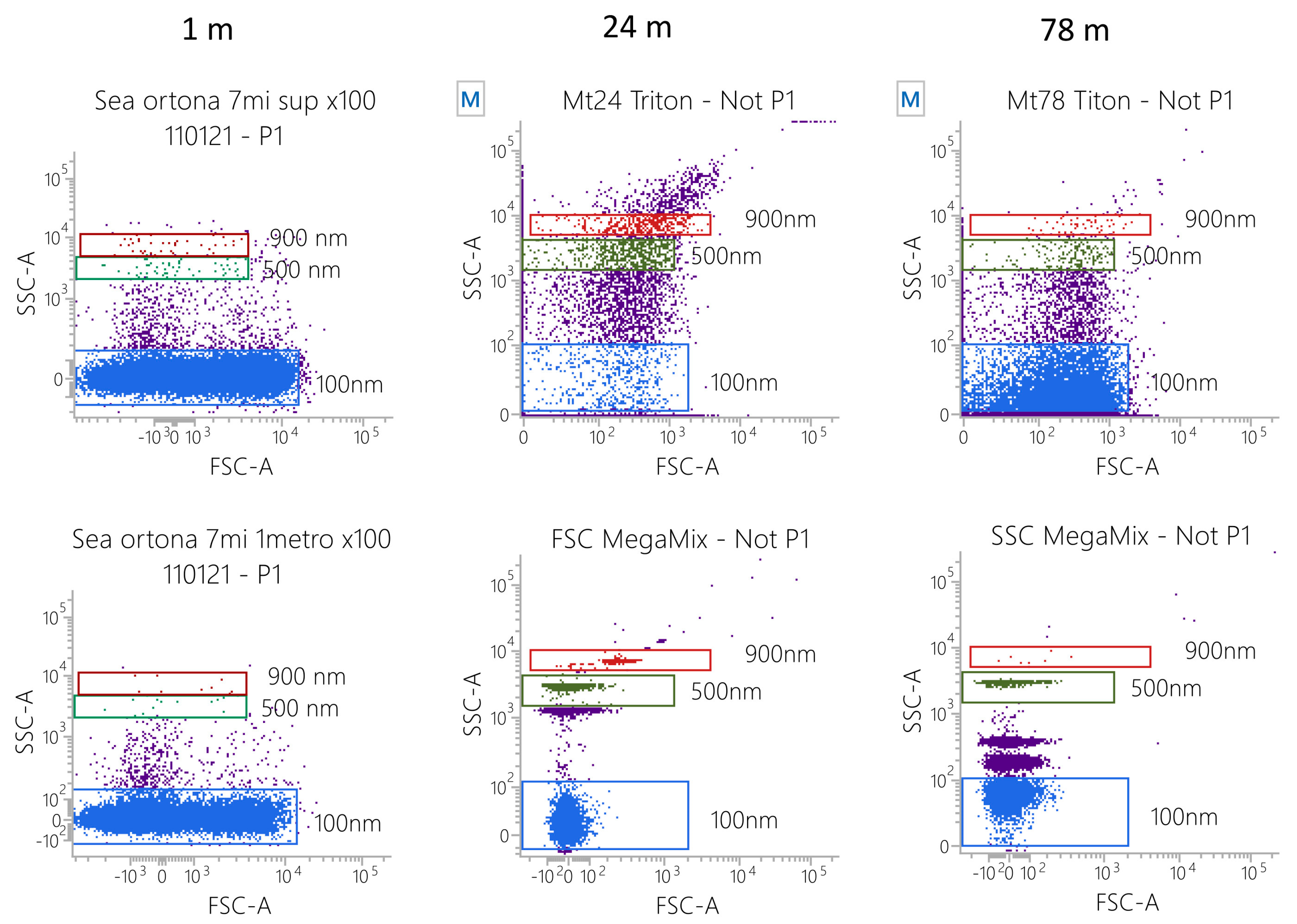
2.3. Flow Cytometry Analyses of Particle Size
2.4. Study Design
- –
- Untreated hGFs, used as control (CTRL);
- –
- hGFs treated with 100 nm micro plastics isolated at 1 m depth (1 m);
- –
- hGFs treated with 600 nm micro plastics isolated at 24 m depth (24 m).
- –
- hGFs treated with 100 nm micro plastics isolated at 78 m depth (78 m);
2.5. Cell Metabolic Activity
2.6. Immunofluorescence Analyses
2.7. RNA Isolation and Real-Time PCR Analysis
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. MPs Characterization
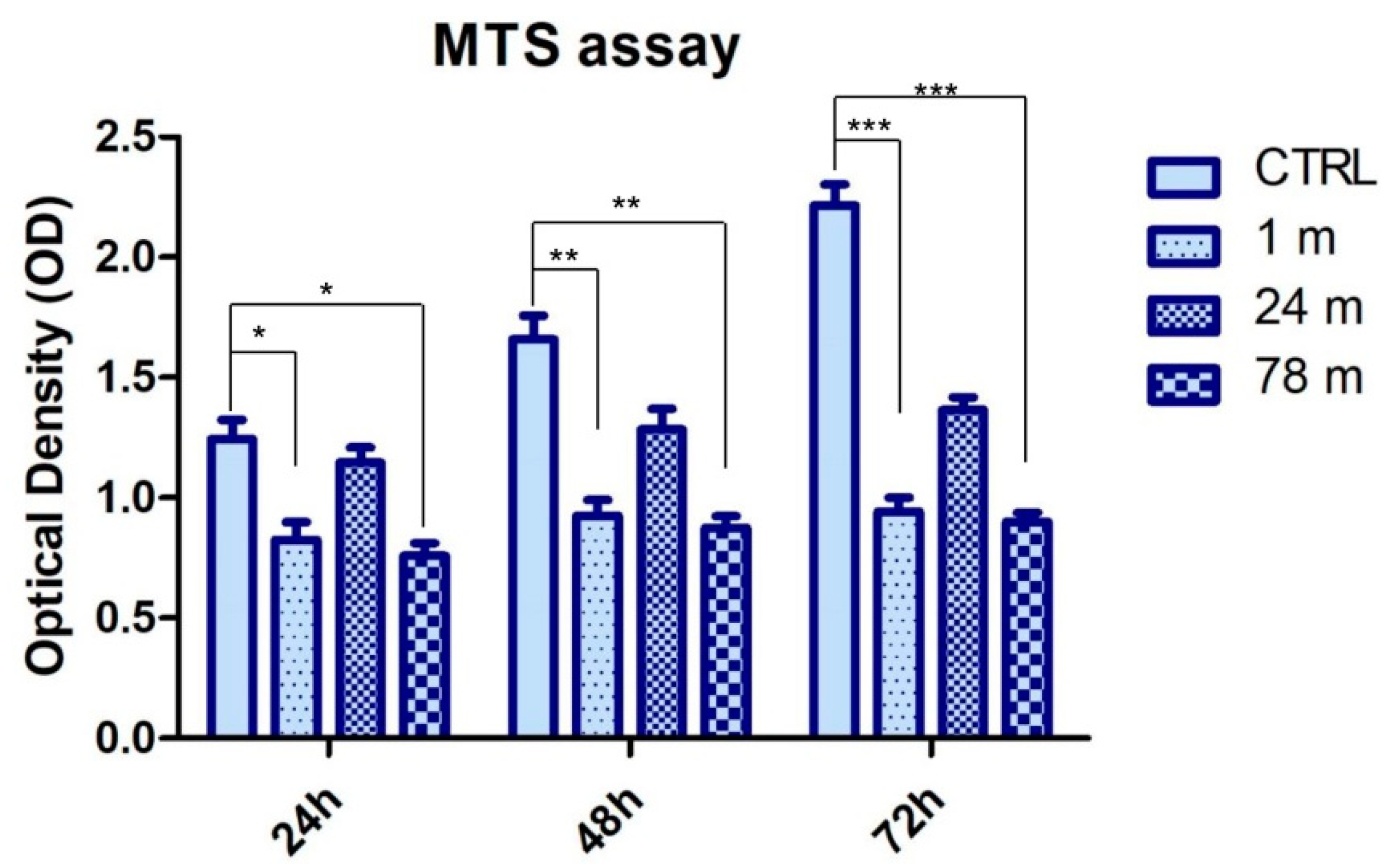
3.2. MPs Attenuated the Cell Viability Rate of hGFs
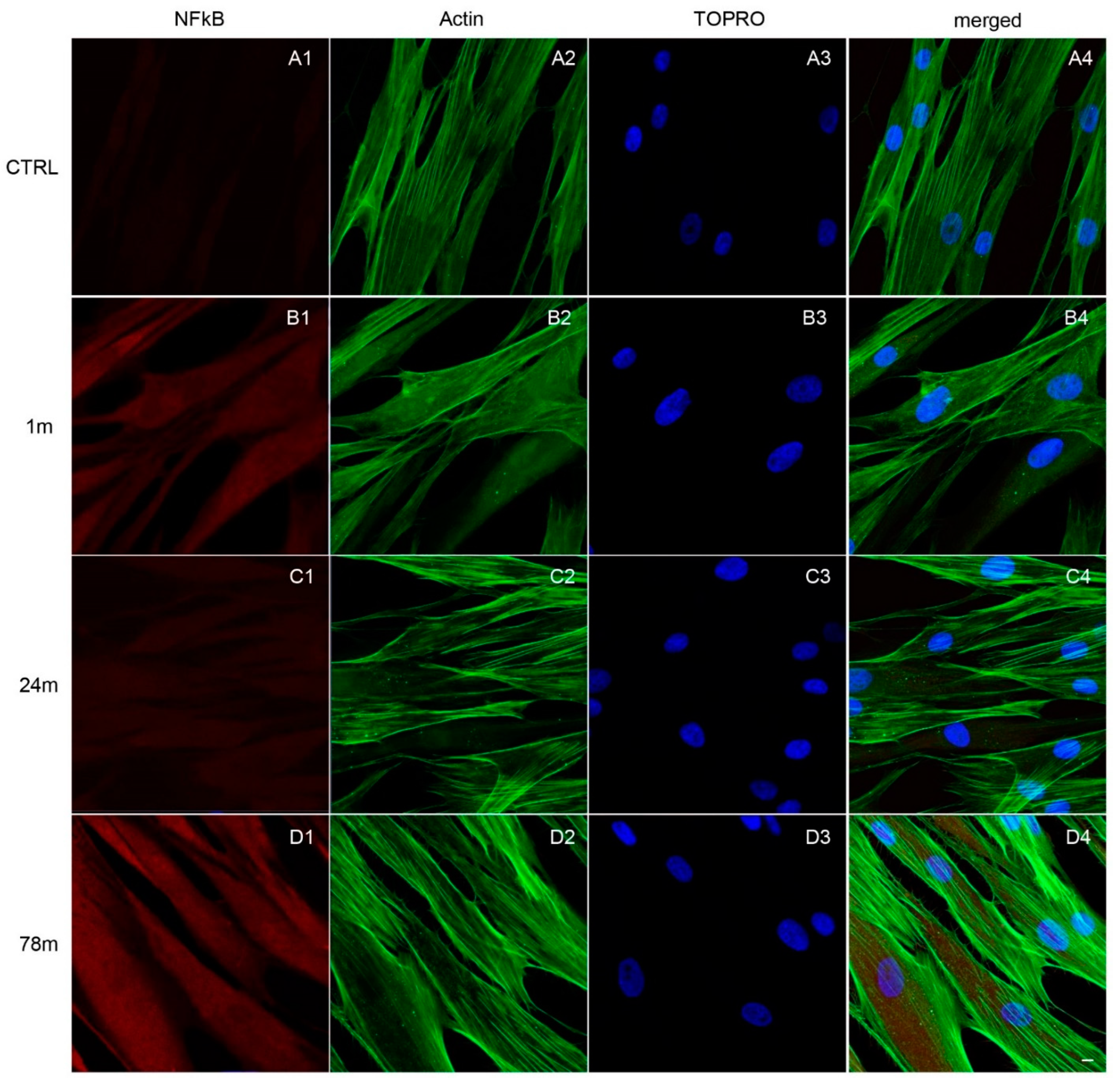
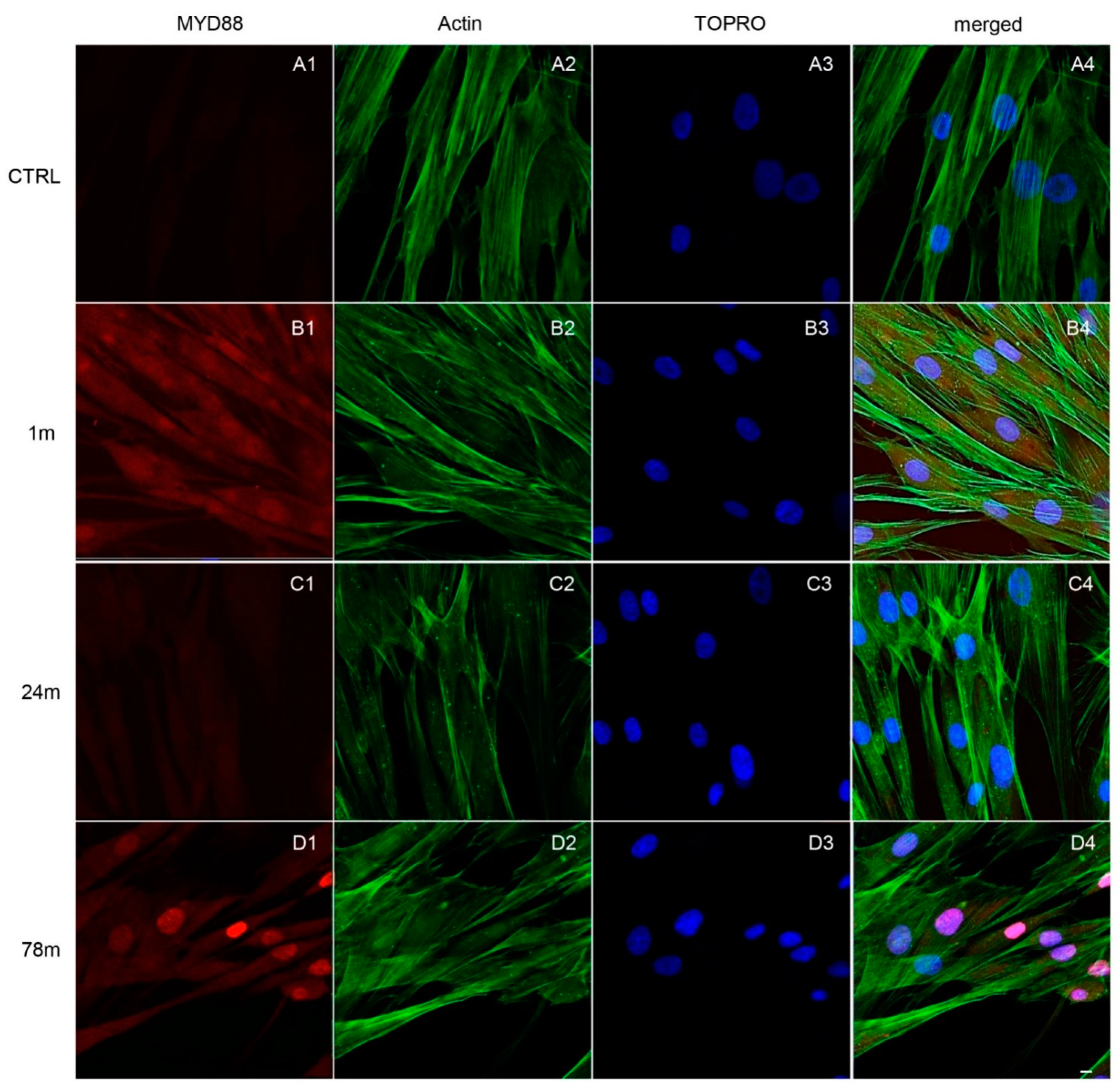
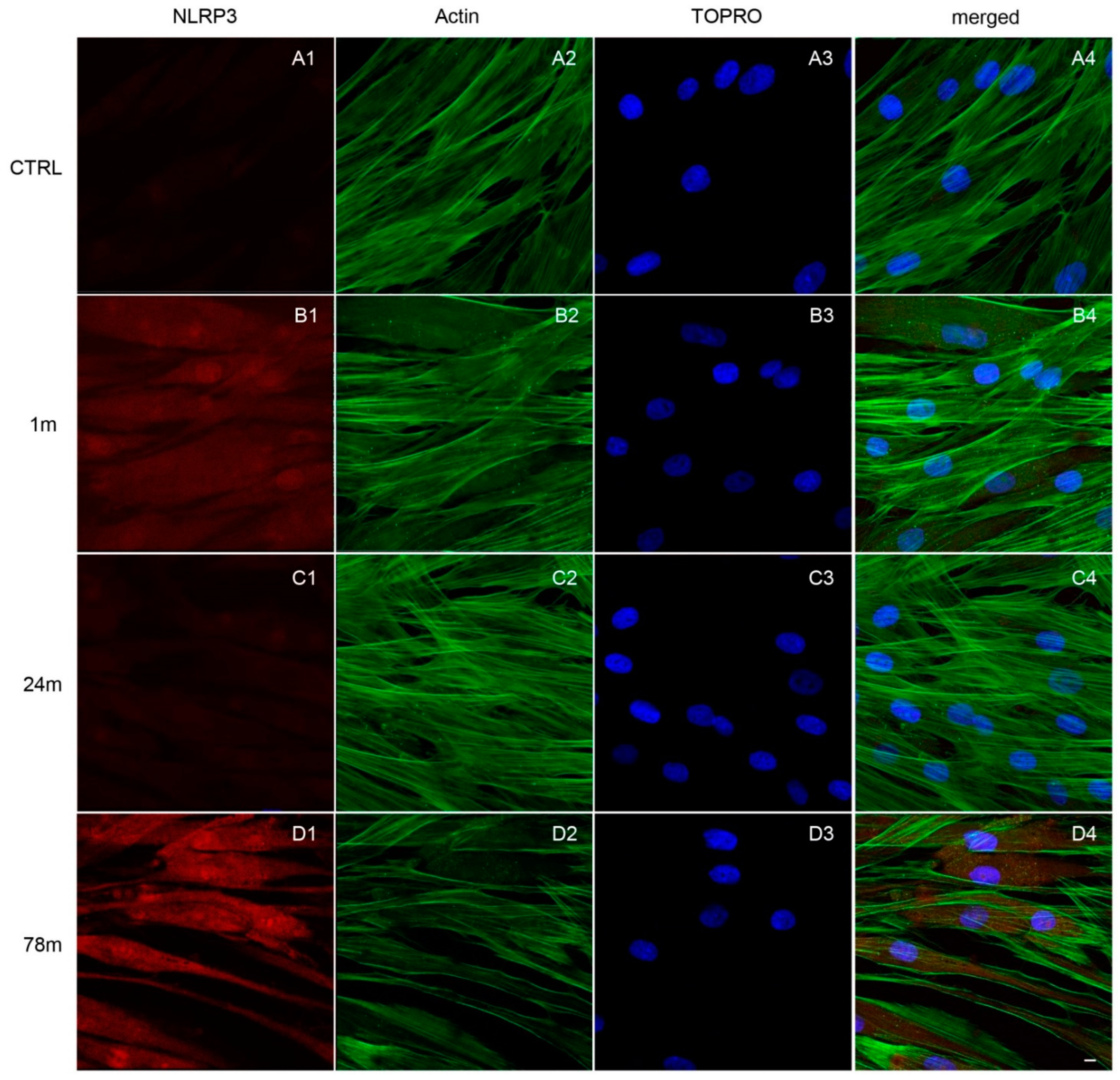
3.3. Upregulation of NFkB, MyD88 and NLRP3 Expression in hGFs Treated with MPs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chapron, L.; Peru, E.; Engler, A.; Ghiglione, J.F.; Meistertzheim, A.L.; Pruski, A.M.; Purser, A.; Vetion, G.; Galand, P.E.; Lartaud, F. Macro- and microplastics affect cold-water corals growth, feeding and behaviour. Sci. Rep. 2018, 8, 15299. [Google Scholar] [CrossRef] [Green Version]
- Barria, C.; Brandts, I.; Tort, L.; Oliveira, M.; Teles, M. Effect of nanoplastics on fish health and performance: A review. Mar. Pollut. Bull. 2020, 151, 110791. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.C.; Fu, D.D.; Qi, H.Y.; Lan, C.Q.; Yu, H.M.; Ge, C.J. Micro- and nano-plastics in marine environment: Source, distribution and threats—A review. Sci. Total Environ. 2020, 698, 134254. [Google Scholar] [CrossRef] [PubMed]
- Zeri, C.; Adamopoulou, A.; Varezic, D.B.; Fortibuoni, T.; Virsek, M.K.; Krzan, A.; Mandic, M.; Mazziotti, C.; Palatinus, A.; Peterlin, M.; et al. Floating plastics in Adriatic waters (Mediterranean Sea): From the macro- to the micro-scale. Mar. Pollut. Bull. 2018, 136, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Gigault, J.; ter Halle, A.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- Ferrero, L.; Scibetta, L.; Markuszewski, P.; Mazurkiewicz, M.; Drozdowska, V.; Makuch, P.; Jutrzenka-Trzebiatowska, P.; Zaleska-Medynska, A.; Ando, S.; Saliu, F.; et al. Airborne and marine microplastics from an oceanographic survey at the Baltic Sea: An emerging role of air-sea interaction? Sci. Total Environ. 2022, 824, 153709. [Google Scholar] [CrossRef]
- Kanhai, L.K.; Gardfeldt, K.; Lyashevska, O.; Hassellov, M.; Thompson, R.C.; O’Connor, I. Microplastics in sub-surface waters of the Arctic Central Basin. Mar. Pollut. Bull. 2018, 130, 8–18. [Google Scholar] [CrossRef]
- Silc, U.; Kuzmic, F.; Cakovic, D.; Stesevic, D. Beach litter along various sand dune habitats in the southern Adriatic (E Mediterranean). Mar. Pollut. Bull. 2018, 128, 353–360. [Google Scholar] [CrossRef]
- Liu, C.G.; Li, J.; Zhang, Y.L.; Wang, L.; Deng, J.; Gao, Y.; Yu, L.; Zhang, J.J.; Sun, H.W. Widespread distribution of PET and PC microplastics in dust in urban China and their estimated human exposure. Environ. Int. 2019, 128, 116–124. [Google Scholar] [CrossRef]
- Stapleton, P.A. Toxicological considerations of nano-sized plastics. AIMS Environ. Sci. 2019, 6, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ma, J.; Ji, R.; Pan, K.; Miao, A.J. Microplastics in aquatic environments: Occurrence, accumulation, and biological effects. Sci. Total Environ. 2020, 703, 134699. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.X.; Deng, Y.F.; Zhang, S.H.; Wolosker, M.B.; Zhu, Q.D.; Ren, H.Q.; Zhang, Y. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef]
- Fueser, H.; Mueller, M.T.; Weiss, L.; Hoss, S.; Traunspurger, W. Ingestion of microplastics by nematodes depends on feeding strategy and buccal cavity size. Environ. Pollut. 2019, 255, 113227. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wu, X.M.; Liu, S.; Wang, Z.Z.; Chen, L. Size-dependent effects of polystyrene microplastics on cytotoxicity and efflux pump inhibition in human Caco-2 cells. Chemosphere 2019, 221, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [Green Version]
- Fonticoli, L.; Della Rocca, Y.; Rajan, T.S.; Murmura, G.; Trubiani, O.; Oliva, S.; Pizzicannella, J.; Marconi, G.D.; Diomede, F. A Narrative Review: Gingival Stem Cells as a Limitless Reservoir for Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 4135. [Google Scholar] [CrossRef]
- Marconi, G.D.; Fonticoli, L.; Rajan, T.S.; Lanuti, P.; Della Rocca, Y.; Pierdomenico, S.D.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Transforming Growth Factor-Beta1 and Human Gingival Fibroblast-to-Myofibroblast Differentiation: Molecular and Morphological Modifications. Front. Physiol. 2021, 12, 676512. [Google Scholar] [CrossRef]
- Marconi, G.D.; Fonticoli, L.; Rajan, T.S.; Pierdomenico, S.D.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in Wound Healing, Tissue Regeneration and Organ Fibrosis. Cells 2021, 10, 1587. [Google Scholar] [CrossRef]
- Zaheer, J.; Kim, H.; Ko, I.O.; Jo, E.K.; Choi, E.J.; Lee, H.J.; Shim, I.; Woo, H.J.; Choi, J.; Kim, G.H.; et al. Pre/post-natal exposure to microplastic as a potential risk factor for autism spectrum disorder. Environ. Int. 2022, 161, 107121. [Google Scholar] [CrossRef]
- Suaria, G.; Avio, C.G.; Mineo, A.; Lattin, G.L.; Magaldi, M.G.; Belmonte, G.; Moore, C.J.; Regoli, F.; Aliani, S. The Mediterranean Plastic Soup: Synthetic polymers in Mediterranean surface waters. Sci. Rep. 2016, 6, 37551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, W.; Dumont, E.; Meybeck, M.; Heussner, S. River discharges of water and nutrients to the Mediterranean and Black Sea: Major drivers for ecosystem changes during past and future decades? Prog. Oceanogr. 2009, 80, 199–217. [Google Scholar] [CrossRef]
- Gallitelli, L.; Cesarini, G.; Cera, A.; Sighicelli, M.; Lecce, F.; Menegoni, P.; Scalici, M. Transport and Deposition of Microplastics and Mesoplastics along the River Course: A Case Study of a Small River in Central Italy. Hydrology 2020, 7, 90. [Google Scholar] [CrossRef]
- Cavaleri, L. The oceanographic tower Acqua Alta—Activity and prediction of sea states at Venice. Coast Eng. 2000, 39, 29–70. [Google Scholar] [CrossRef]
- Paschini, E.; Artegiani, A.; Pinardi, N. The Mesoscale Eddy Field of the Middle Adriatic Sea during Fall 1988. Deep-Sea Res. Part I 1993, 40, 1365–1377. [Google Scholar] [CrossRef]
- Silvestro, S.; Chiricosta, L.; Gugliandolo, A.; Pizzicannella, J.; Diomede, F.; Bramanti, P.; Trubiani, O.; Mazzon, E. Extracellular Vesicles Derived from Human Gingival Mesenchymal Stem Cells: A Transcriptomic Analysis. Genes 2020, 11, 118. [Google Scholar] [CrossRef] [Green Version]
- Ruh, A.C.; Frigo, L.; Cavalcanti, M.; Svidnicki, P.; Vicari, V.N.; Lopes-Martins, R.A.B.; Leal Junior, E.C.P.; De Isla, N.; Diomede, F.; Trubiani, O.; et al. Laser photobiomodulation in pressure ulcer healing of human diabetic patients: Gene expression analysis of inflammatory biochemical markers. Lasers Med. Sci. 2018, 33, 165–171. [Google Scholar] [CrossRef]
- Trubiani, O.; Toniato, E.; Di Iorio, D.; Diomede, F.; Merciaro, I.; D’Arcangelo, C.; Caputi, S. Morphological analysis and interleukin release in human gingival fibroblasts seeded on different denture base acrylic resins. Int. J. Immunopathol. Pharmacol. 2012, 25, 637–643. [Google Scholar] [CrossRef]
- Marchisio, M.; Simeone, P.; Bologna, G.; Ercolino, E.; Pierdomenico, L.; Pieragostino, D.; Ventrella, A.; Antonini, F.; Del Zotto, G.; Vergara, D.; et al. Flow Cytometry Analysis of Circulating Extracellular Vesicle Subtypes from Fresh Peripheral Blood Samples. Int. J. Mol. Sci. 2021, 22, 48. [Google Scholar] [CrossRef]
- Simeone, P.; Celia, C.; Bologna, G.; Ercolino, E.; Pierdomenico, L.; Cilurzo, F.; Grande, R.; Diomede, F.; Vespa, S.; Canonico, B.; et al. Diameters and Fluorescence Calibration for Extracellular Vesicle Analyses by Flow Cytometry. Int. J. Mol. Sci. 2020, 21, 7885. [Google Scholar] [CrossRef]
- Brocco, D.; Lanuti, P.; Pieragostino, D.; Cufaro, M.C.; Simeone, P.; Bologna, G.; Di Marino, P.; De Tursi, M.; Grassadonia, A.; Irtelli, L.; et al. Phenotypic and Proteomic Analysis Identifies Hallmarks of Blood Circulating Extracellular Vesicles in NSCLC Responders to Immune Checkpoint Inhibitors. Cancers 2021, 13, 585. [Google Scholar] [CrossRef] [PubMed]
- Cossarizza, A.; Chang, H.D.; Radbruch, A.; Acs, A.; Adam, D.; Adam-Klages, S.; Agace, W.W.; Aghaeepour, N.; Akdis, M.; Allez, M.; et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur. J. Immunol. 2019, 49, 1457–1973. [Google Scholar] [CrossRef] [Green Version]
- Rajan, T.S.; Diomede, F.; Bramanti, P.; Trubiani, O.; Mazzon, E. Conditioned medium from human gingival mesenchymal stem cells protects motor-neuron-like NSC-34 cells against scratch-injury-induced cell death. Int. J. Immunopathol. Pharmacol. 2017, 30, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Marconi, G.D.; Cavalcanti, M.; Pizzicannella, J.; Pierdomenico, S.D.; Fonticoli, L.; Piattelli, A.; Trubiani, O. VEGF/VEGF-R/RUNX2 Upregulation in Human Periodontal Ligament Stem Cells Seeded on Dual Acid Etched Titanium Disk. Materials 2020, 13, 706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caputi, S.; Trubiani, O.; Sinjari, B.; Trofimova, S.; Diomede, F.; Linkova, N.; Diatlova, A.; Khavinson, V. Effect of short peptides on neuronal differentiation of stem cells. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419828613. [Google Scholar] [CrossRef] [Green Version]
- Digka, N.; Tsangaris, C.; Torre, M.; Anastasopoulou, A.; Zeri, C. Microplastics in mussels and fish from the Northern Ionian Sea. Mar. Pollut. Bull. 2018, 135, 30–40. [Google Scholar] [CrossRef]
- Lu, L.; Luo, T.; Zhao, Y.; Cai, C.H.; Fu, Z.W.; Jin, Y.X. Interaction between microplastics and microorganism as well as gut microbiota: A consideration on environmental animal and human health. Sci. Total Environ. 2019, 667, 94–100. [Google Scholar] [CrossRef]
- Sun, X.X.; Li, Q.J.; Shi, Y.Q.; Zhao, Y.F.; Zheng, S.; Liang, J.H.; Liu, T.; Tian, Z.Y. Characteristics and retention of microplastics in the digestive tracts of fish from the Yellow Sea. Environ. Pollut. 2019, 249, 878–885. [Google Scholar] [CrossRef]
- Waring, R.H.; Harris, R.M.; Mitchell, S.C. Plastic contamination of the food chain: A threat to human health? Maturitas 2018, 115, 64–68. [Google Scholar] [CrossRef]
- Brandon, J.A.; Freibott, A.; Sala, L.M. Patterns of suspended and salp-ingested microplastic debris in the North Pacific investigated with epifluorescence microscopy. Limnol. Oceanogr. Lett. 2020, 5, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Li, W.C.; Tse, H.F.; Fok, L. Plastic waste in the marine environment: A review of sources, occurrence and effects. Sci. Total Environ. 2016, 566, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Hesler, M.; Aengenheister, L.; Ellinger, B.; Drexel, R.; Straskraba, S.; Jost, C.; Wagner, S.; Meier, F.; von Briesen, H.; Buchel, C.; et al. Multi-endpoint toxicological assessment of polystyrene nano- and microparticles in different biological models in vitro. Toxicol. In Vitro 2019, 61, 104610. [Google Scholar] [CrossRef] [PubMed]
- Magri, D.; Sanchez-Moreno, P.; Caputo, G.; Gatto, F.; Veronesi, M.; Bardi, G.; Catelani, T.; Guarnieri, D.; Athanassiou, A.; Pompa, P.P.; et al. Laser Ablation as a Versatile Tool To Mimic Polyethylene Terephthalate Nanoplastic Pollutants: Characterization and Toxicology Assessment. ACS Nano 2018, 12, 7690–7700. [Google Scholar] [CrossRef] [PubMed]
- Stock, V.; Bohmert, L.; Lisicki, E.; Block, R.; Cara-Carmona, J.; Pack, L.K.; Selb, R.; Lichtenstein, D.; Voss, L.; Henderson, C.J.; et al. Uptake and effects of orally ingested polystyrene microplastic particles in vitro and in vivo. Arch. Toxicol. 2019, 93, 1817–1833. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Wilson, M.R.; MacNee, W.; Stone, V.; Donaldson, K. Size-dependent proinflammatory effects of ultrafine polystyrene particles: A role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol. Appl. Pharmacol. 2001, 175, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Fournier, S.B.; D’Errico, J.N.; Adler, D.S.; Kollontzi, S.; Goedken, M.J.; Fabris, L.; Yurkow, E.J.; Stapleton, P.A. Nanopolystyrene translocation and fetal deposition after acute lung exposure during late-stage pregnancy. Part Fibre Toxicol. 2020, 17, 55. [Google Scholar] [CrossRef]
- Jin, H.B.; Ma, T.; Sha, X.X.; Liu, Z.Y.; Zhou, Y.; Meng, X.N.; Chen, Y.B.; Han, X.D.; Ding, J. Polystyrene microplastics induced male reproductive toxicity in mice. J. Hazard. Mater. 2021, 401, 123430. [Google Scholar] [CrossRef]
- Xu, M.; Halimu, G.; Zhang, Q.; Song, Y.; Fu, X.; Li, Y.; Li, Y.; Zhang, H. Internalization and toxicity: A preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci. Total Environ. 2019, 694, 133794. [Google Scholar] [CrossRef]
- Domenech, J.; Hernandez, A.; Rubio, L.; Marcos, R.; Cortes, C. Interactions of polystyrene nanoplastics with in vitro models of the human intestinal barrier. Arch. Toxicol. 2020, 94, 2997–3012. [Google Scholar] [CrossRef]
- Barguilla, I.; Domenech, J.; Rubio, L.; Marcos, R.; Hernandez, A. Nanoplastics and Arsenic Co-Exposures Exacerbate Oncogenic Biomarkers under an In Vitro Long-Term Exposure Scenario. Int. J. Mol. Sci. 2022, 23, 2958. [Google Scholar] [CrossRef]
- Dong, C.D.; Chen, C.W.; Chen, Y.C.; Chen, H.H.; Lee, J.S.; Lin, C.H. Polystyrene microplastic particles: In vitro pulmonary toxicity assessment. J. Hazard. Mater. 2020, 385, 121575. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Choi, D.; Han, S.; Choi, J.; Hong, J. An assessment of the toxicity of polypropylene microplastics in human derived cells. Sci. Total Environ. 2019, 684, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Poma, A.; Vecchiotti, G.; Colafarina, S.; Zarivi, O.; Aloisi, M.; Arrizza, L.; Chichiricco, G.; Di Carlo, P. In Vitro Genotoxicity of Polystyrene Nanoparticles on the Human Fibroblast Hs27 Cell Line. Nanomaterials 2019, 9, 1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prietl, B.; Meindl, C.; Roblegg, E.; Pieber, T.R.; Lanzer, G.; Frohlich, E. Nano-sized and micro-sized polystyrene particles affect phagocyte function. Cell Biol. Toxicol. 2014, 30, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Schirinzi, G.F.; Perez-Pomeda, I.; Sanchis, J.; Rossini, C.; Farre, M.; Barcelo, D. Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environ. Res. 2017, 159, 579–587. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Bai, J.L.; Ning, B.A.; Fan, L.X.; Sun, T.Q.; Fang, Y.J.; Wu, J.; Li, S.; Duan, C.H.; Zhang, Y.C.; et al. Effects of bisphenol A and nanoscale and microscale polystyrene plastic exposure on particle uptake and toxicity in human Caco-2 cells. Chemosphere 2020, 254, 126788. [Google Scholar] [CrossRef]
- Ramsperger, A.F.R.M.; Jasinski, J.; Volkl, M.; Witzmann, T.; Meinhart, M.; Jerome, V.; Kretschmer, W.P.; Freitag, R.; Senker, J.; Fery, A.; et al. Supposedly identical microplastic particles substantially differ in their material properties influencing particle-cell interactions and cellular responses. J. Hazard. Mater. 2022, 425, 127961. [Google Scholar] [CrossRef]
- Im, G.B.; Kim, Y.G.; Jo, I.S.; Yoo, T.Y.; Kim, S.W.; Park, H.S.; Hyeon, T.; Yi, G.R.; Bhang, S.H. Effect of polystyrene nanoplastics and their degraded forms on stem cell fate. J. Hazard. Mater. 2022, 430, 128411. [Google Scholar] [CrossRef]
- Peeters, P.M.; Eurlings, I.M.J.; Perkins, T.N.; Wouters, E.F.; Schins, R.P.F.; Borm, P.J.A.; Drommer, W.; Reynaert, N.L.; Albrecht, C. Silica-induced NLRP3 inflammasome activation in vitro and in rat lungs. Part Fibre Toxicol. 2014, 11, 58. [Google Scholar] [CrossRef] [Green Version]
- Zheng, R.X.; Tao, L.; Jian, H.; Chang, Y.; Cheng, Y.; Feng, Y.L.; Zhang, H.Y. NLRP3 inflammasome activation and lung fibrosis caused by airborne fine particulate matter. Ecotoxicol. Environ. Saf. 2018, 163, 612–619. [Google Scholar] [CrossRef]
- Hou, B.L.; Wang, F.Y.; Liu, T.; Wang, Z.P. Reproductive toxicity of polystyrene microplastics: In vivo experimental study on testicular toxicity in mice. J. Hazard. Mater. 2021, 405, 124028. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.M.; Deng, T.; Duan, J.F.; Xie, J.; Yuan, J.L.; Chen, M.Q. Exposure to polystyrene microplastics causes reproductive toxicity through oxidative stress and activation of the p38 MAPK signaling pathway. Ecotoxicol. Environ. Saf. 2020, 190, 110133. [Google Scholar] [CrossRef]
- Diomede, F.; Thangavelu, S.R.; Merciaro, I.; D’Orazio, M.; Bramanti, P.; Mazzon, E.; Trubiani, O. Porphyromonas gingivalis lipopolysaccharide stimulation in human periodontal ligament stem cells: Role of epigenetic modifications to the inflammation. Eur. J. Histochem. 2017, 61, 2826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, Y.W.; Sun, J.Y.; Li, Z.Y.; Zhang, W.X.; Liu, Z.D.; Li, C.; Peng, C.; Cui, G.Q.; Shao, H.; Du, Z.J. Activation of pyroptosis and ferroptosis is involved in the hepatotoxicity induced by polystyrene microplastics in mice. Chemosphere 2022, 291, 132944. [Google Scholar] [CrossRef] [PubMed]
- Marconi, G.D.; Fonticoli, L.; Guarnieri, S.; Cavalcanti, M.; Franchi, S.; Gatta, V.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Ascorbic Acid: A New Player of Epigenetic Regulation in LPS-gingivalis Treated Human Periodontal Ligament Stem Cells. Oxidative Med. Cell. Longev. 2021, 2021, 6679708. [Google Scholar] [CrossRef]
- Soundara Rajan, T.; Giacoppo, S.; Diomede, F.; Bramanti, P.; Trubiani, O.; Mazzon, E. Human periodontal ligament stem cells secretome from multiple sclerosis patients suppresses NALP3 inflammasome activation in experimental autoimmune encephalomyelitis. Int. J. Immunopathol. Pharmacol. 2017, 30, 238–252. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.A.; Montagnani, M.; Koh, K.K.; Quon, M.J. Reciprocal relationships between insulin resistance and endothelial dysfunction—Molecular and pathophysiological mechanisms. Circulation 2006, 113, 1888–1904. [Google Scholar] [CrossRef]
- Iorio, R.; Castellucci, A.; Ventriglia, G.; Teoli, F.; Cellini, V.; Macchiarelli, G.; Cecconi, S. Ovarian toxicity: From environmental exposure to chemotherapy. Curr. Pharm. Des. 2014, 20, 5388–5397. [Google Scholar] [CrossRef]
- Bianchi, S.; Bernardi, S.; Belli, M.; Varvara, G.; Macchiarelli, G. Exposure to persistent organic pollutants during tooth formation: Molecular mechanisms and clinical findings. Rev. Environ. Health 2020, 35, 303–310. [Google Scholar] [CrossRef]
- Nakajima, S.; Kitamura, M. Bidirectional regulation of NF-kappaB by reactive oxygen species: A role of unfolded protein response. Free Radic. Biol. Med. 2013, 65, 162–174. [Google Scholar] [CrossRef]
- Mohan, S.; Koyoma, K.; Thangasamy, A.; Nakano, H.; Glickman, R.D.; Mohan, N. Low shear stress preferentially enhances IKK activity through selective sources of ROS for persistent activation of NF-kappa B in endothelial cells. Am. J. Physiol. 2007, 292, C362–C371. [Google Scholar] [CrossRef] [PubMed]
- Von Moos, N.; Burkhardt-Holm, P.; Kohler, A. Uptake and Effects of Microplastics on Cells and Tissue of the Blue Mussel Mytilus edulis L. after an Experimental Exposure. Environ. Sci. Technol. 2012, 46, 11327–11335. [Google Scholar] [CrossRef] [PubMed]
- Boozari, M.; Butler, A.E.; Sahebkar, A. Impact of curcumin on toll-like receptors. J. Cell. Physiol. 2019, 234, 12471–12482. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Hoshino, K. Myeloid differentiation factor 88-dependent and -independent pathways in Toll-like receptor signaling. J. Infect. Dis. 2003, 187, S356–S363. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caputi, S.; Diomede, F.; Lanuti, P.; Marconi, G.D.; Di Carlo, P.; Sinjari, B.; Trubiani, O. Microplastics Affect the Inflammation Pathway in Human Gingival Fibroblasts: A Study in the Adriatic Sea. Int. J. Environ. Res. Public Health 2022, 19, 7782. https://doi.org/10.3390/ijerph19137782
Caputi S, Diomede F, Lanuti P, Marconi GD, Di Carlo P, Sinjari B, Trubiani O. Microplastics Affect the Inflammation Pathway in Human Gingival Fibroblasts: A Study in the Adriatic Sea. International Journal of Environmental Research and Public Health. 2022; 19(13):7782. https://doi.org/10.3390/ijerph19137782
Chicago/Turabian StyleCaputi, Sergio, Francesca Diomede, Paola Lanuti, Guya Diletta Marconi, Piero Di Carlo, Bruna Sinjari, and Oriana Trubiani. 2022. "Microplastics Affect the Inflammation Pathway in Human Gingival Fibroblasts: A Study in the Adriatic Sea" International Journal of Environmental Research and Public Health 19, no. 13: 7782. https://doi.org/10.3390/ijerph19137782
APA StyleCaputi, S., Diomede, F., Lanuti, P., Marconi, G. D., Di Carlo, P., Sinjari, B., & Trubiani, O. (2022). Microplastics Affect the Inflammation Pathway in Human Gingival Fibroblasts: A Study in the Adriatic Sea. International Journal of Environmental Research and Public Health, 19(13), 7782. https://doi.org/10.3390/ijerph19137782








