Fractional Ablative Carbon Dioxide Lasers for the Treatment of Morphea: A Case Series and Literature Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Evaluation
2.3. Ultrasound Assessment of Dermal Thickness
2.4. Elasticity Assessment
2.5. Fractional Ablative Carbon Dioxide Laser Treatment
3. Case Series
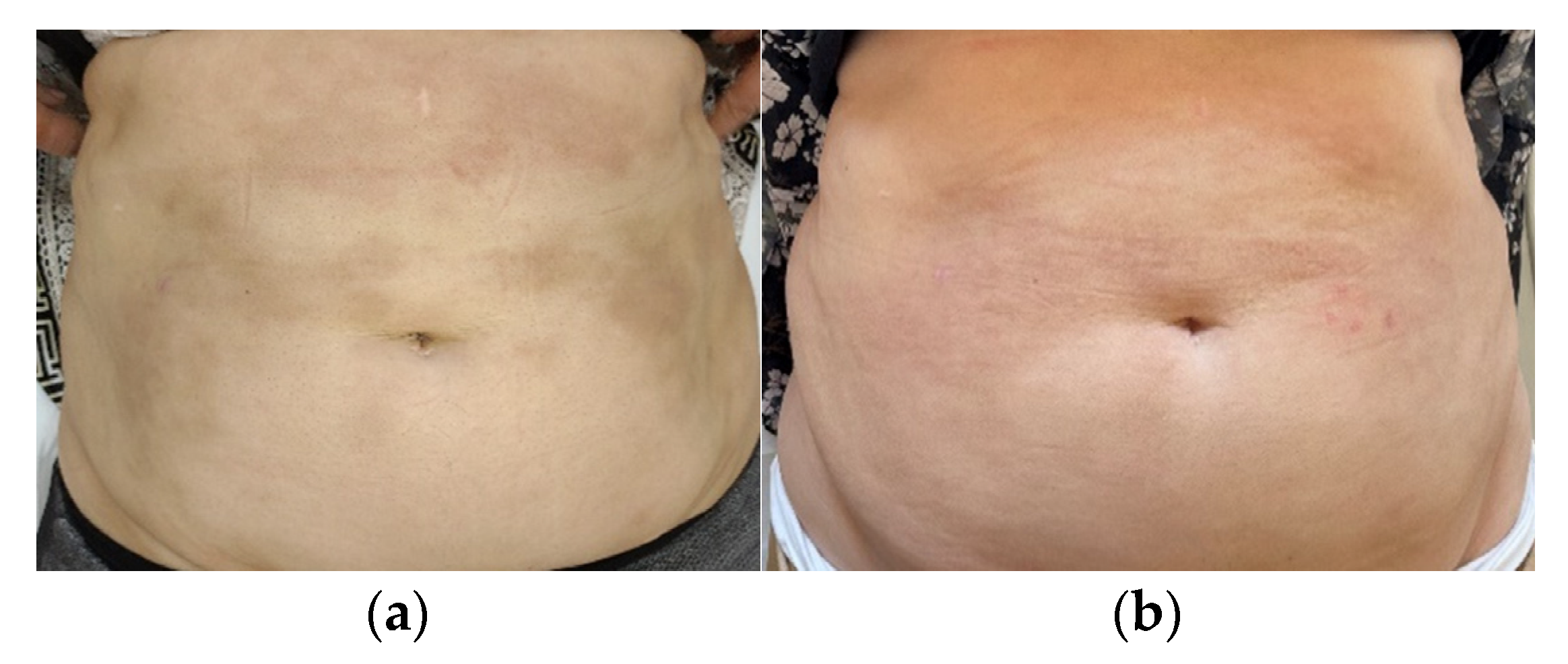
3.1. Case 1
3.2. Case 2
3.3. Case 3
3.4. Case 4
4. Results
4.1. Clinical Evaluation
4.2. Ultrasound Assessment of Dermal Thickness
4.3. Elasticity Assessment
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kreuter, A.; Krieg, T.; Worm, M.; Wenzel, J.; Moinzadeh, P.; Kuhn, A.; Aberer, E.; Scharffetter-Kochanek, K.; Horneff, G.; Reil, E.; et al. German guidelines for the diagnosis and therapy of localized scleroderma. JDDG J. Der Dtsch. Dermatol. Ges. 2016, 14, 199–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fett, N.; Werth, V.P. Update on morphea: Part I. Epidemiology, clinical presentation, and pathogenesis. J. Am. Acad. Dermatol. 2011, 64, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Krasowska, D.; Rudnicka, L.; Dańczak-Pazdrowska, A.; Chodorowska, G.; Woźniacka, A.; Lis-Święty, A.; Czuwara, J.; Maj, J.; Majewski, S.; Sysa-Jędrzejowska, A.; et al. Localized scleroderma (morphea). Diagnostic and therapeutic recommendations of the Polish Dermatological Society. Przegląd Dermatol. 2019, 106, 333–353. [Google Scholar] [CrossRef]
- Saracino, A.M.; Denton, C.P.; Orteu, C.H. The molecular pathogenesis of morphea: From genetics to future treatment targets. Br. J. Dermatol. 2017, 177, 34–46. [Google Scholar] [CrossRef]
- Ihn, H.; Sato, S.; Fujimoto, M.; Kikuchi, K.; Takehara, K. Demonstration of interleukin-2, interleukin-4 and interleukin-6 in sera from patients with localized scleroderma. Arch. Dermatol. Res. 1995, 287, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Leask, A.; Abraham, D.J. TGF-beta signaling and the fibrotic response. FASEB J. 2004, 18, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Niemczyk, M.; Foroncewicz, B.; Mucha, K. Rola TGF beta. Pol. Arch. Med. Wewn. 2005, 113, 401–408. [Google Scholar]
- Knobler, R.; Moinzadeh, P.; Hunzelmann, N.; Kreuter, A.; Cozzio, A.; Mouthon, L.; Cutolo, M.; Rongioletti, F.; Denton, C.P.; Rudnicka, L.; et al. European Dermatology Forum S1-guideline on the diagnosis and treatment of sclerosing diseases of the skin, Part 1: Localized scleroderma, systemic sclerosis and overlap syndromes. J. Eur. Acad. Dermatol. Venereol. JEADV 2017, 31, 1401–1424. [Google Scholar] [CrossRef]
- Szramka-Pawlak, B.; Dańczak-Pazdrowska, A.; Rzepa, T.; Szewczyk, A.; Sadowska-Przytocka, A.; Zaba, R. Quality of Life and Optimism in Patients with Morphea. Appl. Res. Qual. Life 2014, 9, 863–870. [Google Scholar] [CrossRef] [Green Version]
- Wolska-Gawron, K.; Krasowska, D. Localized scleroderma—Classification and tools used for the evaluation of tissue damage and disease activity/severity. Dermatol. Rev. 2017, 104, 269–289. [Google Scholar] [CrossRef] [Green Version]
- Szczepanik-Kułak, P.; Michalska-Jakubus, M.; Krasowska, D. Laser Therapy for the Treatment of Morphea: A Systematic Review of Literature. J. Clin. Med. 2021, 10, 3409. [Google Scholar] [CrossRef]
- Creadore, A.; Watchmaker, J.; Maymone, M.; Pappas, L.; Vashi, N.A.; Lam, C. Cosmetic treatment in patients with autoimmune connective tissue diseases: Best practices for patients with lupus erythematosus. J. Am. Acad. Dermatol. 2020, 83, 343–363. [Google Scholar] [CrossRef] [PubMed]
- Kineston, D.; Kwan, J.M.; Uebelhoer, N.S.; Shumaker, P.R. Use of a fractional ablative 10.6-μm carbon dioxide laser in the treatment of a morphea-related contracture. Arch. Dermatol. 2011, 147, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, S.M.; Bosseila, M.; Fawzy, M.M.; Abdel Halim, D.M.; Sayed, S.S.; Allam, R.S. Fractional carbon dioxide laser versus low-dose UVA-1 phototherapy for treatment of localized scleroderma: A clinical and immunohistochemical randomized controlled study. Lasers Med. Sci. 2016, 31, 1707–1715. [Google Scholar] [CrossRef] [PubMed]
- Farmer, C.; Griffith, J.L.; Lim, H.W.; Ozog, D.M. Fractionated CO2 laser for treatment of linear morphea: A case series. J. Am. Acad. Dermatol. 2018, 79, AB143. [Google Scholar]
- Yeager, D.; Ozog, D.M. Persistent improvement at three year follow-up in a patient with localized deep morphea treated withboth injected and laser-assisted topical poly-l-lactic acid. Lasers Surg. Med. 2019, 51, S11–S12. [Google Scholar]
- Eisen, D.; Alster, T.S. Use of a 585 nm pulsed dye laser for the treatment of morphea. Dermatol. Surg. 2002, 28, 615–616. [Google Scholar] [CrossRef]
- Tawfik, A.A.; Shokir, H.; Soliman, M.; Salah, L.; Fathy, S. Pulsed dye laser in the treatment of localized scleroderma and its effects on CD34+ and factor XIIIa+ cells: An immunohistochemical study. Am. J. Clin. Dermatol. 2013, 14, 235–241. [Google Scholar] [CrossRef]
- Kakimoto, C.V.; Victor Ross, E.; Uebelhoer, N.S. En coup de sabre presenting as a port-wine stain previously treated with pulsed dye laser. Dermatol. Surg. 2009, 35, 165–167. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, J.Y.; Kim, H.O.; Park, Y.M. En coup de sabre presenting as a port-wine stain initially treated with a pulsed dye laser. J. Dermatol. 2011, 38, 209–210. [Google Scholar] [CrossRef]
- Pickert, A.J.; Carpentieri, D.; Price, H.; Hansen, R.C. Early morphea mimicking acquired port-wine stain. Pediatric Dermatol. 2014, 31, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Nijhawan, R.I.; Bard, S.; Blyumin, M.; Smidt, A.C.; Chamlin, S.L.; Connelly, E.A. Early localized morphea mimicking an acquired port-wine stain. J. Am. Acad. Dermatol. 2011, 64, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.S.; Tay, Y.K. Inflammatory morphea mimicking an acquired port-wine stain initially treated with pulsed-dye laser. J. Cosmet. Laser Ther. 2015, 17, 277–280. [Google Scholar] [CrossRef]
- Miura, T.; Yamamoto, T. Pediatric linear scleroderma initially developed with angioma serpiginosum-like appearances. J. Dermatol. 2015, 42, 750–751. [Google Scholar] [CrossRef]
- Nisticò, S.P.; Saraceno, R.; Schipani, C.; Costanzo, A.; Chimenti, S. Different applications of monochromatic excimer light in skin diseases. Photomed. Laser Surg. 2009, 27, 647–654. [Google Scholar] [CrossRef] [Green Version]
- Hanson, A.H.; Fivenson, D.P.; Schapiro, B. Linear scleroderma in an adolescent woman treated with methotrexate and excimer laser. Dermatol. Ther. 2014, 27, 203–205. [Google Scholar] [CrossRef]
- Use of Excimer Laser for Morphea. Available online: https://hsrc.himmelfarb.gwu.edu/researchdays_2014/11/ (accessed on 17 February 2021).
- Tatu, A.; Radaschin, D.; Constantin, V.; Stana, P.; Ardeleanu, V. Laser therapy in superficial morphea lesions—Indications, limitations and therapeutic alternatives. J. Mind Med. Sci. 2020, 7, 46–51. [Google Scholar] [CrossRef]
- Kozarev, J. Fractional Er:YAG Laser Therapy for Localized Scleroderma (Summary). J. Laser Health Acad. 2012, 1, S07. [Google Scholar]
- Ghorbel, H.H.; Lacour, J.P.; Passeron, T. Use of 2940-nm Erbium-Yag fractional laser for treating the skin texture changes in stabilized Parry Romberg syndrome. Eur. J. Dermatol. EJD 2013, 23, 908–909. [Google Scholar] [CrossRef]
- Arpey, C.J.; Patel, D.S.; Stone, M.S.; Qiang-Shao, J.; Moore, K.C. Treatment of atrophoderma of Pasini and Pierini-associated hyperpigmentation with the Q-switched alexandrite laser: A clinical, histologic, and ultrastructural appraisal. Lasers Surg. Med. 2000, 27, 206–212. [Google Scholar] [CrossRef]
- Bimbi, C.; Koumoundourou, D.; Kyriakou, G.; Brzezinski, P. Improvement of linear scleroderma of the limbs after treatment withlong-pulsed 1064 nm Nd: YAG laser: A case report. Dermatol. Online J. 2020, 11, 376–378. [Google Scholar] [CrossRef]
- Owczarczyk-Saczonek, A.; Kasprowicz-Furmańczyk, M.; Kruszewska, A.; Krajewska-Włodarczyk, M.; Bechtold, A.; Klimek, P.; Placek, W. The Correction of Facial Morphea Lesions by Hyaluronic Acid: A Case Series and Literature Review. Dermatol. Ther. 2020, 10, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Saedi, N.; Petelin, A.; Zachary, C. Fractionation: A new era in laser resurfacing. Clin. Plast. Surg. 2011, 38, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Brauer, J.A.; Gordon Spratt, E.A.; Geronemus, R.G. Laser therapy in the treatment of connective tissue diseases: A review. Dermatol. Surg. 2014, 40, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Manstein, D.; Herron, G.S.; Sink, R.K.; Tanner, H.; Anderson, R.R. Fractional photothermolysis: A new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg. Med. 2004, 34, 426–438. [Google Scholar] [CrossRef]
- Helbig, D.; Paasch, U. Molecular changes during skin aging and wound healing after fractional ablative photothermolysis. Ski. Res. Technol. 2011, 17, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Paasch, U. The Future of Fractional Lasers. Facial Plast. Surg. FPS 2016, 32, 261–268. [Google Scholar] [CrossRef] [Green Version]
- El-Hoshy, K.; Abdel-Halim, M.; Dorgham, D.; El-Din Sayed, S.S.; El-Kalioby, M. Efficacy of Fractional Carbon Dioxide Laser in the Treatment of Mature Burn Scars: A Clinical, Histopathological, and Histochemical Study. J. Clin. Aesthetic Dermatol. 2017, 10, 36–43. [Google Scholar]
- Grunewald, S.; Bodendorf, M.; Illes, M.; Kendler, M.; Simon, J.C.; Paasch, U. In vivo wound healing and dermal matrix remodelling in response to fractional CO(2) laser intervention: Clinicopathological correlation in non-facial skin. Int. J. Hyperth. Off. J. Eur. Soc. Hyperthermic Oncol. North Am. Hyperth. Group 2011, 27, 811–818. [Google Scholar] [CrossRef]
- Hantash, B.M.; Bedi, V.P.; Kapadia, B.; Rahman, Z.; Jiang, K.; Tanner, H.; Chan, K.F.; Zachary, C.B. In vivo histological evaluation of a novel ablative fractional resurfacing device. Lasers Surg. Med. 2007, 39, 96–107. [Google Scholar] [CrossRef]
- Makboul, M.; Makboul, R.; Abdelhafez, A.H.; Hassan, S.S.; Youssif, S.M. Evaluation of the effect of fractional CO2 laser on histopathological picture and TGF-β1 expression in hypertrophic scar. J. Cosmet. Dermatol. 2014, 13, 169–179. [Google Scholar] [CrossRef] [PubMed]
- DermaLab® Series SkinLab Combo Instruction Manual; Cortex Technology ApS.: Hadsund, Denmark, 2012.
- Ranosz-Janicka, I.; Lis-Święty, A.; Skrzypek-Salamon, A.; Brzezińska-Wcisło, L. An extended high-frequency ultrasound protocol for assessing and quantifying of inflammation and fibrosis in localized scleroderma. Ski. Res. Technol. 2019, 25, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Roberge, D.; Freeman, C.; Wong, C.; Hines, J.; Turcotte, R.E. Skin elasticity as a measure of radiation fibrosis: Is it reproducible and does it correlate with patient and physician-reported measures? Technol. Cancer Res. Treat. 2014, 13, 469–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Age | Duration of Disease | Subtype of Morphea | Previous Treatment | No. of Treatments | Parameters | LoSDI before FAL | LoSDI after FAL | |
|---|---|---|---|---|---|---|---|---|
| Case 1a * | 30 | 22 years | Linear | Methotrexate, pentoxifylline, chloroquine, topically applied glucocorticoids, physiotherapy, and manual therapy | 4 | Power = 40 W and energy density = 40 mJ/cm2 | 7 | 3 |
| Case 1b * | 4 | Power = 30 W and energy density = 36 mJ/cm2 | 8 | 4 | ||||
| Case 2 | 31 | 1 year | Atrophoderma of Pasini and Pierini | None | 4 | Power = 40 W and energy density = 40 mJ/cm2 | 2 | 0 |
| Case 3 | 51 | 5 years | Plaque | Procaine penicillin, bath PUVA therapy, subcutaneous injections of triamcinolone, and topically applied glucocorticoids | 8 | Power = 40 W and energy density = 40 mJ/cm2 | 4 | 1 |
| Case 4 | 62 | 1 year 3 months | Plaque | PUVA phototherapy and topically applied glucocorticoids | 4 | Power = 40 W and energy density = 40 mJ/cm2 | 2 | 0 |
| Baseline | 4 Weeks after the 4th Procedure | Percentage Increment (%) | |
|---|---|---|---|
| Case 1a * | 976 | 882 | −9.63 |
| Case 1b * | 1369 | 1080 | −21.11 |
| Case 2 | 1043 | 632 | −39.4 |
| Case 3 | 1435 | 1001 | −30.24 |
| Case 4 | 816 | 711 | −12.87 |
| −22.65 | |||
| Baseline | 4 Weeks after the 4th Procedure | Percentage Increment (%) | |
|---|---|---|---|
| Case 1a * | 290 | 254 | −12.41 |
| Case 1b * | 343 | 306 | −10.78 |
| Case 2 | 102 | 98 | −3.92 |
| Case 3 | 131 | 117 | −10.68 |
| Case 4 | 111 | 103 | −7.2 |
| −8.99 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimek, P.; Placek, W.; Owczarczyk-Saczonek, A. Fractional Ablative Carbon Dioxide Lasers for the Treatment of Morphea: A Case Series and Literature Review. Int. J. Environ. Res. Public Health 2022, 19, 8133. https://doi.org/10.3390/ijerph19138133
Klimek P, Placek W, Owczarczyk-Saczonek A. Fractional Ablative Carbon Dioxide Lasers for the Treatment of Morphea: A Case Series and Literature Review. International Journal of Environmental Research and Public Health. 2022; 19(13):8133. https://doi.org/10.3390/ijerph19138133
Chicago/Turabian StyleKlimek, Paulina, Waldemar Placek, and Agnieszka Owczarczyk-Saczonek. 2022. "Fractional Ablative Carbon Dioxide Lasers for the Treatment of Morphea: A Case Series and Literature Review" International Journal of Environmental Research and Public Health 19, no. 13: 8133. https://doi.org/10.3390/ijerph19138133







