The Association between Hypertriglyceridemia and Colorectal Cancer: A Long-Term Community Cohort Study in Taiwan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Statistical Analysis
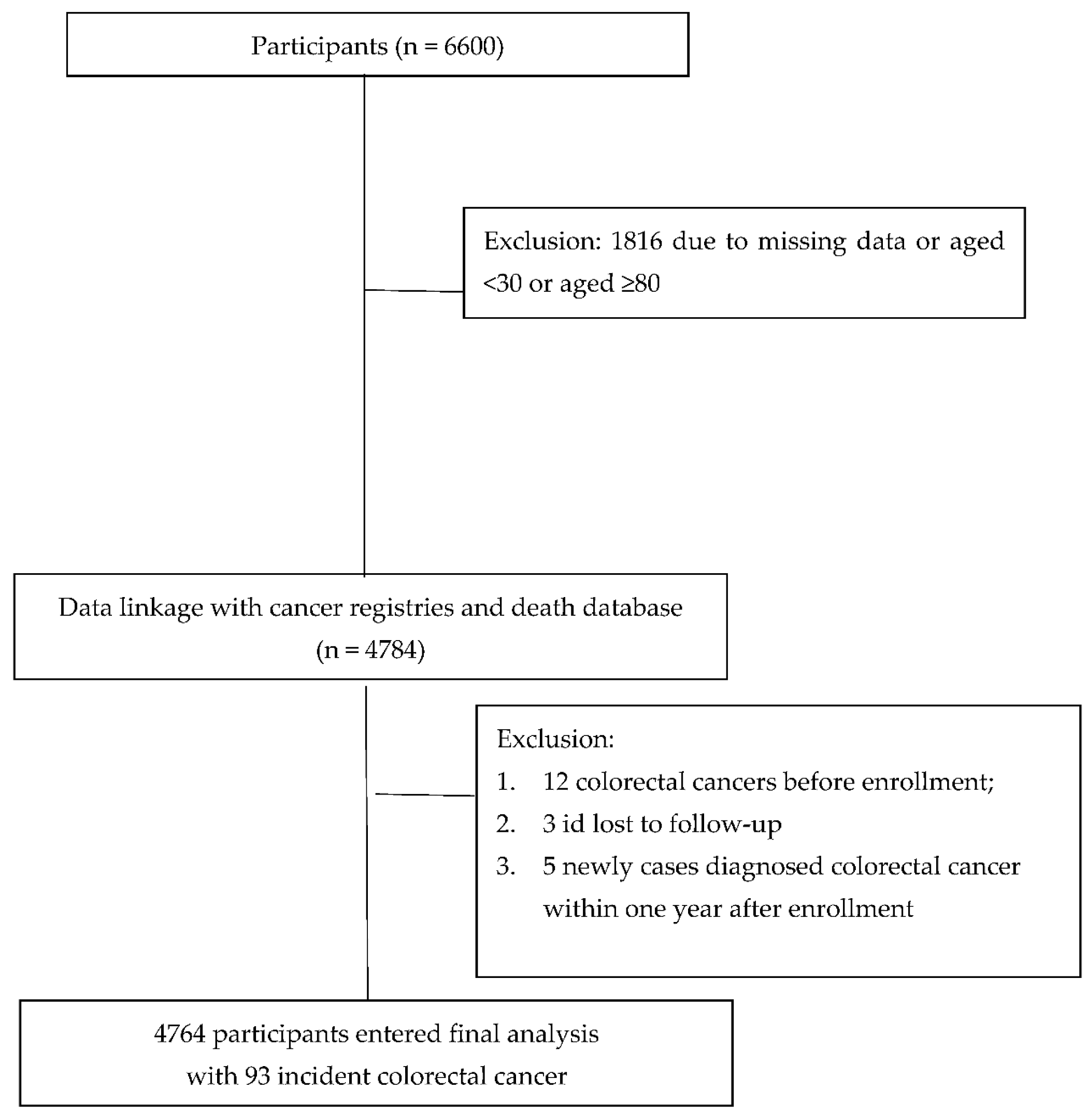
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Agency for Research on Cancer (IARC). Globocan 2018: Cancer Fact Sheets—Colorectal Cancer. 2018. Available online: http://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact-sheet.pdf (accessed on 17 October 2018).
- Yue, X.; Pengfei, X. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar]
- Huang, Y.C.; Chen, Y.H. Cancer incidence characteristic evolution based on the National Cancer Registry in Taiwan. J. Oncol. 2020, 2020, 1408793. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.G. Colorectal cancer in young adults: A difficult challenge. World J. Gastroenterol. 2017, 23, 5041–5044. [Google Scholar] [CrossRef] [PubMed]
- Guren, M.G. The global challenge of colorectal cancer. Lancet Gastroenterol. Hepatol. 2019, 4, 894–895. [Google Scholar] [CrossRef] [Green Version]
- Peterse, E.F.P.; Meester, R.G.S. Comparing the Cost-Effectiveness of Innovative Colorectal Cancer Screening Tests. J. Natl. Cancer Inst. 2021, 113, 154–161. [Google Scholar] [CrossRef]
- Chen, C.J.; You, S.L. Cancer epidemiology and control in Taiwan: A brief review. Jpn. J. Clin. Oncol. 2002, 32, S66–S81. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Cheng, S.C. The risk factors affecting survival in colorectal cancer in Taiwan. Iran. J. Public Health 2018, 47, 519–530. [Google Scholar]
- Tornberg, S.A.; Holm, L.E. Risks of cancer of the colon and rectum in relation to serum cholesterol and beta-lipoprotein. N. Engl. J. Med. 1986, 315, 1629–1633. [Google Scholar] [CrossRef]
- Mamtani, R.; Lewis, J.D. Disentangling the association between statins, cholesterol, and colorectal cancer: A nested case-control study. PLoS Med. 2016, 13, e1002007. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Joo, J.S. Association of serum lipids and glucose with the risk of colorectal adenomatous polyp in men: A case-control study in Korea. J. Korean Med. Sci. 2000, 15, 690–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Broadbent, H.; Law, P.J. Mendelian randomisation implicates hyperlipidaemia as risk factor for colorectal cancer. Int. J. Cancer 2017, 140, 2701–2708. [Google Scholar] [CrossRef] [PubMed]
- Sorlie, P.D.; Fienleib, M. The serum cholesterol-cancer relationship: An analysis of time trends in the Framingham study. J. Natl. Cancer Inst. 1982, 69, 989–996. [Google Scholar] [PubMed]
- Yaari, S.; Goldbourt, E.-Z. Associations of serum high density lipoprotein and total cholesterol with total, cardiovascular, and cancer mortality in a 7-year prospective study of 10,000 men. Lancet 1981, 1, 1011–1015. [Google Scholar] [CrossRef]
- American Diabetes Association. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42 (Suppl. 1), S103–S123. [Google Scholar] [CrossRef] [Green Version]
- Long, J.; Zhang, C.-J. Lipid metabolism and carcinogenesis, cancer development. Am. J. Cancer Res. 2018, 8, 778–791. [Google Scholar]
- Ackerman, D.; Simon, M.C. Hypoxia, lipids, and cancer: Surviving the harsh tumor microenvironment. Trends Cell Biol. 2014, 24, 472–478. [Google Scholar] [CrossRef] [Green Version]
- Laslett, L.J.; Alagona, P., Jr. The worldwide environment of cardiovascular disease: Prevalence, diagnosis, therapy, and policy issues: A report from the American College of Cardiology. J. Am. Coll. Cardiol. 2012, 60, S1–S49. [Google Scholar] [CrossRef] [Green Version]
- Xinghua, Y.; Chaonan, X. Risk prediction model of dyslipidaemia over a 5-year period based on the Taiwan MJ health check-up longitudinal database. Lipids Health Dis. 2018, 17, 259. [Google Scholar]
- Kuo, P.; Syu, J.T. Prevalence and trend of dyslipidaemia from 1996 to 2006 among normal and overweight adolescents in Taiwan. BMJ Open 2014, 4, e003800. [Google Scholar] [CrossRef] [Green Version]
- Cheng, K.C.; Chen, Y.L. Prevalence of dyslipidaemia in patients receiving health checkups: A hospital-based study. Cholesterol 2011, 2011, 314234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opoku, S.; Gan, Y. Awareness, treatment, control, and determinants of dyslipidemia among adults in China. Sci. Rep. 2021, 11, 10056. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kwon, O.D. Impact of a family history of cardiovascular disease on prevalence, awareness, treatment, control of dyslipidemia, and healthy behaviors: Findings from the Korea National Health and Nutrition Examination Survey. PLoS ONE 2021, 16, e0254907. [Google Scholar] [CrossRef] [PubMed]
- Boo, S.; Yoon, Y.J. Evaluating the prevalence, awareness, and control of hypertension, diabetes, and dyslipidemia in Korea using the NHIS-NSC database: A cross-sectional analysis. Medicine 2018, 97, e13713. [Google Scholar] [CrossRef]
- Cheetham, T.C.; Niu, F. Primary nonadherence to statin medications in a managed care organization. J. Manag. Care Pharm. 2013, 19, 367–373. [Google Scholar] [CrossRef]
- Krähenbühl, S.; Pavik-Mezzour, I. Unmet needs in LDL-C lowering: When statins won’t do! Drugs 2016, 76, 1175–1190. [Google Scholar] [CrossRef] [Green Version]
- Agnoli, C.; Grioni, S. Colorectal cancer risk and dyslipidemia: A case-cohort study nested in an Italian multicentre cohort. Cancer Epidemiol. 2014, 38, 144–151. [Google Scholar] [CrossRef]
- Inoue, M.; Noda, M. Impact of metabolic factors on subsequent cancer risk: Results from a large-scale population-based cohort study in Japan. Eur. J. Cancer Prev. 2009, 18, 240–247. [Google Scholar] [CrossRef]
- Strohmaier, S.; Edlinger, M. Total serum cholesterol and cancer incidence in the Metabolic syndrome and Cancer Project (Me-Can). PLoS ONE 2013, 8, e54242. [Google Scholar] [CrossRef] [Green Version]
- van Duijnhoven, F.J.; Bueno-De-Mesquita, H.B. Blood lipid and lipoprotein concentrations and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Gut 2011, 60, 1094–1102. [Google Scholar] [CrossRef]
- Ahmed, R.L.; Schmitz, K.H. The metabolic syndrome and risk of incident colorectal cancer. Cancer 2006, 107, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Bowers, K.; Albanes, D. A prospective study of anthropometric and clinical measurements associated with insulin resistance syndrome and colorectal cancer in male smokers. Am. J. Epidemiol. 2006, 164, 652–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, Y.T.; Hung, Y.T. The design, contents, operation and the characteristics of the respondents of the 2001 National Health Interview Survey in Taiwan. Taiwan J. Public Health 2003, 22, 419–430. [Google Scholar]
- Hwang, L.C.; Bai, C.H. Prevalence of obesity and metabolic syndrome in Taiwan. J. Formos. Med. Assoc. 2006, 105, 626–635. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Physical Status: The Use and Interpretation of Anthropometry, Report of a WHO Expert Committee; WHO: Geneva, Switzerland, 1995; Available online: https://www.who.int/publications/i/item/9241208546 (accessed on 20 December 2019).
- Lin, C.C.; Liu, C.S.; Lai, M.M.; Li, C.I.; Chen, C.C.; Chang, P.C.; Lin, W.-Y.; Lee, Y.-D.; Lin, T.; Li, T.-C. Metabolic syndrome in a Taiwanese metropolitan adult population. BMC Public Health 2007, 7, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, K.C. Obesity and its related diseases in Taiwan. Obes. Rev. 2008, 9 (Suppl. 1), 32–34. [Google Scholar] [CrossRef] [PubMed]
- Kagan, A.; McGee, D.L. Serum cholesterol and mortality in a Japanese-American population: The Honolulu Heart program. Am. J. Epidemiol. 1981, 114, 11–20. [Google Scholar] [CrossRef]
- Circulating cholesterol level and risk of death from cancer in men aged 40 to 69 years: Experience of an international collaborative group. JAMA 1982, 248, 2853–2859. [CrossRef]
- Morris, D.L.; Borhani, N.O. Serum cholesterol and cancer in the hypertension detection and follow-up program. Cancer 1983, 52, 1754–1759. [Google Scholar] [CrossRef]
- Rose, G.; Shipley, M.J. Plasma lipids and mortality: A source of error. Lancet 1980, 315, 523–526. [Google Scholar] [CrossRef]
- Radisauskas, R.; Kuzmickiene, I. Hypertension, serum lipids and cancer risk: A review of epidemiological evidence. Medicina 2016, 52, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Murai, T. Cholesterol lowering: Role in cancer prevention and treatment. Biol. Chem. 2015, 396, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houghton, J.; Lardieri, G.G. Effect of cholesterol levels on villous histology in colonic adenomas. Dig. Dis. Sci. 2000, 45, 896–899. [Google Scholar] [CrossRef]
- Lee, J.W.; You, N.Y. Statin use and site-specific risk of colorectal cancer in individuals with hypercholesterolemia from the National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS). Nutr. Metab. Cardiovasc. Dis. 2019, 29, 701–709. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Tang, W.Z. Association between statin use and colorectal cancer risk: A meta-analysis of 42 studies. Cancer Causes Control 2014, 25, 237–249. [Google Scholar] [CrossRef]
- Ye, P.; Xi, Y. Linking obesity with colorectal cancer: Epidemiology and mechanistic insights. Cancers 2020, 12, 1408. [Google Scholar] [CrossRef] [PubMed]
- Sekharam, M.; Nasir, A. Insulin-like growth factor 1 receptor activates c-SRC and modifies transformation and motility of colorectal cancer in vitro. Anticancer Res. 2003, 23, 1517–1524. [Google Scholar] [PubMed]
- Rouillier, P.; Senesse, P. Dietary patterns and the adenomacarcinoma sequence of colorectal cancer. Eur. J. Nutr. 2005, 44, 311–318. [Google Scholar] [CrossRef]
- Zhang, L.; Theodoropoulos, P.C. Selective targeting of mutant adenomatous polyposis coli (APC) in colorectal cancer. Sci. Transl. Med. 2016, 8, 361ra140. [Google Scholar] [CrossRef]
- Liang, X.; Fan, X. Peroxisome proliferators-activated receptor gamma polymorphisms and colorectal cancer risk. J. Cancer Res. Ther. 2018, 14, S306–S310. [Google Scholar] [CrossRef] [PubMed]
- Niho, N.; Takahashi, M. Concomitant suppression of hyperlipidemia and intestinal polyp formation in Apc-deficient mice by peroxisome proliferator-activated receptor ligands. Cancer Res. 2003, 63, 6090–6095. [Google Scholar]
- Ashbeck, E.L.; Jacobs, E.T. Components of metabolic syndrome and metachronous colorectal neoplasia. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1134–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saydah, S.H.; Platz, E.A. Association of markers of insulin and glucose control with subsequent colorectal cancer risk. Cancer Epidemiol. Biomark. Prev. 2003, 12, 412–418. [Google Scholar]
- Borena, W.; Stocks, T. Serum triglycerides and cancer risk in the metabolic syndrome and cancer (Me-Can) collaborative study. Cancer Causes Control 2011, 22, 291–299. [Google Scholar] [CrossRef]
- Stocks, T.; Lukanova, A. Metabolic factors and the risk of colorectal cancer in 580,000 men and women in the metabolic syndrome and cancer project (Me-Can). Cancer 2011, 117, 2398–2407. [Google Scholar] [CrossRef]
- Ulmer, H.; Borena, W. Serum triglyceride concentrations and cancer risk in a large cohort study in Austria. Br. J. Cancer 2009, 101, 1202–1206. [Google Scholar] [CrossRef] [Green Version]
- McKeown-Eyssen, G. Epidemiology of colorectal cancer revisited: Are serum triglycerides and/or plasma glucose associated with risk? Cancer Epidemiol. Biomark. Prev. 1994, 3, 687–695. [Google Scholar]
- Kundu, J.K.; Surh, Y.J. Inflammation: Gearing the journey to cancer. Mutat. Res. 2008, 659, 15–30. [Google Scholar] [CrossRef]
- Esteve, E.; Ricart, W. Dyslipidemia and inflammation: An evolutionary conserved mechanism. Clin. Nutr. 2005, 24, 16–31. [Google Scholar] [CrossRef]
- Vekic, J.; Zeljkovic, A. Obesity and dyslipidemia. Metabolism 2019, 92, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Pagliassotti, M.J.; Kim, P.Y. Endoplasmic reticulum stress in obesity and obesity-related disorders: An expanded view. Metabolism 2016, 65, 1238–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klop, B.; Elte, J.W. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beloribi-Djefaflia, S.; Vasseur, S. Lipid metabolic reprogramming in cancer cells. Oncogenesis 2016, 5, e189. [Google Scholar] [CrossRef]
- Rolando, V.; Luca, D.L. Hints on ATGL implications in cancer: Beyond bioenergetic clues. Cell Death Dis. 2018, 9, 316. [Google Scholar]
- Zimmermann, R.; Strauss, J.G. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 2004, 306, 1383–1386. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Li, W. Adipose triglyceride lipase promotes the proliferation of. colorectal cancer cells via enhancing the lipolytic pathway. J. Cell. Mol. Med. 2021, 25, 3963–3975. [Google Scholar] [CrossRef]
- Hirano, T. Pathophysiology of Diabetic Dyslipidemia. J. Atheroscler. Thromb. 2018, 25, 771–782. [Google Scholar] [CrossRef] [Green Version]

| Variable | Subject | (%) |
|---|---|---|
| Gender | ||
| Female | 2509 | 52.67 |
| Male | 2255 | 47.33 |
| Age (Years) | ||
| ≤45 | 2064 | 43.32 |
| 46–55 | 1248 | 26.2 |
| ≥55 | 1452 | 30.48 |
| Mean (SD) | 49.37 | 12.67 |
| Educational level | ||
| Elementary | 1330 | 27.97 |
| Illiterate | 369 | 7.76 |
| Junior high school | 774 | 16.28 |
| Senior high school | 1322 | 27.8 |
| College | 960 | 20.19 |
| Missing | 9 |
| Variables | Subjects | Cases | Person-Years | Incidence Rate | Crude HR (95% CI) | p-Value | AHR a (95% CI) | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 4764 | 93 | 73,812.20 | 126 | |||||||
| Gender | |||||||||||
| Female | 2509 | 33 | 39,702.86 | 83.12 | 1 | ||||||
| Male | 2255 | 60 | 34,109.34 | 175.9 | 2.119 | (1.386, 3.241) | 0.0005 | 2.071 | 1.354 | 3.168 | 0.0008 |
| Age (year) | |||||||||||
| ≤45 | 2064 | 16 | 33,877.66 | 47.23 | 1 | ||||||
| 46–54 | 1248 | 25 | 19,812.68 | 126.18 | 2.677 | (1.429, 5.013) | 0.0021 | ||||
| ≥55 | 1452 | 52 | 20,121.85 | 258.43 | 5.53 | (3.157, 9.689) | <0.0001 | ||||
| Educational level | |||||||||||
| Elementary | 1330 | 37 | 19,555.84 | 189.2 | 1 | ||||||
| Illiterate | 369 | 7 | 5094.56 | 137.4 | 0.729 | (0.325, 1.635) | 0.443 | 0.497 | 0.220 | 1.123 | 0.0927 |
| Junior high school | 774 | 21 | 12,179.35 | 172.42 | 0.907 | (0.531, 1.55) | 0.7224 | 1.548 | 0.891 | 2.691 | 0.1213 |
| Senior high school | 1322 | 14 | 21,285.94 | 65.77 | 0.346 | (0.187, 0.64) | 0.0007 | 0.712 | 0.373 | 1.357 | 0.3016 |
| College | 960 | 14 | 15,546.27 | 90.05 | 0.473 | (0.256, 0.876) | 0.0172 | 0.928 | 0.489 | 1.762 | 0.8192 |
| Diabetes Mellitus | |||||||||||
| Never | 4323 | 71 | 67,921.49 | 104.53 | 1 | ||||||
| Have | 437 | 22 | 5823.87 | 377.76 | 3.653 | (2.264, 5.895) | <0.0001 | 2.462 | 1.507 | 4.024 | 0.0003 |
| Hypertension | |||||||||||
| Never | 3568 | 64 | 56,814.56 | 112.65 | 1 | ||||||
| Have | 1194 | 29 | 16,964.07 | 170.95 | 1.528 | (0.985, 2.37) | 0.0584 | 0.883 | 0.556 | 1.402 | 0.5966 |
| Cholesterol (mg/dL) | |||||||||||
| <180 | 2090 | 29 | 32,365.95 | 89.6 | 1 | ||||||
| ≥180 | 2672 | 64 | 41,412.84 | 154.54 | 1.723 | (1.111, 2.671) | 0.0151 | 1.396 | 0.898 | 2.172 | 0.1386 |
| LDL (mg/dL) | |||||||||||
| <110 | 1791 | 26 | 27,879.83 | 93.26 | 1 | ||||||
| 110–129 | 1433 | 32 | 22,161.75 | 144.39 | 1.547 | (0.922, 2.596) | 0.0983 | 1.307 | 0.777 | 2.199 | 0.3125 |
| ≥130 | 1538 | 35 | 23,737.21 | 147.45 | 1.58 | (0.951, 2.625) | 0.0772 | 1.156 | 0.691 | 1.933 | 0.5816 |
| TG (mg/dL) | |||||||||||
| <150 | 3294 | 58 | 51,470.89 | 112.69 | 1 | ||||||
| ≥150 | 506 | 18 | 7798.17 | 230.82 | 2.047 | (1.206, 3.474) | 0.0079 | 1.716 | 1.009 | 2.920 | 0.0463 |
| HDL (mg/dL) | |||||||||||
| Male >40 Female >50 | 3491 | 68 | 54,569.16 | 124.61 | 1 | ||||||
| Male ≤ 40 Female ≤50 | 1271 | 25 | 19,209.63 | 130.14 | 1.048 | (0.662, 1.657) | 0.8422 | 1.077 | 0.681 | 1.703 | 0.7515 |
| Body Mass Index (kg/m2) | |||||||||||
| <24 | 2441 | 38 | 38,528.66 | 98.63 | 1 | ||||||
| 24–27 | 1239 | 26 | 19,153.57 | 135.74 | 1.376 | (0.836, 2.267) | 0.2093 | 1.220 | 0.740 | 2.011 | 0.4356 |
| >27 | 628 | 17 | 9802.24 | 173.43 | 1.757 | (0.992, 3.113) | 0.0534 | 1.596 | 0.900 | 2.828 | 0.1094 |
| Waist circumference (cm) | |||||||||||
| ≤85 | 3025 | 48 | 47,717.51 | 100.59 | 1 | ||||||
| 86–95 | 1286 | 31 | 19,460.29 | 159.30 | 1.585 | (1.009, 2.491) | 0.0455 | 1.215 | 0.768 | 1.923 | 0.4047 |
| >95 | 446 | 14 | 6577.19 | 212.86 | 2.120 | (1.169, 3.845) | 0.0134 | 1.645 | 0.901 | 3.001 | 0.1049 |
| Smoking habit | |||||||||||
| Never | 3383 | 53 | 53,204.58 | 99.62 | 1 | ||||||
| Ever | 1374 | 40 | 20,490.81 | 195.21 | 1.965 | (1.303, 2.963) | 0.0013 | 2.053 | 1.361 | 3.095 | 0.0006 |
| Drinking habits | |||||||||||
| Never | 3426 | 65 | 52,972.80 | 122.7 | 1 | ||||||
| Ever | 1331 | 28 | 20,722.59 | 135.12 | 1.1 | (0.706, 1.713) | 0.6742 | 1.295 | 0.828 | 2.024 | 0.2575 |
| Menopause | |||||||||||
| Never | 1454 | 12 | 23,865.14 | 50.28 | 1 | ||||||
| Ever | 1053 | 20 | 15,814.44 | 126.47 | 2.536 | (1.239, 5.188 | 0.0108 | 1.028 | 0.324 | 3.260 | 0.9627 |
| Metabolic syndrome | |||||||||||
| Never | 3683 | 66 | 58,031.27 | 113.73 | 1 | ||||||
| Have | 1027 | 27 | 14,989.93 | 180.12 | 1.590 | (1.016, 2.488) | 0.0424 | 1.139 | 0.720 | 1.800 | 0.5786 |
| Exercise | |||||||||||
| Never | 3635 | 66 | 56,283.23 | 117.26 | 1 | ||||||
| Have | 1127 | 27 | 17,495.07 | 154.33 | 1.316 | (0.841, 2.059) | 0.2298 | 1.017 | 0.647 | 1.598 | 0.9432 |
| Animal fat diet | |||||||||||
| Low | 2758 | 50 | 42,901.11 | 116.55 | 1 | ||||||
| High | 2005 | 43 | 30,894.37 | 139.18 | 1.195 | (0.795, 1.796) | 0.3921 | 1.168 | 0.777 | 1.757 | 0.4541 |
| DM | Non-DM | |||
|---|---|---|---|---|
| TG < 150 mg/dL | TG ≥ 150 mg/dL | TG < 150 mg/dL | TG ≥ 150 mg/dL | |
| Cholesterol | ||||
| <180 mg/dL | 1.00 | 1.326 | 1.00 | 0.800 |
| (Referent) | (0.137–12.820) | (Referent) | (0.186–3.449) | |
| ≥180 mg/dL | 1.531 | 4.118 * | 1.339 | 1.761 |
| (0.381–6.160) | (1.061–15.975) | (0.751–2.388) | (0.768–4.035) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, S.-H.; Syu, D.-K.; Chen, Y.-C.; Liu, C.-K.; Sun, C.-A.; Chen, M. The Association between Hypertriglyceridemia and Colorectal Cancer: A Long-Term Community Cohort Study in Taiwan. Int. J. Environ. Res. Public Health 2022, 19, 7804. https://doi.org/10.3390/ijerph19137804
Hsu S-H, Syu D-K, Chen Y-C, Liu C-K, Sun C-A, Chen M. The Association between Hypertriglyceridemia and Colorectal Cancer: A Long-Term Community Cohort Study in Taiwan. International Journal of Environmental Research and Public Health. 2022; 19(13):7804. https://doi.org/10.3390/ijerph19137804
Chicago/Turabian StyleHsu, Shu-Hua, De-Kai Syu, Yong-Chen Chen, Chih-Kuang Liu, Chien-An Sun, and Mingchih Chen. 2022. "The Association between Hypertriglyceridemia and Colorectal Cancer: A Long-Term Community Cohort Study in Taiwan" International Journal of Environmental Research and Public Health 19, no. 13: 7804. https://doi.org/10.3390/ijerph19137804
APA StyleHsu, S. -H., Syu, D. -K., Chen, Y. -C., Liu, C. -K., Sun, C. -A., & Chen, M. (2022). The Association between Hypertriglyceridemia and Colorectal Cancer: A Long-Term Community Cohort Study in Taiwan. International Journal of Environmental Research and Public Health, 19(13), 7804. https://doi.org/10.3390/ijerph19137804






