Prevention of Loss of Muscle Mass and Function in Older Adults during COVID-19 Lockdown: Potential Role of Dietary Essential Amino Acids
Abstract
:1. Introduction
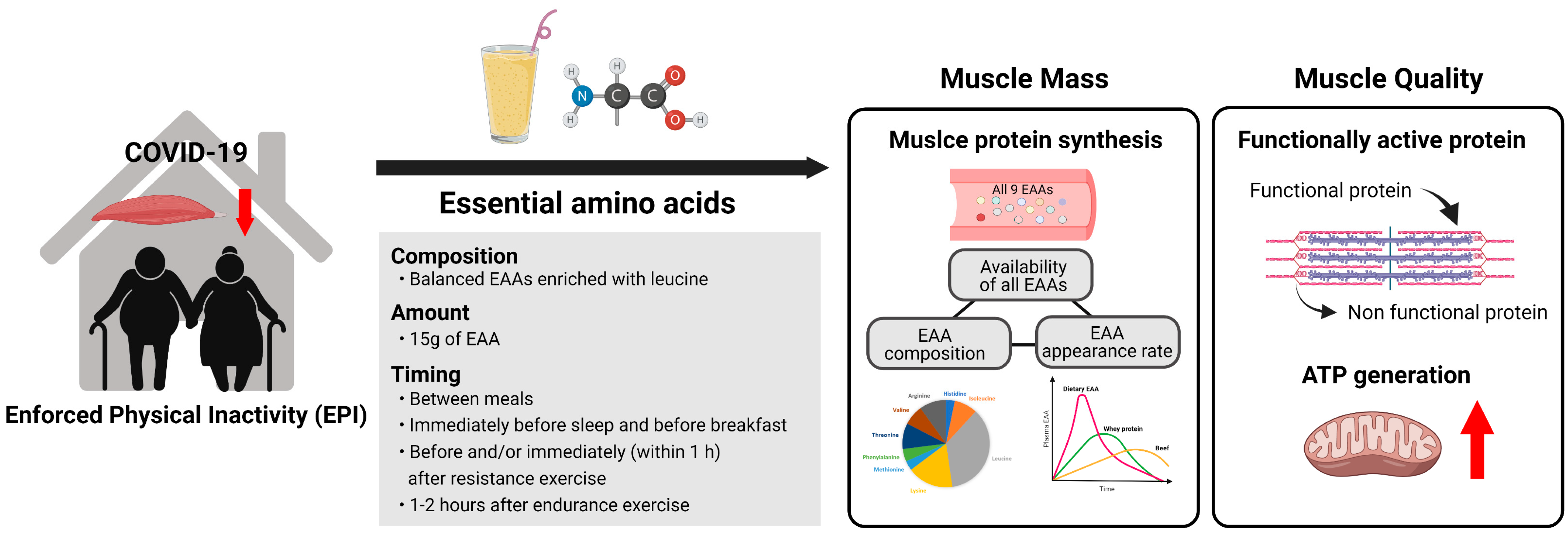
2. COVID-19-Induced Physical Inactivity and Its Influence on Sarcopenia
3. Superiority of Dietary EAAs for Muscle Growth
3.1. All EAAs Work as a Team for Making New Proteins
3.2. Role of EAAs in Stimulation of MPS
3.3. Role of Plasma EAA Appearance Rate on Stimulation of MPS
3.4. Role of EAAs on Stimulation of MPS Signaling
3.5. Clinical Evidence: Dietary EAAs Are More Effective in Inducing MPS Than High-Quality Protein
4. Roles of EAAs in Improving Muscle Quality
4.1. Functional Contractile Proteins in Skeletal Muscle
4.2. Mitochondrial Biogenesis
5. Practical Guidelines for Consumption of Free EAA Supplemental Intake for Older Adults
5.1. Dosage
5.2. Composition
5.3. Timing and Frequency
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Kirwan, R.; McCullough, D.; Butler, T.; Perez de Heredia, F.; Davies, I.G.; Stewart, C. Sarcopenia during COVID-19 lockdown restrictions: Long-term health effects of short-term muscle loss. GeroScience 2020, 42, 1547–1578. [Google Scholar] [CrossRef]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am. J. Clin. Nutr. 2005, 82, 1065–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.-Y.; Park, S.; Smeets, E.T.H.C.; Schutzler, S.; Azhar, G.; Wei, J.Y.; Ferrando, A.A.; Wolfe, R.R. Consumption of a Specially-Formulated Mixture of Essential Amino Acids Promotes Gain in Whole-Body Protein to a Greater Extent than a Complete Meal Replacement in Older Women with Heart Failure. Nutrients 2019, 11, 1360. [Google Scholar] [CrossRef] [Green Version]
- Paddon-Jones, D.; Sheffield-Moore, M.; Katsanos, C.S.; Zhang, X.-J.; Wolfe, R.R. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp. Gerontol. 2006, 41, 215–219. [Google Scholar] [CrossRef]
- Azhar, G.; Wei, J.Y.; Schutzler, S.E.; Coker, K.; Gibson, R.V.; Kirby, M.F.; Ferrando, A.A.; Wolfe, R.R. Daily Consumption of a Specially Formulated Essential Amino Acid-Based Dietary Supplement Improves Physical Performance in Older Adults with Low Physical Functioning. J. Gerontol. Ser. A 2021, 76, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Børsheim, E.; Bui, Q.U.T.; Tissier, S.; Kobayashi, H.; Ferrando, A.A.; Wolfe, R.R. Effect of amino acid supplementation on muscle mass, strength and physical function in elderly. Clin. Nutr. 2008, 27, 189–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rondanelli, M.; Opizzi, A.; Antoniello, N.; Boschi, F.; Iadarola, P.; Pasini, E.; Aquilani, R.; Dioguardi, F.S. Effect of essential amino acid supplementation on quality of life, Amino acid profile and strength in institutionalized elderly patients. Clin. Nutr. 2011, 30, 571–577. [Google Scholar] [CrossRef]
- World Health Organization. Recommended Population Levels of Physical Activity for Health. In Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010; pp. 15–34. ISBN 9789241599979. [Google Scholar]
- Breen, L.; Stokes, K.A.; Churchward-Venne, T.A.; Moore, D.R.; Baker, S.K.; Smith, K.; Atherton, P.J.; Phillips, S.M. Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J. Clin. Endocrinol. Metab. 2013, 98, 2604–2612. [Google Scholar] [CrossRef] [Green Version]
- Ferrando, A.A.; Paddon-Jones, D.; Hays, N.P.; Kortebein, P.; Ronsen, O.; Williams, R.H.; McComb, A.; Symons, T.B.; Wolfe, R.R.; Evans, W. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin. Nutr. 2010, 29, 18–23. [Google Scholar] [CrossRef]
- Moga, T.D.; Nistor-Cseppento, C.D.; Bungau, S.G.; Tit, D.M.; Sabau, A.M.; Behl, T.; Nechifor, A.C.; Bungau, A.F.; Negrut, N. The Effects of the “Catabolic Crisis” on Patients’ Prolonged Immobility after COVID-19 Infection. Medicina 2022, 58, 828. [Google Scholar] [CrossRef]
- da Rocha, A.Q.; Lobo, P.C.B.; Pimentel, G.D. Muscle Function Loss and Gain of Body Weight during the COVID-19 Pandemic in Elderly Women: Effects of One Year of Lockdown. J. Nutr. Health Aging 2021, 25, 1028–1029. [Google Scholar] [CrossRef]
- Pleguezuelos, E.; Del Carmen, A.; Llorensi, G.; Carcole, J.; Casarramona, P.; Moreno, E.; Ortega, P.; Serra-Prat, M.; Palomera, E.; Miravitlles, M.M.; et al. Severe loss of mechanical efficiency in COVID-19 patients. J. Cachexia Sarcopenia Muscle 2021, 12, 1056–1063. [Google Scholar] [CrossRef]
- Pinto, A.J.; Dunstan, D.W.; Owen, N.; Bonfá, E.; Gualano, B. Combating physical inactivity during the COVID-19 pandemic. Nat. Rev. Rheumatol. 2020, 16, 347–348. [Google Scholar] [CrossRef] [PubMed]
- The Impact of Coronavirus on Global Activity—Fitbit Blog. Available online: https://blog.fitbit.com/covid-19-global-activity/ (accessed on 16 January 2022).
- Devries, M.C.; Breen, L.; Von Allmen, M.; MacDonald, M.J.; Moore, D.R.; Offord, E.A.; Horcajada, M.N.; Breuillé, D.; Phillips, S.M. Low-load resistance training during step-reduction attenuates declines in muscle mass and strength and enhances anabolic sensitivity in older men. Physiol. Rep. 2015, 3, e12493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.Y.; Lin, X.L.; Fang, A.P.; Zhu, H.L. Eating Habits and Lifestyles during the Initial Stage of the COVID-19 Lockdown in China: A Cross-Sectional Study. Nutrients 2021, 13, 970. [Google Scholar] [CrossRef] [PubMed]
- Damanti, S.; Cristel, G.; Ramirez, G.A.; Bozzolo, E.P.; Da Prat, V.; Gobbi, A.; Centurioni, C.; Di Gaeta, E.; Del Prete, A.; Calabrò, M.G.; et al. Influence of reduced muscle mass and quality on ventilator weaning and complications during intensive care unit stay in COVID-19 patients. Clin. Nutr. 2021. [Google Scholar] [CrossRef]
- Sullivan, D.H.; Sun, S.; Walls, R.C. Protein-energy undernutrition among elderly hospitalized patients: A prospective study. JAMA 1999, 281, 2013–2019. [Google Scholar] [CrossRef] [Green Version]
- Lieffers, J.R.; Bathe, O.F.; Fassbender, K.; Winget, M.; Baracos, V.E. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br. J. Cancer 2012, 107, 931–936. [Google Scholar] [CrossRef] [Green Version]
- Roubenoff, R.; Hughes, V.A. SarcopeniaCurrent Concepts. J. Gerontol. Ser. A 2000, 55, M716–M724. [Google Scholar] [CrossRef] [Green Version]
- Beaudart, C.; Zaaria, M.; Pasleau, F.; Reginster, J.Y.; Bruyère, O. Health Outcomes of Sarcopenia: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0169548. [Google Scholar] [CrossRef] [Green Version]
- Roubenoff, R. Sarcopenia: A major modifiable cause of frailty in the elderly. J. Nutr. Health Aging 2000, 4, 140–142. [Google Scholar] [PubMed]
- Sallis, R.; Young, D.R.; Tartof, S.Y.; Sallis, J.F.; Sall, J.; Li, Q.; Smith, G.N.; Cohen, D.A. Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: A study in 48 440 adult patients. Br. J. Sports Med. 2021, 55, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Park, S.; Jang, J.; Wolfe, R.R. Understanding Muscle Protein Dynamics: Technical Considerations for Advancing Sarcopenia Research. Ann. Geriatr. Med. Res. 2020, 24, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Markofski, M.M.; Jennings, K.; Timmerman, K.L.; Dickinson, J.M.; Fry, C.S.; Borack, M.S.; Reidy, P.T.; Deer, R.R.; Randolph, A.; Rasmussen, B.B.; et al. Effect of Aerobic Exercise Training and Essential Amino Acid Supplementation for 24 Weeks on Physical Function, Body Composition, and Muscle Metabolism in Healthy, Independent Older Adults: A Randomized Clinical Trial. J. Gerontol. Ser. A 2018, 74, 1598–1604. [Google Scholar] [CrossRef] [PubMed]
- Dillon, E.L.; Sheffield-Moore, M.; Paddon-Jones, D.; Gilkison, C.; Sanford, A.P.; Casperson, S.L.; Jiang, J.; Chinkes, D.L.; Urban, R.J. Amino Acid Supplementation Increases Lean Body Mass, Basal Muscle Protein Synthesis, and Insulin-Like Growth Factor-I Expression in Older Women. J. Clin. Endocrinol. Metab. 2009, 94, 1630–1637. [Google Scholar] [CrossRef] [Green Version]
- Katsanos, C.S.; Chinkes, D.L.; Paddon-Jones, D.; Zhang, X.; Aarsland, A.; Wolfe, R.R. Whey protein ingestion in elderly persons results in greater muscle protein accrual than ingestion of its constituent essential amino acid content. Nutr. Res. 2008, 28, 651–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volpi, E.; Kobayashi, H.; Sheffield-Moore, M.; Mittendorfer, B.; Wolfe, R.R. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am. J. Clin. Nutr. 2003, 78, 250–258. [Google Scholar] [CrossRef]
- Park, S.; Church, D.D.; Azhar, G.; Schutzler, S.E.; Ferrando, A.A.; Wolfe, R.R. Anabolic response to essential amino acid plus whey protein composition is greater than whey protein alone in young healthy adults. J. Int. Soc. Sports Nutr. 2020, 17, 9. [Google Scholar] [CrossRef] [Green Version]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am. J. Physiol. Metab. 2006, 291, E381–E387. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, R.R. Regulation of muscle protein by amino acids. J. Nutr. 2002, 132, 3219S–3224S. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, K.R.; Church, D.D.; Kim, I.-Y.; Park, S.; Wolfe, R.R.; Ferrando, A.A. Comparison of basal whole-body protein kinetics and muscle protein synthesis between young and older adults. Physiol. Rep. 2020, 8, e14633. [Google Scholar] [CrossRef] [PubMed]
- Louard, R.J.; Barrett, E.J.; Gelfand, R.A. Effect of Infused Branched-Chain Amino Acids on Muscle and Whole-Body Amino Acid Metabolism in Man. Clin. Sci. 1990, 79, 457–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhoeven, S.; Vanschoonbeek, K.; Verdijk, L.B.; Koopman, R.; Wodzig, W.K.; Dendale, P.; van Loon, L.J. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am. J. Clin. Nutr. 2009, 89, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Fitts, R.H.; Romatowski, J.G.; Peters, J.R.; Paddon-Jones, D.; Wolfe, R.R.; Ferrando, A.A. The deleterious effects of bed rest on human skeletal muscle fibers are exacerbated by hypercortisolemia and ameliorated by dietary supplementation. Am. J. Physiol. Physiol. 2007, 293, C313–C320. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, B.B.; Tipton, K.D.; Miller, S.L.; Wolf, S.E.; Wolfe, R.R. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J. Appl. Physiol. 2000, 88, 386–392. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Jang, J.; Choi, M.D.; Shin, Y.-A.; Schutzler, S.; Azhar, G.; Ferrando, A.A.; Wolfe, R.R.; Kim, I.-Y. The Anabolic Response to Dietary Protein Is Not Limited by the Maximal Stimulation of Protein Synthesis in Healthy Older Adults: A Randomized Crossover Trial. Nutrients 2020, 12, 3276. [Google Scholar] [CrossRef]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.P.; Maubois, J.L.; Beaufrère, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef] [Green Version]
- Dangin, M.; Guillet, C.; Garcia-Rodenas, C.; Gachon, P.; Bouteloup-Demange, C.; Reiffers-Magnani, K.; Fauquant, J.; Ballèvre, O.; Beaufrère, B. The rate of protein digestion affects protein gain differently during aging in humans. J. Physiol. 2003, 549, 635–644. [Google Scholar] [CrossRef]
- Park, S.; Church, D.D.; Starck, C.; Schutzler, S.E.; Azhar, G.; Kim, I.-Y.; Ferrando, A.A.; Moughan, P.J.; Wolfe, R.R. The impact of Hayward green kiwifruit on dietary protein digestion and protein metabolism. Eur. J. Nutr. 2020, 60, 1141–1148. [Google Scholar] [CrossRef]
- West, D.W.; Burd, N.A.; Coffey, V.G.; Baker, S.K.; Burke, L.M.; Hawley, J.A.; Moore, D.R.; Stellingwerff, T.; Phillips, S.M. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. Am. J. Clin. Nutr. 2011, 94, 795–803. [Google Scholar] [CrossRef] [Green Version]
- Churchward-Venne, T.A.; Burd, N.A.; Mitchell, C.J.; West, D.W.D.; Philp, A.; Marcotte, G.R.; Baker, S.K.; Baar, K.; Phillips, S.M. Supplementation of a suboptimal protein dose with leucine or essential amino acids: Effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J. Physiol. 2012, 590, 2751–2765. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.C.; Lang, C.H.; Crozier, S.J.; Anthony, T.G.; MacLean, D.A.; Kimball, S.R.; Jefferson, L.S. Contribution of insulin to the translational control of protein synthesis in skeletal muscle by leucine. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E1092–E1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimball, S.R.; Jefferson, L.S. Signaling Pathways and Molecular Mechanisms through which Branched-Chain Amino Acids Mediate Translational Control of Protein Synthesis. J. Nutr. 2006, 136, 227S–231S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anthony, J.C.; Reiter, A.K.; Anthony, T.G.; Crozier, S.J.; Lang, C.H.; MacLean, D.A.; Kimball, S.R.; Jefferson, L.S. Orally administered leucine enhances protein synthesis in skeletal muscle of diabetic rats in the absence of increases in 4E-BP1 or S6K1 phosphorylation. Diabetes 2002, 51, 928–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulla, H.; Smith, K.; Atherton, P.J.; Idris, I. Role of insulin in the regulation of human skeletal muscle protein synthesis and breakdown: A systematic review and meta-analysis. Diabetologia 2016, 59, 44–55. [Google Scholar] [CrossRef]
- Sacheck, J.M.; Ohtsuka, A.; McLary, S.C.; Goldberg, A.L. IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E591–E601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, B.T.; Lee, K.Y.; Klaus, K.; Softic, S.; Krumpoch, M.T.; Fentz, J.; Stanford, K.I.; Robinson, M.M.; Cai, W.; Kleinridders, A.; et al. Insulin and IGF-1 receptors regulate FoxO-mediated signaling in muscle proteostasis. J. Clin. Investig. 2016, 126, 3433–3446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bukhari, S.S.I.; Phillips, B.E.; Wilkinson, D.J.; Limb, M.C.; Rankin, D.; Mitchell, W.K.; Kobayashi, H.; Greenhaff, P.L.; Smith, K.; Atherton, P.J. Intake of low-dose leucine-rich essential amino acids stimulates muscle anabolism equivalently to bolus whey protein in older women at rest and after exercise. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E1056–E1065. [Google Scholar] [CrossRef]
- Jang, J.; Park, S.; Kim, Y.; Jung, J.; Lee, J.; Chang, Y.; Lee, S.P.; Park, B.C.; Wolfe, R.R.; Choi, C.S.; et al. Myostatin Inhibition-Induced Increase in Muscle Mass and Strength Was Amplified by Resistance Exercise Training, and Dietary Essential Amino Acids Improved Muscle Quality in Mice. Nutrients 2021, 13, 1508. [Google Scholar] [CrossRef] [PubMed]
- Balagopal, P.; Rooyackers, O.E.; Adey, D.B.; Ades, P.A.; Nair, K.S. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy- chain and sarcoplasmic protein in humans. Am. J. Physiol. Endocrinol. Metab. 1997, 273, E790–E800. [Google Scholar] [CrossRef]
- Kim, Y.; Park, S.; Lee, J.; Jang, J.; Jung, J.; Koh, J.-H.; Choi, C.S.; Wolfe, R.R.; Kim, I.-Y. Essential Amino Acid-Enriched Diet Alleviates Dexamethasone-Induced Loss of Muscle Mass and Function through Stimulation of Myofibrillar Protein Synthesis and Improves Glucose Metabolism in Mice. Metabolites 2022, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Chen Scarabelli, C.; McCauley, R.B.; Yuan, Z.; Di Rezze, J.; Patel, D.; Putt, J.; Raddino, R.; Allebban, Z.; Abboud, J.; Scarabelli, G.M.; et al. Oral Administration of Amino Acidic Supplements Improves Protein and Energy Profiles in Skeletal Muscle of Aged Rats: Elongation of Functional Performance and Acceleration of Mitochondrial Recovery in Adenosine Triphosphate After Exhaustive Exertion. Am. J. Cardiol. 2008, 101, S42–S48. [Google Scholar] [CrossRef]
- Hollenberg, M.; Ngo, L.H.; Turner, D.; Tager, I.B. Treadmill exercise testing in an epidemiologic study of elderly subjects. J. Gerontol. A. Biol. Sci. Med. Sci. 1998, 53, B259–B267. [Google Scholar] [CrossRef] [PubMed]
- D’Antona, G.; Ragni, M.; Cardile, A.; Tedesco, L.; Dossena, M.; Bruttini, F.; Caliaro, F.; Corsetti, G.; Bottinelli, R.; Carruba, M.O.; et al. Branched-Chain Amino Acid Supplementation Promotes Survival and Supports Cardiac and Skeletal Muscle Mitochondrial Biogenesis in Middle-Aged Mice. Cell Metab. 2010, 12, 362–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Breen, L.; Burd, N.A.; Hector, A.J.; Churchward-Venne, T.A.; Josse, A.R.; Tarnopolsky, M.A.; Phillips, S.M. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br. J. Nutr. 2012, 108, 1780–1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohé, J.; Low, A.; Wolfe, R.R.; Rennie, M.J. Human Muscle Protein Synthesis is Modulated by Extracellular, Not Intramuscular Amino Acid Availability: A Dose-Response Study. J. Physiol. 2003, 552, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.J.; Glynn, E.L.; Fry, C.S.; Timmerman, K.L.; Volpi, E.; Rasmussen, B.B. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E1011. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.-Y.; Schutzler, S.E.; Schrader, A.; Spencer, H.J.; Azhar, G.; Deutz, N.E.P.; Wolfe, R.R. Acute ingestion of citrulline stimulates nitric oxide synthesis but does not increase blood flow in healthy young and older adults with heart failure. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E915–E924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mioche, L.; Bourdiol, P.; Monier, S.; Martin, J.-F.; Cormier, D. Changes in jaw muscles activity with age: Effects on food bolus properties. Physiol. Behav. 2004, 82, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Mioche, L.; Bourdiol, P.; Peyron, M.-A. Influence of age on mastication: Effects on eating behaviour. Nutr. Res. Rev. 2004, 17, 43–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudson, J.L.; Paddon-Jones, D.; Campbell, W.W. Whey protein supplementation 2 hours after a lower protein breakfast restores plasma essential amino acid availability comparable to a higher protein breakfast in overweight adults. Nutr. Res. 2017, 47, 90–97. [Google Scholar] [CrossRef]
- Trommelen, J.; Kouw, I.W.K.; Holwerda, A.M.; Snijders, T.; Halson, S.L.; Rollo, I.; Verdijk, L.B.; van Loon, L.J.C. Pre-sleep dietary protein-derived amino acids are incorporated in myofibrillar protein during post-exercise overnight recovery. Am. J. Physiol. Metab. 2017, 314, E457–E467. [Google Scholar]
- Groen, B.B.L.; Res, P.T.; Pennings, B.; Hertle, E.; Senden, J.M.G.; Saris, W.H.M.; van Loon, L.J.C. Intragastric protein administration stimulates overnight muscle protein synthesis in elderly men. Am. J. Physiol. Metab. 2012, 302, E52–E60. [Google Scholar] [CrossRef] [PubMed]
- Kouw, I.W.; Holwerda, A.M.; Trommelen, J.; Kramer, I.F.; Bastiaanse, J.; Halson, S.L.; Wodzig, W.K.; Verdijk, L.B.; van Loon, L.J. Protein Ingestion before Sleep Increases Overnight Muscle Protein Synthesis Rates in Healthy Older Men: A Randomized Controlled Trial. J. Nutr. 2017, 147, 2252–2261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarasheski, K.E.; Zachwieja, J.J.; Bier, D.M. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am. J. Physiol. Metab. 1993, 265, E210–E214. [Google Scholar] [CrossRef] [PubMed]
- Esmarck, B.; Andersen, J.L.; Olsen, S.; Richter, E.A.; Mizuno, M.; Kjær, M. Timing of postexercise protein intake is important for muscle hypertrophy with resistance training in elderly humans. J. Physiol. 2001, 535, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Tipton, K.D.; Rasmussen, B.B.; Miller, S.L.; Wolf, S.E.; Owens-Stovall, S.K.; Petrini, B.E.; Wolfe, R.R. Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise. Am. J. Physiol. Metab. 2001, 281, E197–E206. [Google Scholar] [CrossRef] [Green Version]
- Beelen, M.; Koopman, R.; Gijsen, A.P.; Vandereyt, H.; Kies, A.K.; Kuipers, H.; Saris, W.H.M.; Van Loon, L.J.C. Protein coingestion stimulates muscle protein synthesis during resistance-type exercise. Am. J. Physiol. Endocrinol. Metab. 2008, 295, 70–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norton, L.E.; Layman, D.K. Leucine Regulates Translation Initiation of Protein Synthesis in Skeletal Muscle after Exercise. J. Nutr. 2006, 136, 533S–537S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuthbertson, D.; Smith, K.; Babraj, J.; Leese, G.; Waddell, T.; Atherton, P.; Wackerhage, H.; Taylor, P.M.; Rennie, M.J. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005, 19, 422–424. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Chang, Y.; Wolfe, R.R.; Kim, I.-Y. Prevention of Loss of Muscle Mass and Function in Older Adults during COVID-19 Lockdown: Potential Role of Dietary Essential Amino Acids. Int. J. Environ. Res. Public Health 2022, 19, 8090. https://doi.org/10.3390/ijerph19138090
Park S, Chang Y, Wolfe RR, Kim I-Y. Prevention of Loss of Muscle Mass and Function in Older Adults during COVID-19 Lockdown: Potential Role of Dietary Essential Amino Acids. International Journal of Environmental Research and Public Health. 2022; 19(13):8090. https://doi.org/10.3390/ijerph19138090
Chicago/Turabian StylePark, Sanghee, Yewon Chang, Robert R. Wolfe, and Il-Young Kim. 2022. "Prevention of Loss of Muscle Mass and Function in Older Adults during COVID-19 Lockdown: Potential Role of Dietary Essential Amino Acids" International Journal of Environmental Research and Public Health 19, no. 13: 8090. https://doi.org/10.3390/ijerph19138090
APA StylePark, S., Chang, Y., Wolfe, R. R., & Kim, I.-Y. (2022). Prevention of Loss of Muscle Mass and Function in Older Adults during COVID-19 Lockdown: Potential Role of Dietary Essential Amino Acids. International Journal of Environmental Research and Public Health, 19(13), 8090. https://doi.org/10.3390/ijerph19138090







