SARS-CoV-2 Viral Load Analysis at Low and High Altitude: A Case Study from Ecuador
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
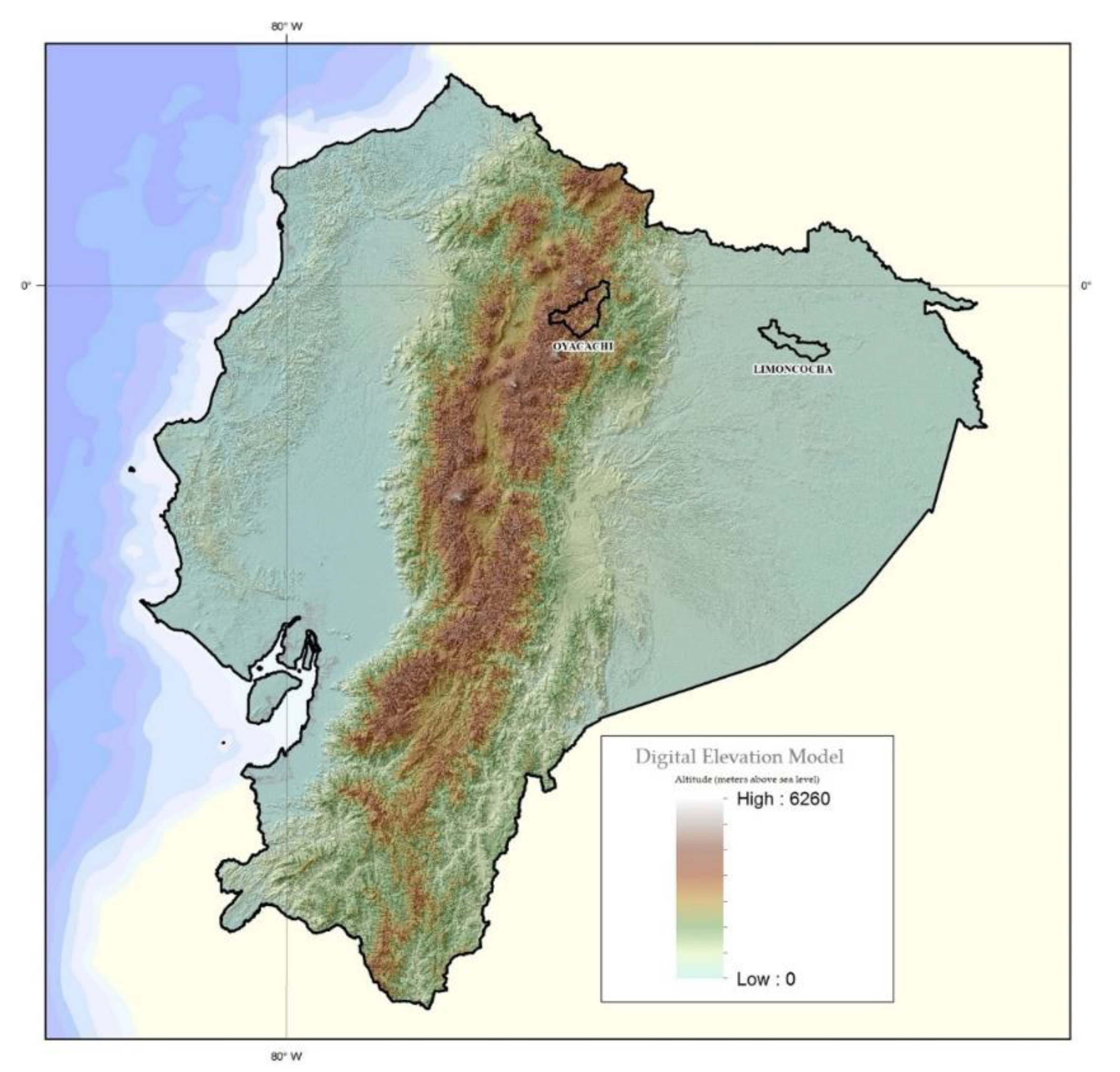
2.2. Settings
2.3. Participants
2.4. Data Source and Variables
2.5. Study and Sample Size
2.6. Statistical Methods
2.7. RNA Extraction and RT-qPCR for SARS-CoV-2 Detection
2.8. Nasopharyngeal Sample Collection
2.9. Bias
2.10. Ethics Statement
3. Results
3.1. Demographic Results
3.2. Positive Testing Rates
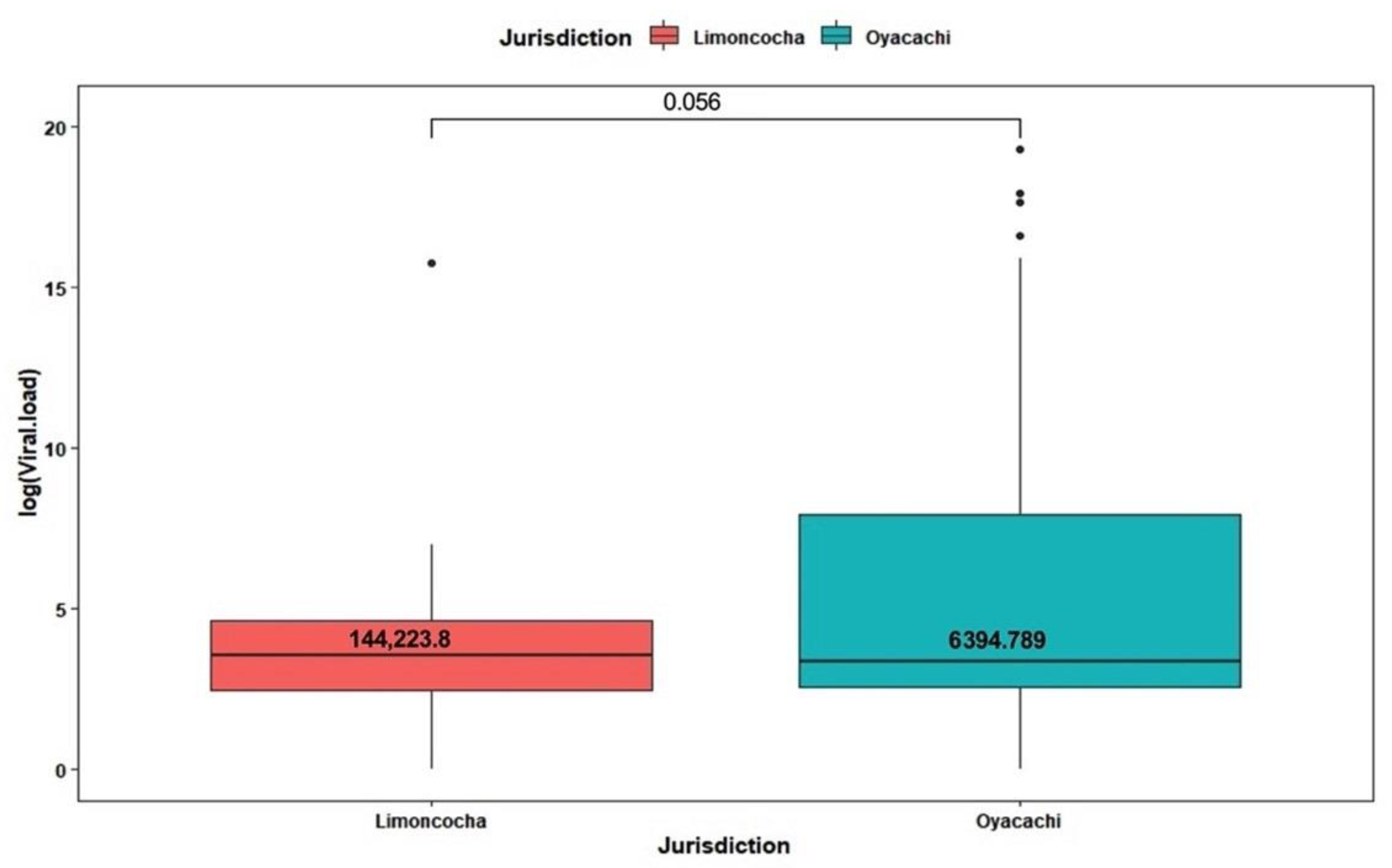
3.3. Viral Load Analysis by Altitude
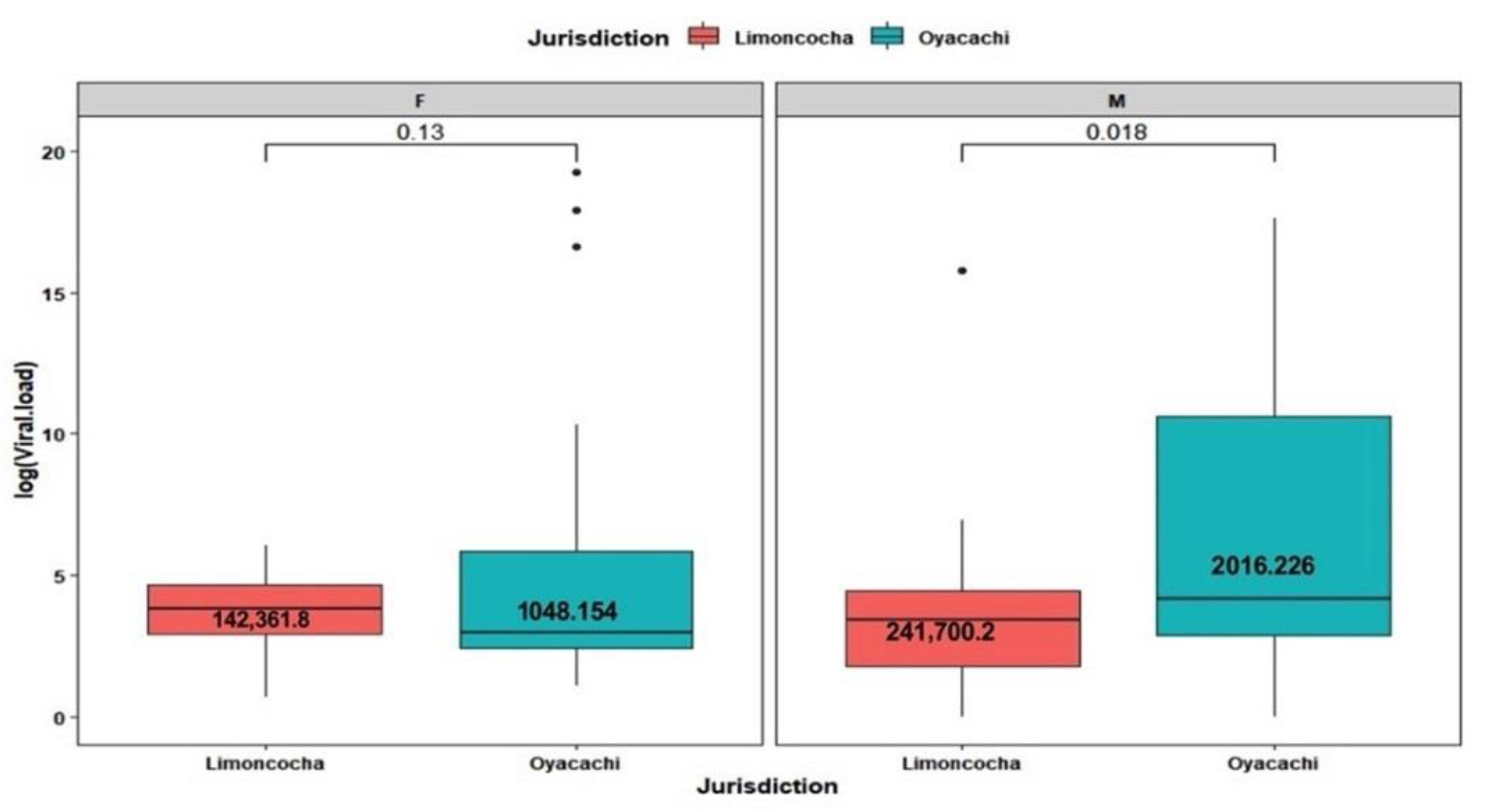
3.4. Viral Load Analysis by Age and Sex
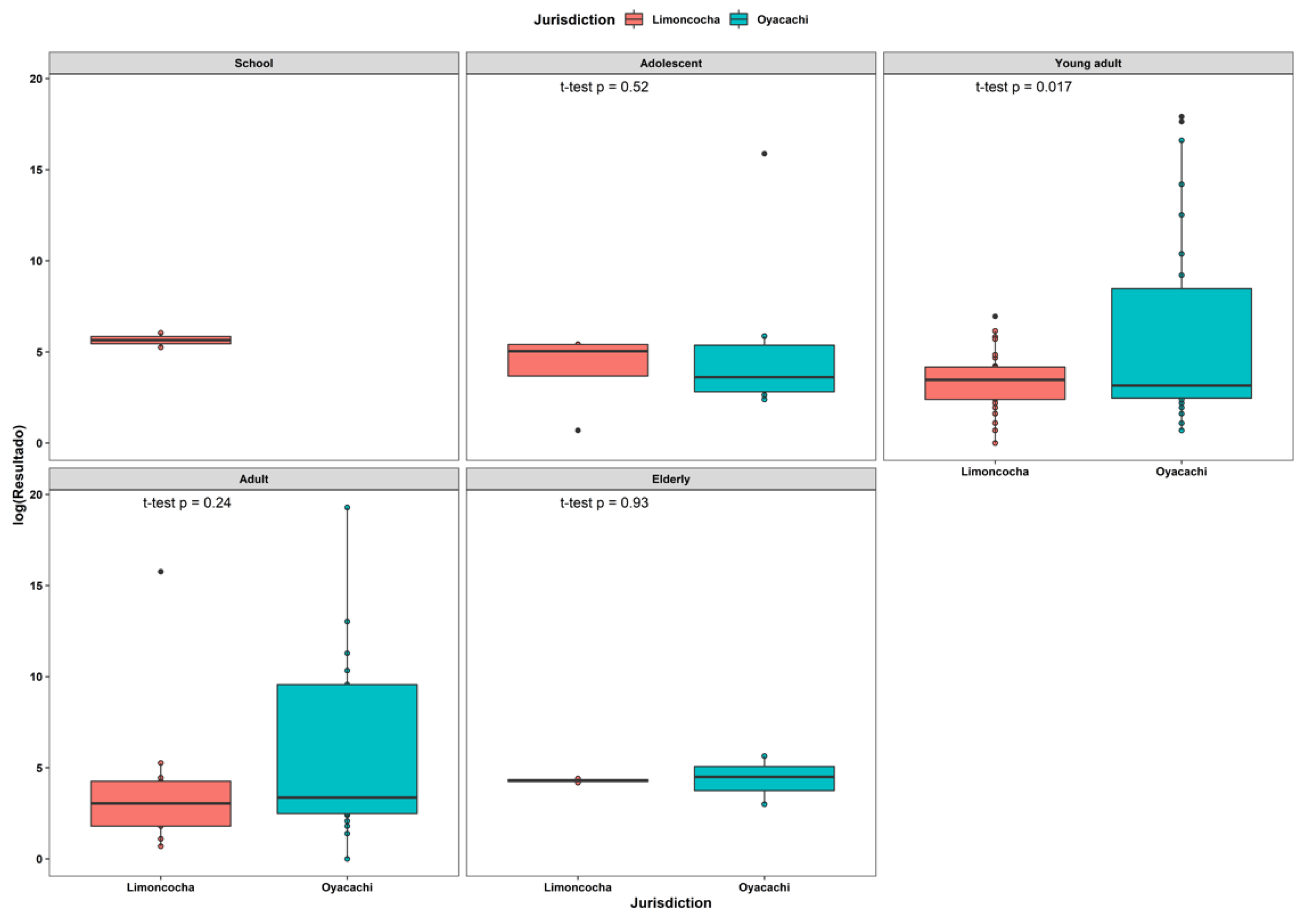
3.5. Viral Load Analysis by Age
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Millet, G.P.; Debevec, T.; Brocherie, F.; Burtscher, M.; Burtscher, J. Altitude and COVID-19: Friend or Foe? A Narrative Review. Physiol. Rep. 2021, 8, e14615. [Google Scholar] [CrossRef]
- Srivastava, A. COVID-19 and Air Pollution and Meteorology-an Intricate Relationship: A Review. Chemosphere 2021, 263, 128297. [Google Scholar] [CrossRef]
- Arias-Reyes, C.; Zubieta-DeUrioste, N.; Poma-Machicao, L.; Aliaga-Raduan, F.; Carvajal-Rodriguez, F.; Dutschmann, M.; Schneider-Gasser, E.M.; Zubieta-Calleja, G.; Soliz, J. Does the Pathogenesis of SARS-CoV-2 Virus Decrease at High-Altitude? Respir. Physiol. Neurobiol. 2020, 277, 103443. [Google Scholar] [CrossRef]
- Pun, M.; Turner, R.; Strapazzon, G.; Brugger, H.; Swenson, E.R. Lower Incidence of COVID-19 at High Altitude: Facts and Confounders. High Alt. Med. Biol. 2020, 21, 217–222. [Google Scholar] [CrossRef]
- Castilla, J.; Fresán, U.; Trobajo-Sanmartín, C.; Guevara, M. Altitude and SARS-CoV-2 Infection in the First Pandemic Wave in Spain. Int. J. Environ. Res. Public Health 2021, 18, 2578. [Google Scholar] [CrossRef]
- Ortiz-Prado, E.; Simbaña-Rivera, K.; Barreno, L.G.; Diaz, A.M.; Barreto, A.; Moyano, C.; Arcos, V.; Vásconez-González, E.; Paz, C.; Simbaña-Guaycha, F.; et al. Epidemiological, Socio-Demographic and Clinical Features of the Early Phase of the COVID-19 Epidemic in Ecuador. PLoS Negl. Trop. Dis. 2021, 15, e0008958. [Google Scholar] [CrossRef]
- Cano-Pérez, E.; Torres-Pacheco, J.; Fragozo-Ramos, M.C.; García-Díaz, G.; Montalvo-Varela, E.; Pozo-Palacios, J.C. Negative Correlation between Altitude and COVID-19 Pandemic in Colombia: A Preliminary Report. Am. J. Trop. Med. Hyg. 2020, 103, 2347–2349. [Google Scholar] [CrossRef]
- Canales-Gutiérrez, A.; Canales-Manchuria, G.P.-M.; Canales-Manchuria, F. Adaptation to Hypobaric Hypoxia of Residents at High Altitude, to Counteract COVID-19 Disease. Enferm. Clin. Engl. Ed. 2021, 31, 130–131. [Google Scholar] [CrossRef]
- Arias-Reyes, C.; Carvajal-Rodriguez, F.; Poma-Machicao, L.; Aliaga-Raduán, F.; Marques, D.A.; Zubieta-DeUrioste, N.; Accinelli, R.A.; Schneider-Gasser, E.M.; Zubieta-Calleja, G.; Dutschmann, M.; et al. Decreased Incidence, Virus Transmission Capacity, and Severity of COVID-19 at Altitude on the American Continent. PLoS ONE 2021, 16, e0237294. [Google Scholar] [CrossRef]
- Segovia-Juarez, J.; Castagnetto, J.M.; Gonzales, G.F. High Altitude Reduces Infection Rate of COVID-19 but Not Case-Fatality Rate. Respir. Physiol. Neurobiol. 2020, 281, 103494. [Google Scholar] [CrossRef]
- Bhadra, A.; Mukherjee, A.; Sarkar, K. Impact of Population Density on COVID-19 Infected and Mortality Rate in India. Model. Earth Syst. Environ. 2020, 7, 623–629. [Google Scholar] [CrossRef]
- Calderón-Larrañaga, A.; Vetrano, D.L.; Rizzuto, D.; Bellander, T.; Fratiglioni, L.; Dekhtyar, S. High Excess Mortality in Areas with Young and Socially Vulnerable Populations during the COVID-19 Outbreak in Stockholm Region, Sweden. BMJ Glob. Health 2020, 5, e003595. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.A.; Williams, D.R. Excess Deaths from COVID-19, Community Bereavement, and Restorative Justice for Communities of Color. JAMA 2020, 324, 1491–1492. [Google Scholar] [CrossRef] [PubMed]
- Choquenaira-Quispe, C.; Saldaña-Bobadilla, V.; Ramirez, J.K. Factors Involved in Low Susceptibility to COVID-19: An Adaptation of High Altitude Inhabitants. Med. Hypotheses 2020, 143, 110068. [Google Scholar] [CrossRef]
- Pujadas, E.; Chaudhry, F.; McBride, R.; Richter, F.; Zhao, S.; Wajnberg, A.; Nadkarni, G.; Glicksberg, B.S.; Houldsworth, J.; Cordon-Cardo, C. SARS-CoV-2 Viral Load Predicts COVID-19 Mortality. Lancet Respir. Med. 2020, 8, e70. [Google Scholar] [CrossRef]
- Freire-Paspuel, B.; Vega-Mariño, P.; Velez, A.; Castillo, P.; Gomez-Santos, E.E.; Cruz, M.; Garcia-Bereguiain, M.A. Cotton-Tipped Plastic Swabs for SARS-CoV-2 RT-QPCR Diagnosis to Prevent Supply Shortages. Front. Cell. Infect. Microbiol. 2020, 10, 356. [Google Scholar] [CrossRef]
- Ortiz-Prado, E.; Portilla, D.; Mosquera, J.; Simbaña-Rivera, K.L.; Duta, D.; Ochoa, I.; Burgos, G.; Izquierdo-Condoy, J.S.; Vasconez, E.; Calvopiña, M. Hematological Parameters, Lipid Profile and Cardiovascular Risk Analysis among Genotype Controlled Indigenous Kichwa Men and Women Living at Low and High Altitude. Front. Physiol. 2021, 1769, 749006. [Google Scholar] [CrossRef]
- Ortiz-Prado, E.; Simbaña-Rivera, K.; Duta, D.; Ochoa, I.; Izquierdo-Condoy, J.S.; Vasconez, E.; Carrasco, K.; Calvopiña, M.; Viscor, G.; Paz, C. Optimism and Health Self-Perception-Related Differences in Indigenous Kiwchas of Ecuador at Low and High Altitude: A Cross-Sectional Analysis. High Alt. Med. Biol. 2022, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Prado, E.; Encalada, S.; Mosquera, J.; Simbaña-Rivera, K.; Gomez-Barreno, L.; Duta, D.; Ochoa, I.; Izquierdo-Condoy, J.S.; Vasconez, E.; Burgos, G. A Comparative Analysis of Lung Function and Spirometry Parameters in Genotype-Controlled Natives Living at Low and High Altitude. BMC Pulm. Med. 2022, 22, 100. [Google Scholar] [CrossRef]
- Freire-Paspuel, B.; Vega-Mariño, P.; Velez, A.; Cruz, M.; Garcia-Bereguiain, M.A. Sample Pooling of RNA Extracts to Speed up SARS-CoV-2 Diagnosis Using CDC FDA EUA RT-QPCR Kit. Virus Res. 2020, 290, 198173. [Google Scholar] [CrossRef] [PubMed]
- CDC Labs. Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html (accessed on 13 November 2021).
- Freire-Paspuel, B.; Garcia-Bereguiain, M.A. Analytical Sensitivity and Clinical Performance of a Triplex RT-QPCR Assay Using CDC N1, N2, and RP Targets for SARS-CoV-2 Diagnosis. Int. J. Infect. Dis. 2021, 102, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.; Barbanti, F.; Scaturro, M.; Fontana, S.; Di Martino, A.; Marsili, G.; Puzelli, S.; Calzoletti, L.; Facchini, M.; Di Mario, G.; et al. Multiplex Real-Time Reverse-Transcription Polymerase Chain Reaction Assays for Diagnostic Testing of Severe Acute Respiratory Syndrome Coronavirus 2 and Seasonal Influenza Viruses: A Challenge of the Phase 3 Pandemic Setting. J. Infect. Dis. 2021, 223, 765–774. [Google Scholar] [CrossRef]
- Semple, J.L.; Moore, G.W.K. High Levels of Ambient Ozone (O3) May Impact COVID-19 in High Altitude Mountain Environments. Respir. Physiol. Neurobiol. 2020, 280, 103487. [Google Scholar] [CrossRef]
- Haque, S.E.; Rahman, M. Association between Temperature, Humidity, and COVID-19 Outbreaks in Bangladesh. Environ. Sci. Policy 2020, 114, 253–255. [Google Scholar] [CrossRef]
- Ortiz-Prado, E.; Rivera-Olivero, I.A.; Freire-Paspuel, B.; Lowe, R.; Lozada, T.; Henriquez-Trujillo, A.R.; Garcia-Bereguiain, M.A. Testing for SARS-CoV-2 at the Core of Voluntary Collective Isolation: Lessons from the Indigenous Populations Living in the Amazon Region in Ecuador. Int. J. Infect. Dis. 2021, 105, 234–235. [Google Scholar] [CrossRef]
- Santander-Gordon, D.; Iturralde, G.A.; Freire-Paspuel, B.; Zambrano-Mila, M.S.; Morales-Jadan, D.; Vallejo-Janeta, P.A.; Coronel, B.; Galvis, H.; Jaramillo-Vivanco, T.; Bilvao, C.D. Crucial Contribution of the Universities to SARS-CoV-2 Surveillance in Ecuador: Lessons for Developing Countries. One Health 2021, 13, 100267. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Garg, I.; Bansal, A.; Kumar, B. SARS-CoV-2 Infection: Physiological and Environmental Gift Factors at High Altitude. Virus Dis. 2020, 31, 450. [Google Scholar] [CrossRef] [PubMed]
- Nicastro, F.; Sironi, G.; Antonello, E.; Bianco, A.; Biasin, M.; Brucato, J.R.; Ermolli, I.; Pareschi, G.; Salvati, M.; Tozzi, P.; et al. Solar UV-B/A Radiation Is Highly Effective in Inactivating SARS-CoV-2. Sci. Rep. 2021, 11, 14805. [Google Scholar] [CrossRef]
- Seyer, A.; Sanlidag, T. Solar Ultraviolet Radiation Sensitivity of SARS-CoV-2. Lancet Microbe 2020, 1, e8–e9. [Google Scholar] [CrossRef]
- AccuWeather. Air Quality in Limoncocha and Oyacachi. Available online: https://www.accuweather.com/es/ec/limoncocha/1239184/air-quality-index/1239184 (accessed on 30 April 2022).
- Ortiz-Prado, E.; Dunn, J.F.; Vasconez, J.; Castillo, D.; Viscor, G. Partial Pressure of Oxygen in the Human Body: A General Review. Am. J. Blood Res. 2019, 9, 1. [Google Scholar]
- GAD Oyacachi. Actualización Del Plan de Desarrollo y Ordenamiento Terrritorial de Oyacachi 2014–2019. 2019. Available online: http://app.sni.gob.ec/sni-link/sni/PORTAL_SNI/data_sigad_plus/sigadplusdocumentofinal/1768098760001_PDyOT%20DIAGNOSTICO%20OYACACHI%201_30-10-2015_23-37-32.pdf (accessed on 30 April 2022).
- GAG Limoncocha. Actualización Del Plan de Desarrollo y Ordenamiento Territorial de Limoncocha 2014–2019. 2019. Available online: http://app.sni.gob.ec/sni-link/sni/PORTAL_SNI/data_sigad_plus/sigadplusdocumentofinal/1768086160001_ACTUALIZACION%20PDOT%20LIMONCOCHA%202015%20-%202019_29-10-2015_15-41-36.pdf (accessed on 30 April 2022).
- Sobral, M.F.F.; Duarte, G.B.; da Penha Sobral, A.I.G.; Marinho, M.L.M.; de Souza Melo, A. Association between Climate Variables and Global Transmission oF SARS-CoV-2. Sci. Total Environ. 2020, 729, 138997. [Google Scholar] [CrossRef]
- Adedokun, K.A.; Olarinmoye, A.O.; Mustapha, J.O.; Kamorudeen, R.T. A Close Look at the Biology of SARS-CoV-2, and the Potential Influence of Weather Conditions and Seasons on COVID-19 Case Spread. Infect. Dis. Poverty 2020, 9, 77. [Google Scholar] [CrossRef]
- Scafetta, N. Distribution of the SARS-CoV-2 Pandemic and Its Monthly Forecast Based on Seasonal Climate Patterns. Int. J. Environ. Res. Public Health 2020, 17, 3493. [Google Scholar] [CrossRef]
- Jaramillo, P.R.M.; Simbaña-Rivera, K.; Silva, J.V.V.; Gómez-Barreno, L.; Campoverde, A.B.V.; Cevallos, J.F.N.; Guanoquiza, W.E.A.; Guevara, S.L.C.; Castro, L.G.I.; Puerta, N.A.M.; et al. High-Altitude Is Associated with Better Short-Term Survival in Critically Ill COVID-19 Patients Admitted to the ICU. PLoS ONE 2022, 17, e0262423. [Google Scholar] [CrossRef]
- Collaco, J.M.; Aoyama, B.C.; Rice, J.L.; McGrath-Morrow, S.A. Influences of Environmental Exposures on Preterm Lung Disease. Expert Rev. Respir. Med. 2021, 15, 1271–1279. [Google Scholar] [CrossRef]
- Woolcott, O.O.; Bergman, R.N. Mortality Attributed to COVID-19 in High-Altitude Populations. High Alt. Med. Biol. 2020, 21, 409–416. [Google Scholar] [CrossRef]
- Fajnzylber, J.; Regan, J.; Coxen, K.; Corry, H.; Wong, C.; Rosenthal, A.; Worrall, D.; Giguel, F.; Piechocka-Trocha, A.; Atyeo, C.; et al. SARS-CoV-2 Viral Load Is Associated with Increased Disease Severity and Mortality. Nat. Commun. 2020, 11, 5493. [Google Scholar] [CrossRef]
- Wu, Y.; Cheng, X.; Jiang, G.; Tang, H.; Ming, S.; Tang, L.; Lu, J.; Guo, C.; Shan, H.; Huang, X. Altered Oral and Gut Microbiota and Its Association with SARS-CoV-2 Viral Load in COVID-19 Patients during Hospitalization. npj Biofilm. Microbiomes 2021, 7, 61. [Google Scholar] [CrossRef]
- Mahallawi, W.H.; Alsamiri, A.D.; Dabbour, A.F.; Alsaeedi, H.; Al-Zalabani, A.H. Association of Viral Load in SARS-CoV-2 Patients with Age and Gender. Front. Med. 2021, 8, 39. [Google Scholar] [CrossRef]
| Limoncocha | Oyacachi | ||||||
|---|---|---|---|---|---|---|---|
| Age Range | N | Viral Load | SD (±) | N | Viral Load | SD (±) | p-Value * |
| 5 to 9 | 1 | 191.0 | N/A | 0 | 0 | 0 | N/A |
| 10 to 14 | 1 | 422.0 | N/A | 2 | 3,947,564.0 | 5,582,629.0 | N/A |
| 15 to 19 | 3 | 110.6 | 1,105,456.0 | 2 | 190.5 | 229.8 | 0.64 |
| 20 to 24 | 6 | 57.3 | 86.7 | 5 | 757.6 | 1667.2 | 0.64 |
| 25 to 29 | 4 | 124.5 | 229.7 | 9 | 7,093,211.0 | 15,456,697.0 | 0.023 |
| 30 to 34 | 13 | 149.2 | 284.6 | 10 | 6,049,341.0 | 19,125,937.0 | 0.39 |
| 35 to 39 | 7 | 61.4 | 106.5 | 6 | 194.6 | 430.2 | 0.51 |
| 40 to 44 | 7 | 1,000,949.0 | 2,648,156.0 | 8 | 125,185.6 | 204,549.2 | 0.29 |
| 45 to 49 | 4 | 49 | 34.6 | 5 | 47,526,374.0 | 1,067,567 | 0.53 |
| 50 to 54 | 0 | 0 | 0 | 3 | 29.3 | 14.0 | N/A |
| 55 to 59 | 1 | 44 | 3 | 101.6 | 165.7 | N/A | |
| 60 to 64 | 1 | 2 | 2 | 15,421.0 | 21,787.3 | N/A | |
| 65 to 69 | 1 | 66 | 2 | 185.5 | 135.0 | N/A | |
| 70 to 74 | 0 | 0 | 0 | 1 | 20 | N/A | N/A |
| >80 | 1 | 82 | 0 | 0 | 0 | N/A | |
| Variable | Oyacachi | Limoncocha |
|---|---|---|
| Adult population | 739 | 6817 |
| Road access | Cobblestone road | Asphalt road |
| Altitude (meters) | 3800 to 4300 m | 228–2800 m |
| Barometric pressure (BP) | 487 mmHg (65 kPa) | 739 mmHg (98 kPa) |
| Oxygen availability compared to sea level | 64% | 97% |
| Weather | Upper montane rainforest | Lowland rainforest |
| Temperature (°C) | −2–17 °C | 18–26 °C |
| Rainfall (mm) | 1200–3000 | 3200–3400 |
| Relative humidity (%) | 89% | >90% |
| Ozone (O3) | 27 µg/m3 | 27 µg/m3 |
| Particulate matter 2.5 | 4 µg/m3 | 5 µg/m3 |
| Particulate matter 10 | 8 µg/m3 | 8 µg/m3 |
| NO2 | 3 µg/m3 | 4 µg/m3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Prado, E.; Simbaña-Rivera, K.; Fernandez-Naranjo, R.; Vásconez, J.E.; Henriquez-Trujillo, A.R.; Vallejo-Janeta, A.P.; Rivera-Olivero, I.A.; Lozada, T.; Viscor, G.; Garcia-Bereguiain, M.A., on behalf of the UDLA-COVID-19 Team. SARS-CoV-2 Viral Load Analysis at Low and High Altitude: A Case Study from Ecuador. Int. J. Environ. Res. Public Health 2022, 19, 7945. https://doi.org/10.3390/ijerph19137945
Ortiz-Prado E, Simbaña-Rivera K, Fernandez-Naranjo R, Vásconez JE, Henriquez-Trujillo AR, Vallejo-Janeta AP, Rivera-Olivero IA, Lozada T, Viscor G, Garcia-Bereguiain MA on behalf of the UDLA-COVID-19 Team. SARS-CoV-2 Viral Load Analysis at Low and High Altitude: A Case Study from Ecuador. International Journal of Environmental Research and Public Health. 2022; 19(13):7945. https://doi.org/10.3390/ijerph19137945
Chicago/Turabian StyleOrtiz-Prado, Esteban, Katherine Simbaña-Rivera, Raul Fernandez-Naranjo, Jorge Eduardo Vásconez, Aquiles R. Henriquez-Trujillo, Alexander Paolo Vallejo-Janeta, Ismar A. Rivera-Olivero, Tannya Lozada, Gines Viscor, and Miguel Angel Garcia-Bereguiain on behalf of the UDLA-COVID-19 Team. 2022. "SARS-CoV-2 Viral Load Analysis at Low and High Altitude: A Case Study from Ecuador" International Journal of Environmental Research and Public Health 19, no. 13: 7945. https://doi.org/10.3390/ijerph19137945
APA StyleOrtiz-Prado, E., Simbaña-Rivera, K., Fernandez-Naranjo, R., Vásconez, J. E., Henriquez-Trujillo, A. R., Vallejo-Janeta, A. P., Rivera-Olivero, I. A., Lozada, T., Viscor, G., & Garcia-Bereguiain, M. A., on behalf of the UDLA-COVID-19 Team. (2022). SARS-CoV-2 Viral Load Analysis at Low and High Altitude: A Case Study from Ecuador. International Journal of Environmental Research and Public Health, 19(13), 7945. https://doi.org/10.3390/ijerph19137945








