Determining Optimal Temperature Combination for Effective Pretreatment and Anaerobic Digestion of Corn Stalk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock and Inoculum
2.2. Pretreatment with NaOH
2.3. Experimental Set-Up
2.4. Operating Conditions
2.5. Analytical Methods
2.6. Assessment of Energy Balances
3. Results and Discussion
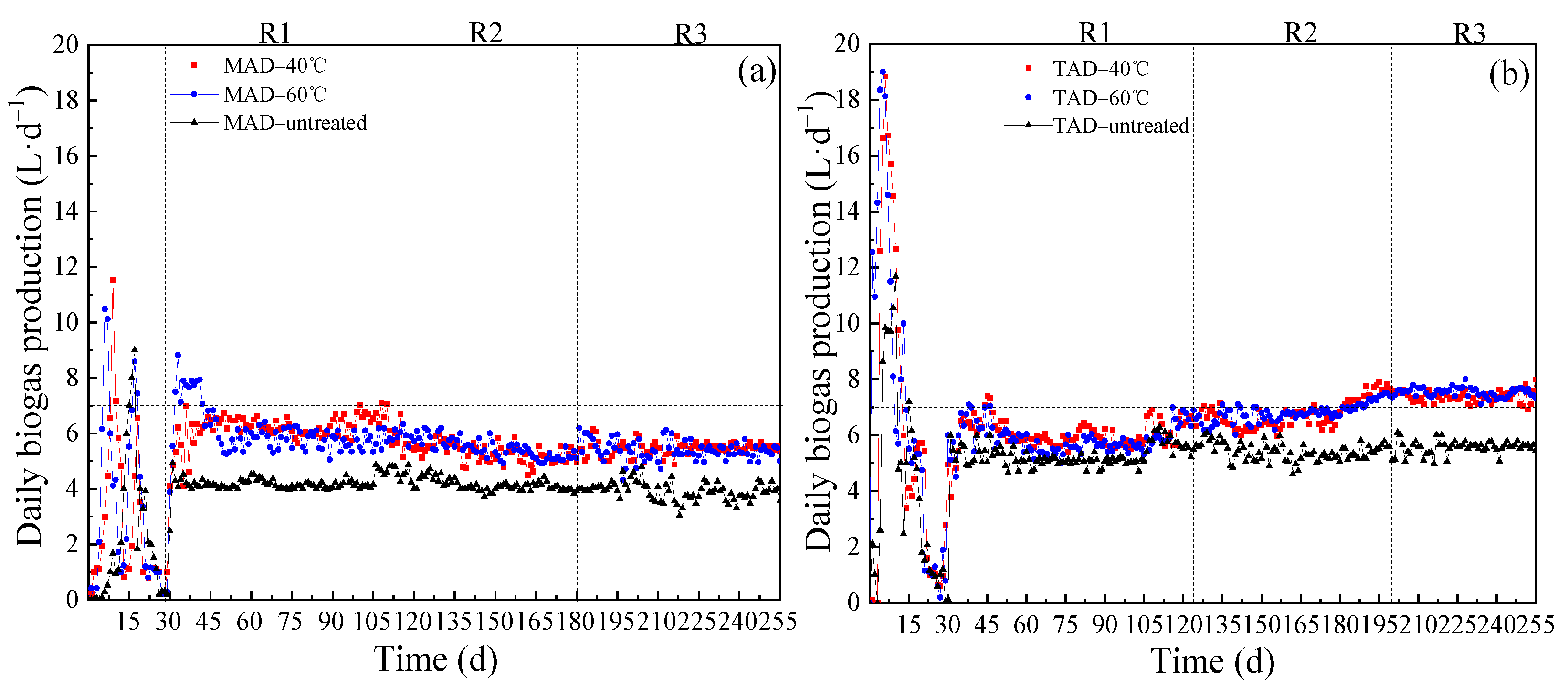
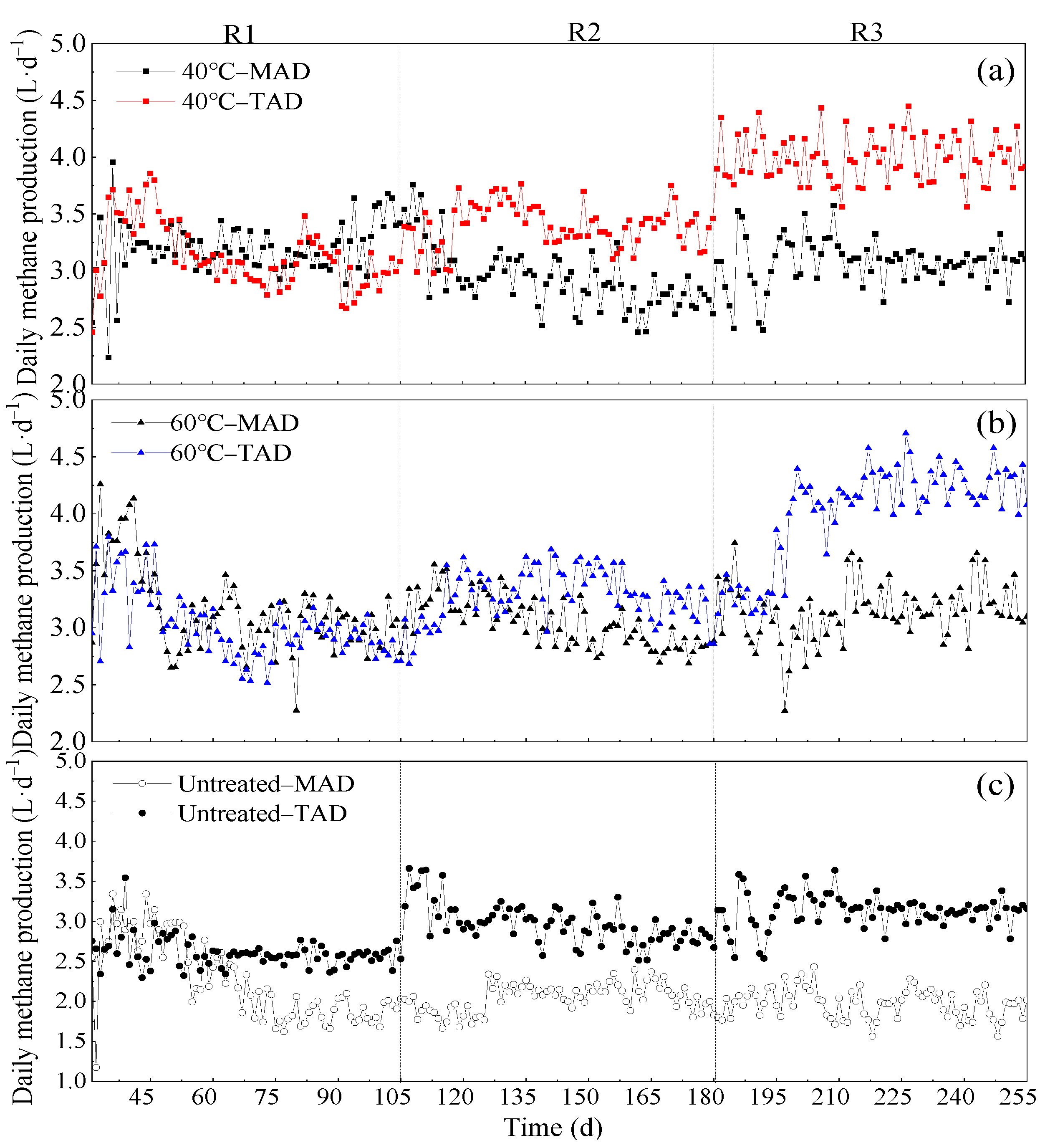
3.1. Daily Biogas Production and Average Methane Content
3.2. Influence of OLR on Reactor Performance
3.3. Comparison of Different Pretreatment and AD Temperatures
3.3.1. Comparison of Different Pretreatment Conditions
3.3.2. Comparison of Different AD Conditions
3.4. Changes of Main Compositions
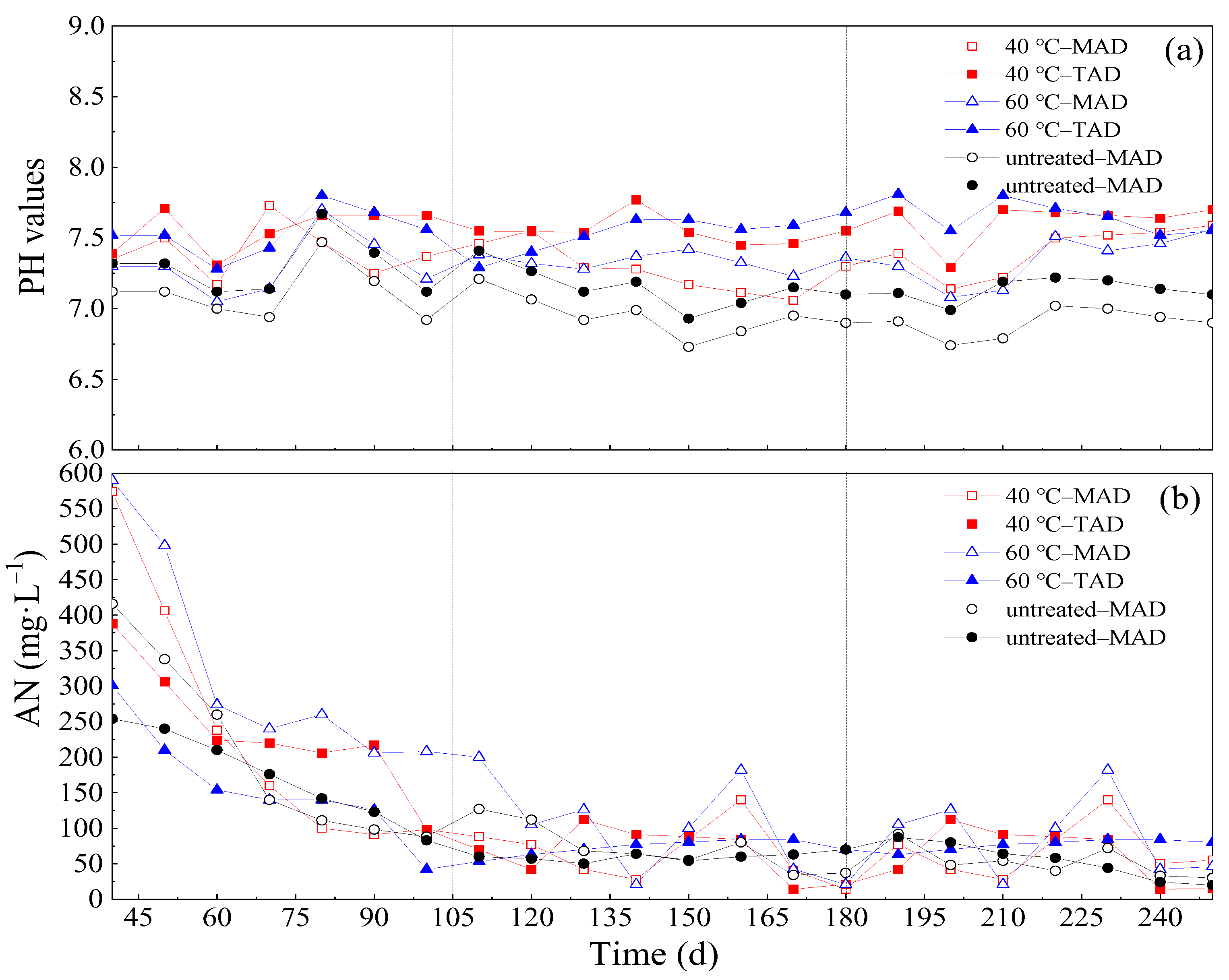
3.5. Evaluation of System Stability
3.5.1. pH
3.5.2. Ammonia Nitrogen and TVFAs/TAC
3.6. Energy Balance
3.7. Economic Aspect of Pretreatment and Anaerobic Digestion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CS | corn stalk |
| AD | anaerobic digestion |
| MAD | mesophilic anaerobic digestion |
| TAD | thermophilic anaerobic digestion |
| CSTR | completely stirred tank reactor |
| HRT | hydraulic retention time |
| OL | organic loading rate |
| MLSS | mixed liquid suspended solid |
| TS | total solids |
| VS | volatile solids |
| TC | total carbon |
| TN | total nitrogen |
| LCH | lignin, cellulose, hemicellulose |
| DBP | daily biogas production |
| DMP | daily methane production |
| AN | ammonia nitrogen |
| VFA | volatile fatty acid |
| TAC | total alkalinity concentration |
| Ei | energy input |
| Ei,h | energy for heating |
| Ei,m | energy for mixing |
| Ei,p | energy for pumping |
| Eo | energy output |
| Ro/I | ratio of Eo to Ei |
References
- Li, J.; Wachemo, A.C.; Yuan, H.; Zuo, X.; Li, X. Natural freezing-thawing pretreatment of corn stalk for enhancing anaerobic digestion performance. Bioresour. Technol. 2019, 288, 121518. [Google Scholar] [CrossRef]
- Xie, Z.; Zou, H.; Zheng, Y.; Fu, S.F. Improving anaerobic digestion of corn straw by using solid-state urea pretreatment. Chemosphere 2022, 293, 133559. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, H.; He, S.; Shi, C.; Yuan, H.; Zuo, X.; Li, X. Utilizing hydrolysis and acidification via liquid fraction of digestate (LFD-HA) for methane production enhancement of corn straw: Physicochemical and microbial community characterization. J. Clean. Prod. 2021, 326, 129282. [Google Scholar] [CrossRef]
- Veluchamy, C.; Kalamdhad, A.S. Enhancement of hydrolysis of lignocellulose waste pulp and paper mill sludge through different heating processes on thermal pretreatment. J. Clean. Prod. 2017, 168, 219–226. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.; Hua, D.; Zhao, Y.; Mu, H.; Chen, H.; Chen, G. Enhancing the anaerobic digestion of corn stover by chemical pretreatment with the black liquor from the paper industry. Bioresour. Technol. 2020, 306, 123090. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S. A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process. Technol. 2017, 160, 196–206. [Google Scholar] [CrossRef]
- Scherzinger, M.; Kaltschmitt, M. Thermal pre-treatment options to enhance anaerobic digestibility—A review. Renew. Sustain. Energy Rev. 2021, 137, 110627. [Google Scholar] [CrossRef]
- Frigon, J.-C.; Mehta, P.; Guiot, S.R. Impact of mechanical, chemical and enzymatic pre-treatments on the methane yield from the anaerobic digestion of switchgrass. Biomass Bioenergy 2012, 36, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zborowska, M.; Waliszewska, H.; Waliszewska, B.; Borysiak, S.; Brozdowski, J.; Stachowiak-Wencek, A. Conversion of Carbohydrates in Lignocellulosic Biomass after Chemical Pretreatment. Energies 2021, 15, 254. [Google Scholar] [CrossRef]
- Saha, B.C.; Yoshida, T.; Cotta, M.A.; Sonomoto, K. Hydrothermal pretreatment and enzymatic saccharification of corn stover for efficient ethanol production. Ind. Crops Prod. 2013, 44, 367–372. [Google Scholar] [CrossRef]
- Veluchamy, C.; Kalamdhad, A.S. Enhanced methane production and its kinetics model of thermally pretreated lignocellulose waste material. Bioresour. Technol. 2017, 241, 1–9. [Google Scholar] [CrossRef]
- Guan, R.; Yuan, H.; Zhang, L.; Zuo, X.; Li, X. Combined pretreatment using CaO and liquid fraction of digestate of rice straw: Anaerobic digestion performance and electron transfer. Chin. J. Chem. Eng. 2021, 36, 223–232. [Google Scholar] [CrossRef]
- Yuan, H.; Song, X.; Guan, R.; Zhang, L.; Li, X.; Zuo, X. Effect of low severity hydrothermal pretreatment on anaerobic digestion performance of corn stover. Bioresour. Technol. 2019, 294, 122238. [Google Scholar] [CrossRef]
- Katsimpouras, C.; Zacharopoulou, M.; Matsakas, L.; Rova, U.; Christakopoulos, P.; Topakas, E. Sequential high gravity ethanol fermentation and anaerobic digestion of steam explosion and organosolv pretreated corn stover. Bioresour. Technol. 2017, 244, 1129–1136. [Google Scholar] [CrossRef]
- Shimada, M.; Na, R.; Kushima, K.; Shimizu, N. Effects of Pyrolysis on Biogas Production during Anaerobic Co-digestion of Corn Stover. In Proceedings of the 18th Asian Pacific Confederation of Chemical Engineering Congress (APCChE 2019), Sapporo, Japan, 23–27 September 2019; Volume 333, p. 07011. [Google Scholar]
- Cai, Y.; Zheng, Z.; Schäfer, F.; Stinner, W.; Yuan, X.; Wang, H.; Cui, Z.; Wang, X. A review about pretreatment of lignocellulosic biomass in anaerobic digestion: Achievement and challenge in Germany and China. J. Clean. Prod. 2021, 299, 126885. [Google Scholar] [CrossRef]
- Sołowski, G.; Konkol, I.; Cenian, A. Production of hydrogen and methane from lignocellulose waste by fermentation. A review of chemical pretreatment for enhancing the efficiency of the digestion process. J. Clean. Prod. 2020, 267, 121721. [Google Scholar] [CrossRef]
- Feng, R.; Zaidi, A.A.; Li, Q.; Zhang, K.; Shi, Y. NaOH–urea pretreatment for biogas enhancement from algal biomass anaerobic digestion. J. Renew. Sustain. Energy 2021, 13, 033102. [Google Scholar] [CrossRef]
- Li, Q.; Yang, F.; Zheng, G.; Guan, Z. Effects of urea ammonia pretreatment on the batch anaerobic fermentation efficiency of corn stovers. Int. J. Agric. Biol. Eng. 2019, 12, 169–173. [Google Scholar] [CrossRef]
- Niu, X.; Zhong, J.; Lei, D.; Zhang, H.; Wang, W. A Highly Effective Inorganic Composite Promoter: Synergistic Effect of Boric Acid and Calcium Hydroxide in Promoting Methane Hydrate Formation under Static Conditions. Ind. Eng. Chem. Res. 2022, 61, 3775–3780. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.J. Lignocellulosic biomass pretreatment by deep eutectic solvents on lignin extraction and saccharification enhancement: A review. Bioresour. Technol. 2021, 339, 125587. [Google Scholar] [CrossRef]
- Tan, Y.T.; Ngoh, G.C.; Chua, A.S.M. Evaluation of fractionation and delignification efficiencies of deep eutectic solvents on oil palm empty fruit bunch. Ind. Crops Prod. 2018, 123, 271–277. [Google Scholar] [CrossRef]
- Basak, B.; Patil, S.; Kumar, R.; Ha, G.S.; Park, Y.K.; Ali Khan, M.; Kumar Yadav, K.; Fallatah, A.M.; Jeon, B.H. Integrated hydrothermal and deep eutectic solvent-mediated fractionation of lignocellulosic biocomponents for enhanced accessibility and efficient conversion in anaerobic digestion. Bioresour. Technol. 2022, 351, 127034. [Google Scholar] [CrossRef]
- Omar, K.A.; Sadeghi, R. Physicochemical Properties of Deep Eutectic Solvents: A Review. J. Mol. Liq. 2022, 360, 119524. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- You, Z.; Wei, T.; Cheng, J.J. Improving Anaerobic Codigestion of Corn Stover Using Sodium Hydroxide Pretreatment. Energy Fuels 2014, 28, 549–554. [Google Scholar] [CrossRef]
- Li, G.; Sun, Y.; Guo, W.; Yuan, L. Comparison of various pretreatment strategies and their effect on chemistry and structure of sugar beet pulp. J. Clean. Prod. 2018, 181, 217–223. [Google Scholar] [CrossRef]
- Menya, E.; Olupot, P.W.; Storz, H.; Lubwama, M.; Kiros, Y. Characterization and alkaline pretreatment of rice husk varieties in Uganda for potential utilization as precursors in the production of activated carbon and other value-added products. Waste Manag. 2018, 81, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, C.; Zahoor; Chen, X.; Yu, Q.; Wang, Z.; Zhuang, X.; Yuan, Z. Effect of a Nonionic Surfactant on Enzymatic Hydrolysis of Lignocellulose Based on Lignocellulosic Features and Enzyme Adsorption. ACS Omega 2020, 5, 15812–15820. [Google Scholar] [CrossRef]
- Li, L.; Yang, X.; Li, X.; Zheng, M.; Chen, J.; Zhang, Z. The Influence of Inoculum Sources on Anaerobic Biogasification of NaOH-treated Corn Stover. Energy Sources Part A Recovery Util. Environ. Eff. 2011, 33, 138–144. [Google Scholar] [CrossRef]
- Zheng, M.; Li, L.; Li, X.; Xiong, J.; Mei, T.; Chen, G. The Effects of Alkaline Pretreatment Parameters on Anaerobic Biogasification of Corn Stover. Energy Sources Part A Recovery Util. Environ. Eff. 2010, 32, 1918–1925. [Google Scholar] [CrossRef]
- Wei, Y.; Li, X.; Yu, L.; Zou, D.; Yuan, H. Mesophilic anaerobic co-digestion of cattle manure and corn stover with biological and chemical pretreatment. Bioresour. Technol. 2015, 198, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wachemo, A.C.; Yuan, H.; Li, X. Anaerobic digestion performance and microbial community structure of corn stover in three-stage continuously stirred tank reactors. Bioresour. Technol. 2019, 287, 121339. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Han, S.K.; Shin, H.S. Feasibility of biohydrogen production by anaerobic co-digestion of food waste and sewage sludge. Int. J. Hydrogen Energy 2004, 29, 1607–1616. [Google Scholar] [CrossRef]
- Ren, N.; Wang, A. Anaerobic Biotechnology Principles and Applications; Chemical Industry Press: Beijing, China, 2004. [Google Scholar]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef]
- Ahring, B.K. Perspectives for Anaerobic Digestion. In Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2003; Volume 81, pp. 1–30. [Google Scholar]
- Duran, M.; Speece, R.E. Temperature-Staged Anaerobic Processes. Environ. Technol. 1997, 18, 747–753. [Google Scholar] [CrossRef]
- Watanabe, K.; Koyama, M.; Ueda, J.; Ban, S.; Kurosawa, N.; Toda, T. Effect of operating temperature on anaerobic digestion of the Brazilian waterweed Egeria densa and its microbial community. Anaerobe 2017, 47, 8–17. [Google Scholar] [CrossRef]
- Shen, F.; Tian, L.; Yuan, H.; Pang, Y.; Chen, S.; Zou, D.; Zhu, B.; Liu, Y.; Li, X. Improving the Mixing Performances of Rice Straw Anaerobic Digestion for Higher Biogas Production by Computational Fluid Dynamics (CFD) Simulation. Appl. Biochem. Biotechnol. 2013, 171, 626–642. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z. Biogasification of rice straw with an anaerobic-phased solids digester system. Bioresour. Technol. 1999, 68, 235–245. [Google Scholar] [CrossRef]
- Karim, K.; Hoffmann, R.; Klasson, K.T.; Al-Dahhan, M.H. Anaerobic digestion of animal waste: Effect of mode of mixing. Water Res. 2005, 39, 3597–3606. [Google Scholar] [CrossRef]
- Miner, G. Standard Methods for the Examination of Water and Wastewater, 21st ed.; Journal American Water Works Association: Denver, CO, USA, 2006; p. 130. [Google Scholar]
- Eddy, M. Wastewater Engineering: Treatment and Reuse, 4th ed.; McGraw-Hill Inc.: New York, NY, USA, 2003. [Google Scholar]
- Passos, F.; Ferrer, I. Influence of hydrothermal pretreatment on microalgal biomass anaerobic digestion and bioenergy production. Water Res. 2015, 68, 364–373. [Google Scholar] [CrossRef] [Green Version]
- Dumas, C.; Perez, S.; Paul, E.; Lefebvre, X. Combined thermophilic aerobic process and conventional anaerobic digestion: Effect on sludge biodegradation and methane production. Bioresour. Technol. 2010, 101, 2629–2636. [Google Scholar] [CrossRef] [PubMed]
- Hun, K.S.; Kafle, G.K. Effective Treatment of Swine Manure with Chinese Cabbage Silage through Two Serial Anaerobic Digestion. J. Biosyst. Eng. 2010, 35, 53–63. [Google Scholar]
- Kim, J.; Lee, C. Response of a continuous biomethanation process to transient organic shock loads under controlled and uncontrolled pH conditions. Water Res. 2015, 73, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Franke-Whittle, I.H.; Walter, A.; Ebner, C.; Insam, H. Investigation into the effect of high concentrations of volatile fatty acids in anaerobic digestion on methanogenic communities. Waste Manag. 2014, 34, 2080–2089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, L.; Zou, D.; Yuan, H.; Wang, L.; Zhang, X.; Li, X. Identifying proper agitation interval to prevent floating layers formation of corn stover and improve biogas production in anaerobic digestion. Bioresour. Technol. 2015, 186, 1–7. [Google Scholar] [CrossRef]
- Li, J.; Wachemo, A.C.; Yu, G.; Li, X. Enhanced anaerobic digestion performance of corn stalk pretreated with freezing-thawing and ammonia: An experimental and theoretical study. J. Clean. Prod. 2020, 247, 119112. [Google Scholar] [CrossRef]
- Zheng, M.; Li, X.; Li, L.; Yang, X.; He, Y. Enhancing anaerobic biogasification of corn stover through wet state NaOH pretreatment. Bioresour. Technol. 2009, 100, 5140–5145. [Google Scholar] [CrossRef]
- Li, J.; Wachemo, A.C.; Yuan, H.; Zuo, X.; Li, X. Evaluation of system stability and anaerobic conversion performance for corn stover using combined pretreatment. Waste Manag. 2019, 97, 52–62. [Google Scholar] [CrossRef]
- Böske, J.; Wirth, B.; Garlipp, F.; Mumme, J.; Van den Weghe, H. Upflow anaerobic solid-state (UASS) digestion of horse manure: Thermophilic vs. mesophilic performance. Bioresour. Technol. 2015, 175, 8–16. [Google Scholar] [CrossRef]
- Jiménez, J.; Guardia-Puebla, Y.; Cisneros-Ortiz, M.E.; Morgan-Sagastume, J.M.; Guerra, G.; Noyola, A. Optimization of the specific methanogenic activity during the anaerobic co-digestion of pig manure and rice straw, using industrial clay residues as inorganic additive. Chem. Eng. J. 2015, 259, 703–714. [Google Scholar] [CrossRef]
- Pohl, M.; Mumme, J.; Heeg, K.; Nettmann, E. Thermo- and mesophilic anaerobic digestion of wheat straw by the upflow anaerobic solid-state (UASS) process. Bioresour. Technol. 2012, 124, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dang, F.; Zhang, Y.; Zou, D.; Yuan, H. Anaerobic digestion prformance and mechanism of ammoniation pretreatment of corn stover. Bioresources 2015, 10, 1930–2126. [Google Scholar] [CrossRef] [Green Version]
- Zhong, W.; Zhang, Z.; Luo, Y.; Sun, S.; Wei, Q.; Meng, X. Effect of biological pretreatments in enhancing corn straw biogas production. Bioresour. Technol. 2011, 102, 11177–11182. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I.; Ponsá, S.; Vázquez, F.; Font, X. Increasing biogas production by thermal (70 °C) sludge pre-treatment prior to thermophilic anaerobic digestion. Biochem. Eng. J. 2008, 42, 186–192. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.; Hwang, K.; Shin, S.G.; Lee, S.; Hwang, S. Effect of high temperature on bacterial community dynamics in anaerobic acidogenesis using mesophilic sludge inoculum. Bioresour. Technol. 2010, 101, S17–S22. [Google Scholar] [CrossRef]
- Hejnfelt, A.; Angelidaki, I. Anaerobic digestion of slaughterhouse by-products. Biomass Bioenergy 2009, 33, 1046–1054. [Google Scholar] [CrossRef]
- Ye, C.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar]
- Pilli, S.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Anaerobic digestion of ultrasonicated sludge at different solids concentrations-Computation of mass-energy balance and greenhouse gas emissions. J. Environ. Manag. 2016, 166, 374–386. [Google Scholar] [CrossRef]


| Parameter | Value (%) | |
|---|---|---|
| Corn Stalk | Inoculum | |
| TS (%) a | 91.05 ± 0.23 | 8.25 ± 0.03 |
| VS (%) a | 87.80 ± 0.15 | 4.18 ± 0.05 |
| TC (%) b | 43.42 ± 0.54 | 27.05 ± 0.25 |
| TN (%) b | 0.68 ± 0.08 | 2.3 ± 0.53 |
| C/N(%) b | 63.85 ± 0.56 | 11.76 ± 0.89 |
| MLSS (g L−1) a | ND | 109.00 ± 2.18 |
| Cellulose (%) b | 40.36 ± 0.60 | ND |
| Hemicellulose (%) b | 20.07 ± 0.03 | ND |
| Lignin (%) b | 11.41 ± 0.13 | ND |
| LCH (%) b | 71.84 ± 0.76 | ND |
| Pretreatment Temperature (°C) | AD Temperature (°C) | OLR (g·L−1·d−1) | OLR (g·L−1·d−1) | OLR (g·L−1·d−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1.6 | 1.8 | 2.0 | 1.6 | 1.8 | 2.0 | 1.6 | 1.8 | 2.0 | ||
| Biogas Yield (mL·gvs−1) | Methane Yield (mL·gvs−1) | Average Methane Content (%) | ||||||||
| 40 | 35 | 524 ± 30 | 409 ± 20 | 319 ± 16 | 275 ± 20 | 220 ± 20 | 181 ± 16 | 52.49 ± 0.79 | 53.67 ± 0.95 | 56.90 ± 1.31 |
| 55 | 513 ± 29 | 488 ± 27 | 450 ± 24 | 270 ± 19 | 255 ± 17 | 236 ± 14 | 52.46 ± 0.74 | 52.43 ± 0.62 | 52.55 ± 0.80 | |
| 60 | 35 | 528 ± 31 | 417 ± 22 | 320 ± 16 | 280 ± 21 | 229 ± 12 | 184 ± 9 | 53.05 ± 0.86 | 55.01 ± 1.08 | 57.52 ± 1.52 |
| 55 | 495 ± 28 | 478 ± 26 | 449 ± 24 | 258 ± 18 | 247 ± 16 | 236 ± 14 | 52.17 ± 0.59 | 51.66 ± 0.51 | 52.45 ± 0.71 | |
| untreated | 35 | 383 ± 19 | 299 ± 17 | 230 ± 14 | 194 ± 11 | 153 ± 5 | 118 ± 16 | 50.60 ± 0.46 | 51.17 ± 0.58 | 51.30 ± 0.52 |
| 55 | 444 ± 23 | 417 ± 22 | 340 ± 18 | 223 ± 11 | 213 ± 10 | 180 ± 9 | 50.22 ± 0.44 | 51.08 ± 0.41 | 52.94 ± 0.73 | |
| Pretreatment Temperature (°C) | Ethanol and Acetic Acid (mg·L−1) | Individual Acid Concentration (mg·L−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ethanol | Acetic Acid | Propionic Acid | Isobutyric Acid | Butyric Acid | Isovaleric Acid | Valeric Acid | Total Acid | ||
| 40 | 6326 ± 30 | 2156 ± 13 | 4270 ± 27 | 103 ± 10 | 47 ± 13 | 35 ± 3 | 4 ± 0.2 | 11 ± 0.6 | 6630 ± 32 |
| 60 | 6944 ± 41 | 1210 ± 9 | 5734 ± 12 | 115 ± 4 | 76 ± 4 | 86 ± 5 | 50 ± 3.6 | 30 ± 1.1 | 8303 ± 45 |
| Pretreatment Temperature (°C) | Cellulose | Hemicellulose | Lignin | LCH |
|---|---|---|---|---|
| 40 | 36.5 ± 0.4 | 13.6 ± 0.1 | 10.6 ± 0.1 | 56.4 ± 0.3 |
| 60 | 35.4 ± 0.3 | 13.1 ± 0.1 | 10.5 ± 0.1 | 55.6 ± 0.2 |
| untreated | 40.3 ± 0.6 | 20.0 ± 0.2 | 11.4 ± 0.2 | 71.8 ± 0.7 |
| Pretreatment Temperature (°C) | AD Temperature (°C) | OLR (g·L−1·d−1) | Conversion Rate (%) * | ||||
|---|---|---|---|---|---|---|---|
| Cellulose | Hemicellulose | Lignin | TS | VS | |||
| 40 | 35 | 1.6 | 64.3 ± 1.2 | 60.1 ± 1.6 | 25.1 ± 0.7 | 50.0 ± 2.2 | 61.2 ± 1.7 |
| 1.8 | 60.4 ± 1.6 | 56.7 ± 3.2 | 24.7 ± 0.6 | 48.7 ± 2.5 | 56.9 ± 2.6 | ||
| 2.0 | 53.5 ± 3.3 | 49.2 ± 3.0 | 21.2 ± 0.4 | 46.3 ± 3.1 | 53.5 ± 2.5 | ||
| 55 | 1.6 | 64.3 ± 3.5 | 60.2 ± 3.4 | 26.2 ± 0.8 | 50.6 ± 1.5 | 60.7 ± 2.8 | |
| 1.8 | 62.7 ± 2.8 | 58.6 ± 2.1 | 25.6 ± 0.7 | 48.9 ± 2.7 | 58.3 ± 1.5 | ||
| 2.0 | 59.5 ± 3.7 | 55.4 ± 3.2 | 21.4 ± 0.4 | 47.1 ± 2.4 | 54.4 ± 3.0 | ||
| 60 | 35 | 1.6 | 64.9 ± 2.1 | 60.4 ± 3.1 | 25.4 ± 0.7 | 50.2 ± 1.8 | 61.9 ± 2.6 |
| 1.8 | 59.3 ± 2.4 | 55.6 ± 4.2 | 21.6 ± 0.4 | 48.0 ± 2.2 | 55.7 ± 1.9 | ||
| 2.0 | 53.5 ± 4.1 | 49.8 ± 3.4 | 19.8 ± 0.3 | 46.3 ± 2.4 | 54.2 ± 1.7 | ||
| 55 | 1.6 | 61.7 ± 3.2 | 57.3 ± 2.2 | 25.3 ± 0.9 | 49.6 ± 2.6 | 57.7 ± 2.4 | |
| 1.8 | 60.2 ± 3.6 | 56.9 ± 3.0 | 24.9 ± 0.7 | 48.2 ± 1.7 | 56.0 ± 1.6 | ||
| 2.0 | 59.4 ± 3.8 | 55.4 ± 2.1 | 20.4 ± 0.3 | 47.1 ± 2.3 | 55.1 ± 1.8 | ||
| untreated | 35 | 1.6 | 55.6 ± 2.0 | 53.2 ± 2.1 | 18.4 ± 0.2 | 46.1 ± 1.7 | 57.4 ± 2.6 |
| 1.8 | 52.5 ± 2.2 | 51.6 ± 2.2 | 17.7 ± 0.2 | 45.2 ± 1.5 | 55.7 ± 1.8 | ||
| 2.0 | 47.5 ± 2.1 | 45.5 ± 1.4 | 15.6 ± 0.1 | 43.3 ± 1.4 | 53.5 ± 1.5 | ||
| 55 | 1.6 | 59.3 ± 3.3 | 55.4 ± 2.2 | 19.4 ± 0.2 | 47.5 ± 2.2 | 58.7 ± 2.3 | |
| 1.8 | 58.1 ± 3.1 | 52.7 ± 2.0 | 18.9 ± 0.2 | 46.8 ± 1.7 | 56.2 ± 1.9 | ||
| 2.0 | 56.4 ± 3.0 | 49.4 ± 1.5 | 16.3 ± 0.1 | 44.9 ± 1.3 | 53.6 ± 1.6 | ||
| Process | Item | MAD | TAD |
|---|---|---|---|
| Anaerobic digestion | Eo (kWh/mg VS) | 3855.4 | 4012.6 |
| Pretreatment | Ei,h (kWh/mg VS) | 0 (untreated) | 0 (untreated) |
| 298.67 (40 °C) | 298.67 (40 °C) | ||
| 597.34 (60 °C) | 597.34 (60 °C) | ||
| Mixing | Ei,m (kWh/mg VS) | 108.0 | 276.3 |
| Pumping | Ei,p (kWh/mg VS) | 21.6 | 43.9 |
| Anaerobic digestion | Ro/i | 29.7 (untreated) | 12.5 (untreated) |
| 9.0 (40 °C) | 6.48 (40 °C) | ||
| 5.3 (60 °C) | 4.4 (60 °C) | ||
| Anaerobic digestion | ΔE (kWh/mg VS) | 3725.8 (untreated) | 3692.4 (untreated) |
| 3427.13 (40 °C) | 3393.73 (40 °C) |
| Pretreatment Temperature (°C) | AD Temperature (°C) | Amount (Ton) | Reagent Cost (USD) | Water (USD) | Electricity Cost (USD) | Staff Cost (USD) | Sum (USD) | Methane Yield (m3·t−1) | Unit Methane Production Cost (USD·m3·CH4−1) |
|---|---|---|---|---|---|---|---|---|---|
| 40 | 35 | 0.02 | 3.56 | 0.4266 | 3.5936 | 7.485 | 21.0852 | 181 | 0.0833 |
| 55 | 0.02 | 3.56 | 0.4266 | 5.6603 | 7.485 | 23.1519 | 236 | 0.0726 | |
| 60 | 35 | 0.02 | 3.56 | 0.4266 | 4.4762 | 7.485 | 21.9678 | 184 | 0.0867 |
| 55 | 0.02 | 3.56 | 0.4266 | 6.9981 | 7.485 | 24.4897 | 236 | 0.0783 | |
| untreated | 35 | 0 | 0 | 0.2558 | 3.3455 | 7.485 | 11.0863 | 118 | 0.1241 |
| 55 | 0 | 0 | 0.2558 | 4.6282 | 7.485 | 12.369 | 180 | 0.0884 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, X.; Wachemo, A.C.; Chen, W.; Zuo, X. Determining Optimal Temperature Combination for Effective Pretreatment and Anaerobic Digestion of Corn Stalk. Int. J. Environ. Res. Public Health 2022, 19, 8027. https://doi.org/10.3390/ijerph19138027
Li J, Li X, Wachemo AC, Chen W, Zuo X. Determining Optimal Temperature Combination for Effective Pretreatment and Anaerobic Digestion of Corn Stalk. International Journal of Environmental Research and Public Health. 2022; 19(13):8027. https://doi.org/10.3390/ijerph19138027
Chicago/Turabian StyleLi, Juan, Xiujin Li, Akiber Chufo Wachemo, Weiwei Chen, and Xiaoyu Zuo. 2022. "Determining Optimal Temperature Combination for Effective Pretreatment and Anaerobic Digestion of Corn Stalk" International Journal of Environmental Research and Public Health 19, no. 13: 8027. https://doi.org/10.3390/ijerph19138027






