A Review of the Presence of SARS-CoV-2 in Wastewater: Transmission Risks in Mexico
Abstract
:1. Introduction
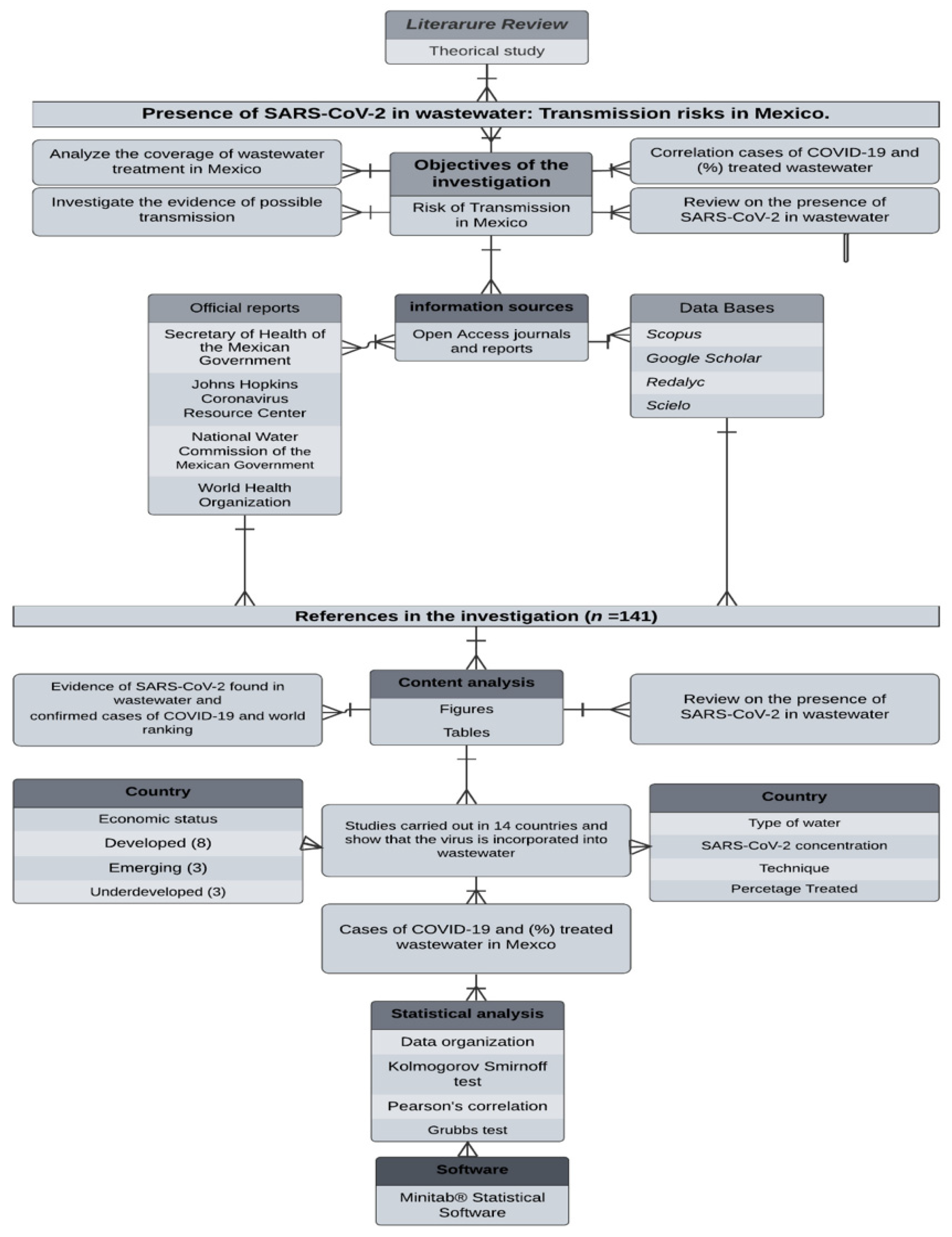
2. Materials and Methods
Statistical Analysis
3. Results and Discussion
3.1. Presence of SARs-CoV-2 in Municipal Wastewater
3.2. Potential Risks from Wastewater Management
3.3. Wastewater Treatment in Mexico and SARS-CoV-2 Risks
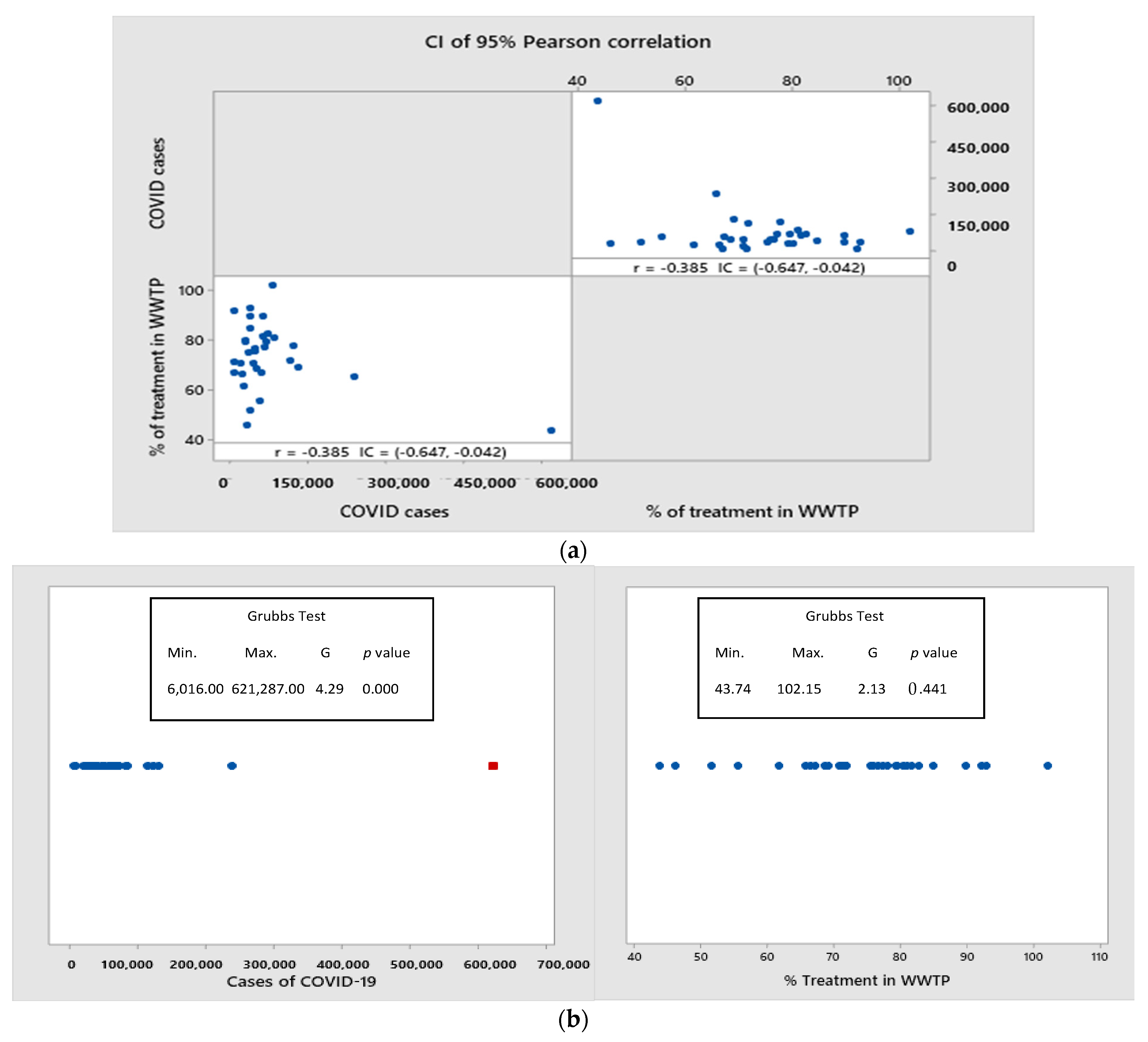
3.4. Is It Possible to Find a Relationship between COVID-19-Positive Cases and the Level of Wastewater Treatment in Mexico?
3.5. How to Reduce the Risk from Wastewater
3.6. Can SARS-CoV-2 Survive in the Environment in the Form of Bioaresols?
- ❖ 4 h on copper surfaces;
- ❖ 24 h in cardboard;
- ❖ Two or three days in stainless steel;
- ❖ Three days in plastics.
3.7. Is the Virus Present in the Wastewater Infectious Enough to Cause the Disease Regardless of the Means of Transmission?
3.8. Possible Solutions to Minimize the Risk
- Trends/changes in occurrence;
- Evaluation of community prevalence;
- Risk assessment;
- Viral evolution.
3.9. Added Value of this Study
3.10. Implications of All the Available Evidence
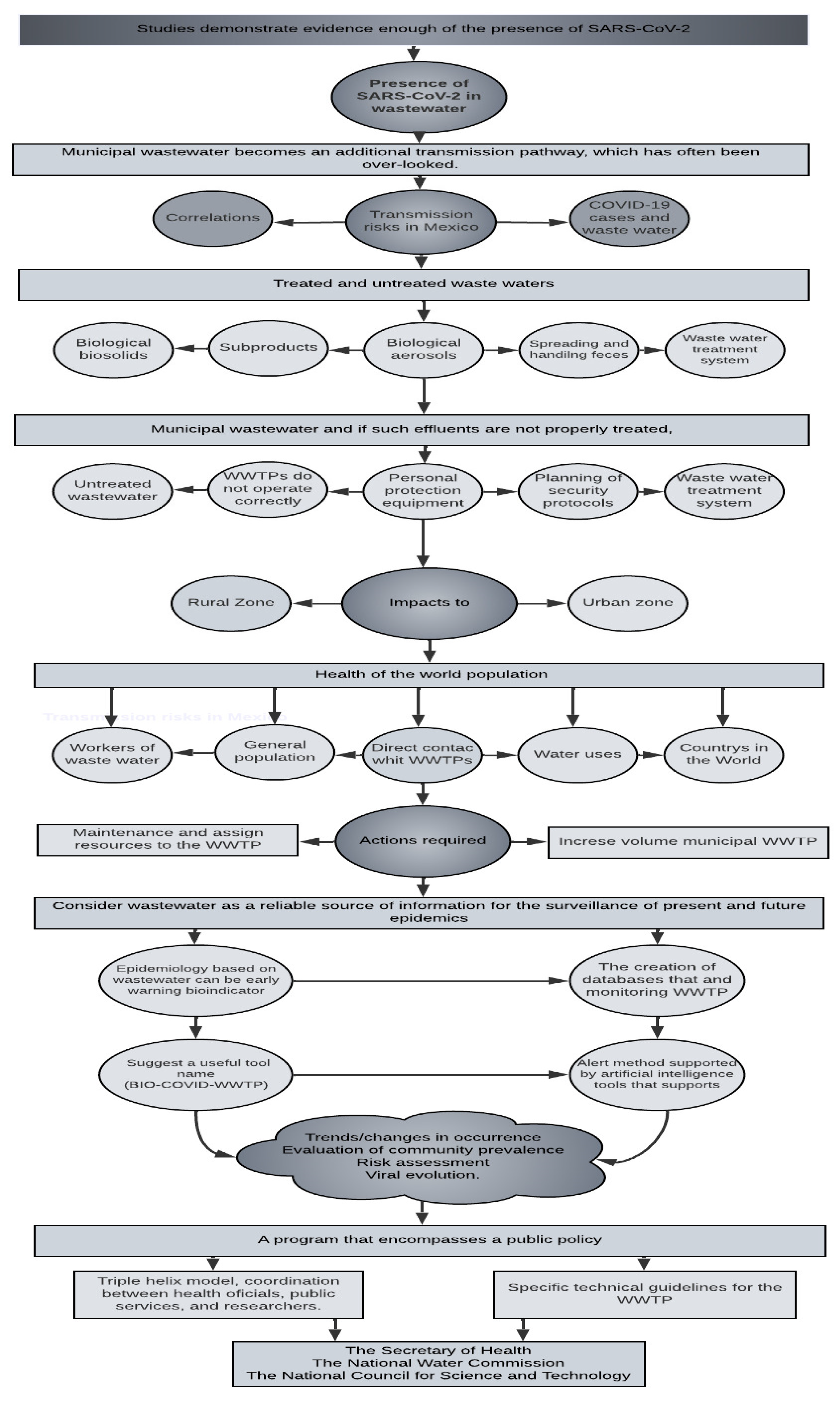
4. Conclusions
- (1)
- SARS-CoV-2 is present in municipal wastewater, and if such effluents are not properly treated, the virus can reach the receiving aquatic bodies. Thus, municipal wastewater becomes an additional transmission pathway, which has often been overlooked.
- (2)
- In the rapid review of scientific articles, where it was classified by country and the degree of development, to date, nothing similar was found, and this aspect is forceful: “SARS-CoV-2 despite the economic status of the countries, even the virus is incorporated, regardless of the economic potential of the country, where it would be assumed that its treatment systems are robust and modern”.
- (3)
- Although a correlation was found between the variables of %TWW by state and positive cases of COVID-19 in Mexico, the cause–effect relationship should be considered with caution since wastewater is not the main route of transmission of SARS-CoV-2. However, it should serve to emphasize the importance of increasing the level of wastewater treatment to reduce exposure of the population and contamination of drinking water sources.
- (4)
- Further confirmatory studies of fecal–air, fecal–oral, and fecal–nasal transmission or by sewage, by inhalation of fecal particles with the presence of viable viruses in the form of aerosols, and by the presence of the virus in receiving water bodies are still necessary. On the other hand, it is conclusive in the review carried out that these poorly investigated pathways may constitute a potential source of transmission.
5. Recommendations
6. Future Lines of Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Travaglio, M.; Yu, Y.; Popovic, R.; Selley, L.; Leal, N.S.; Martins, L.M. Links between air pollution and COVID-19 in England. Environ. Pollut. 2021, 268, 115859. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Singh, R.P. Decline in PM2. 5 concentrations over major cities around the world associated with COVID-19. Environ. Res. 2020, 187, 109634. [Google Scholar] [CrossRef] [PubMed]
- Farkas, K.; Hillary, L.S.; Malham, S.K.; McDonald, J.E.; Jones, D.L. Wastewater and public health: The potential of wastewater surveillance for monitoring COVID-19. Curr. Opin. Environ. Sci. Health 2020, 17, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Casanova, L.; Rutala, W.A.; Weber, D.J.; Sobsey, M.D. Survival of surrogate coronaviruses in water. Water Res. 2009, 43, 1893–1898. [Google Scholar] [CrossRef]
- Fernández-Vargas, G. Water governance as an integrating framework for the fulfillment of the sustainable development goals clean in Latin America. Rev. Udcaactual. Divulg. Cient. 2020, 23, e1561. Available online: http://www.scielo.org.co/scielo.php?pid=S0123-42262020000200022&script=sci_abstract&tlng=en (accessed on 3 July 2021).
- Ramos-Alvariño, C. Behavior of health and ecotoxicological indicators of wastewater with drug traces. Cuba. Rev. Chem. 2013, 25, 180–205. Available online: https://www.redalyc.org/articulo.oa?id=443543735008 (accessed on 3 July 2021).
- Lahrich, S.; Laghrib, F.; Farahi, A.; Bakasse, M.; Saqrane, S.; Mhammedi, E.M.A. Review on COVID-19 virus contamination of wastewater: Impact and treatment. Rev. Int. Contam. Environ. 2020, 751, 142325. Available online: https://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S1135-57272005000200012 (accessed on 3 July 2021). [CrossRef]
- National Water Comission. Numeragua México 2018. Available online: http://sina.conagua.gob.mx/sina/index.php?publicaciones=126 (accessed on 30 December 2020).
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/table (accessed on 20 June 2022).
- Johns Hopkins Coronavirus Resource Center, JHCRC. Mortality in the Most Affected Countries 2021b. Available online: https://coronavirus.jhu.edu/data/mortality (accessed on 4 May 2021).
- Garg, S.; Kim, L.; Whitaker, M.; O’Halloran, A.; Cummings, C.; Holstein, R.; Fry, A. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019-COVID- NET, 14 States, 1–30 March 2020. MMWR Morb Mortal. Wkly. Rep. 2020, 69, 458–464. [Google Scholar] [CrossRef]
- With Water. National Inventory of Municipal Potabilization and Wastewater Treatment Plants in Operation. 2018. Available online: https://www.gob.mx/cms/uploads/attachment/file/563375/Inventario_2018.pdf (accessed on 3 July 2021).
- World Health Organization. Laboratory Testing for 2019 Novel Coronavirus (2019-nCoV) in Suspected Human Cases: Interim Guidance, 14 January 2020 (No. WHO/2019-nCoV/laboratory/2020.2); World Health Organization: Geneva, Switzerland, 2020; Available online: https://apps.who.int/iris/bitstream/handle/10665/332300/WHO-2019-nCoV-laboratory-2020.2-eng.pdf (accessed on 3 July 2021).
- Kniffin, K.M.; Narayanan, J.; Anseel, F.; Antonakis, J.; Ashford, S.P.; Bakker, A.B.; Vugt, M.V. COVID-19 and the workplace: Implications, issues, and insights for future research and action. Am. Psychol. 2021, 76, 63. [Google Scholar] [CrossRef]
- Baz, E.S.; Imziln, B. Can Aerosols and Wastewater be Considered as Potential Transmissional Sources of COVID-19 to Humans? Eur. J. Public Health 2020, 4, em0047. [Google Scholar] [CrossRef]
- Rabi, F.A.; Zoubi, A.M.S.; Kasasbeh, G.A.; Salameh, D.M.; Al-Nasser, A.D. SARS-CoV-2 and coronavirus disease 2019: What we know so far. Pathogens 2020, 9, 231. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Zhang, Y.; Kong, D.; Li, S.; Yang, N. The effects of social support on sleep quality of medical staff treating patients with coronavirus disease 2019 (COVID-19) in January and February 2020 in China. Clin. Exp. Med. Res. 2020, 26, e923549-1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ling, H.; Huang, X.; Li, J.; Li, W.; Yi, C.; Qu, J. Potential spreading risks and disinfection challenges of medical wastewater by the presence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) viral RNA in septic tanks of Fangcang Hospital. Sci. Total Environ. 2020, 741, 140445. [Google Scholar] [CrossRef] [PubMed]
- Quilliam, R.S.; Weidmann, M.; Moresco, V.; Purshouse, H.; O’Hara, Z.; Oliver, D.M. COVID-19: The environmental implications of shedding SARS-CoV-2 in human faeces. Environ. Int. 2020, 140, 105790. [Google Scholar] [CrossRef] [PubMed]
- Treacy, J. Drinking Water Treatment and Challenges in Developing Countries. The relevance of Hygiene to Health in Developing Countries 2019. Available online: https://bit.ly/3MYtkDL (accessed on 3 July 2021).
- Heller, L.; Mota, C.R.; Greco, D.B. COVID-19 faecal-oral transmission: Are we asking the right questions? Sci. Total Environ. 2020, 729, 138919. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, R.; Kassiri, H. A brief review on the possible role of houseflies and cockroaches in the mechanical transmission of coronavirus disease 2019 (COVID-19). Arch. Clin. Infect. Dis. 2020, 15, e102863. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.W.; Li, J.; Guo, T.; Zhen, B.; Kong, Q.; Yi, B.; Li, Z.; Song, N.; Jin, M.; Xiao, W.; et al. Concentration and detection of SARS coronavirus in sewage from Xiao Tang Shan Hospital and the 309th Hospital of the Chinese People’s Liberation Army. Water Sci. Technol. Water Supply 2005, 52, 213–221. [Google Scholar] [CrossRef]
- Wang, J.; Feng, H.; Zhang, S.; Ni, Z.; Ni, L.; Chen, Y.; Qu, T. SARS-CoV-2 RNA detection of hospital isolation wards hygiene monitoring during the Coronavirus Disease 2019 outbreak in a Chinese hospital. Int. J. Infect. Dis. 2020, 94, 103–106. [Google Scholar] [CrossRef]
- Lodder, W.; de Roda Husman, A.M. SARS-CoV-2 in wastewater: Potential health risk, but also data source. Lancet Gastroenterol. Hepatol. 2020, 5, 533–534. [Google Scholar] [CrossRef]
- Núñez-Delgado, A. What do we know about the SARS-CoV-2 coronavirus in the environment? Sci. Total Environ. 2020, 727, 138647. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, J.; Xiao, A.; Gu, X.; Lee, W.L.; Armas, F.; Alm, J.E. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. Msystems 2020, 5, e00614-20. [Google Scholar] [CrossRef] [PubMed]
- Aquino González, S.D.; Moyano Villafuerte, S.J. Evaluation of Sars-CoV-2 in Two Wastewater Treatment Plants in the Provinces of Guayas and Santa Elena. Bachelor′s Thesis, Faculty of Chemical Engineering, University of Guayaquil, Ecuador, Guayaquil, 2020. Available online: https://doi.org/repositorio.ug.edu.ec/handle/redug/51092 (accessed on 3 July 2021).
- Medema, G.; Heijnen, L.; Elsinga, G.; Italiaander, R.; Brouwer, A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environment. Sci. Technol. Lett. 2020, 7, 511–516. [Google Scholar] [CrossRef]
- Westhaus, S.; Weber, F.A.; Schiwy, S.; Linnemann, V.; Brinkmann, M.; Widera, M.; Ciesek, S. Detection of SARS-CoV-2 in raw and treated wastewater in Germany–suitability for COVID-19 surveillance and potential transmission risks. Sci. Total Environ. 2021, 751, 141750. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Angel, N.; Edson, J.; Bibby, K.; Bivins, A.; O’Brien, J.W.; Mueller, J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: A proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020, 728, 138764. [Google Scholar] [CrossRef] [PubMed]
- Wurtzer, S.; Marechal, V.; Mouchel, J.M.; Moulin, L. Time course quantitative detection of SARS-CoV-2 in Parisian wastewaters correlates with COVID-19 confirmed cases. MedRxiv 2020. [Google Scholar] [CrossRef]
- Hasan, S.W.; Ibrahim, Y.; Daou, M.; Kannout, H.; Jan, N.; Lopes, A.; Yousef, A.F. Detection and quantification of SARS-CoV-2 RNA in wastewater and treated effluents: Surveillance of COVID-19 epidemic in the United Arab Emirates. Sci. Total Environ. 2021, 764, 142929. [Google Scholar] [CrossRef]
- Haramoto, E.; Malla, B.; Thakali, O.; Kitajima, M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020, 737, 140405. [Google Scholar] [CrossRef]
- Hata, A.; Honda, R.; Hara-Yamamura, H.; Meuchi, Y. Detection of SARS-CoV-2 in wastewater in Japan by multiple molecular assays-implication for wastewater-based epidemiology (WBE). MedRxiv 2020. [Google Scholar] [CrossRef]
- Kujawski, S.A.; Wong, K.K.; Collins, J.P.; Epstein, L.; Killerby, M.E.; Midgley, C.M.; Stoecker, W. First 12 patients with coronavirus disease 2019 (COVID-19) in the United States. MedRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Randazzo, W.; Cuevas-Ferrando, E.; Sanjuán, R.; Domingo-Calap, P.; Sánchez, G. Metropolitan wastewater analysis for COVID-19 epidemiological surveillance. Int. J. Hyg. Environ. Health. 2020, 230, 113621. [Google Scholar] [CrossRef]
- Randazzo, W.; Truchado, P.; Cuevas-Ferrando, E.; Simón, P.; Allende, A.; Sánchez, G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020, 181, 115942. [Google Scholar] [CrossRef] [PubMed]
- Balboa, S.; Mauricio-Iglesias, M.; Rodríguez, S.; Martínez-Lamas, L.; Vasallo, F.J.; Regueiro, B.; Lema, J.M. The fate of SARS-CoV-2 in wastewater treatment plants points out the sludge line as a suitable spot for incidence monitoring. MedRxiv 2020. [Google Scholar] [CrossRef]
- Bar-Or, I.; Yaniv, K.; Shagan, M.; Ozer, E.; Erster, O.; Mendelson, E.; Kushmaro, A. Regressing SARS-CoV-2 sewage measurements onto COVID-19 burden in the population: A proof-of-concept for quantitative environmental surveillance. MedRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, G.; Iaconelli, M.; Mancini, P.; Ferraro, G.B.; Veneri, C.; Bonadonna, L.; Suffredini, E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020, 736, 139652. [Google Scholar] [CrossRef]
- Carrillo-Reyes, J.; Barragán-Trinidad, M.; Buitrón, G. Surveillance of SARS-CoV-2 in sewage and wastewater treatment plants in Mexico. J. Water Process. Eng. 2021, 40, 101815. [Google Scholar] [CrossRef]
- Kocamemi, B.A.; Kurt, H.; Sait, A.; Sarac, F.; Saatci, A.M.; Pakdemirli, B. SARS-CoV-2 detection in Istanbul wastewater treatment plant sludges. Medrxiv 2020. [Google Scholar] [CrossRef]
- Guerrero-Latorre, L.; Ballesteros, I.; Villacrés-Granda, I.; Granda, M.G.; Freire-Paspuel, B.; Ríos-Touma, B. SARS-CoV-2 in river water: Implications in low sanitation countries. Sci. Total Environ. 2020, 743, 140832. [Google Scholar] [CrossRef]
- Bhowmick, G.D.; Dhar, D.; Nath, D.; Ghangrekar, M.M.; Banerjee, R.; Das, S.; Chatterjee, J. Coronavirus disease 2019 (COVID-19) outbreak: Some serious consequences with urban and rural water cycle. NPJ Clean Water 2020, 3, 1–8. [Google Scholar] [CrossRef]
- Han, J.; He, S. Urban flooding events pose risks of virus spread during the novel coronavirus (COVID-19) pandemic. Sci. Total Environ. 2021, 755, 142491. [Google Scholar] [CrossRef]
- Paleologos, E.K.; O’Kelly, B.C.; Tang, C.S.; Cornell, K.; Rodríguez-Chueca, J.; Abuel-Naga, H.; Singh, D.N. Post Covid-19 water and waste water management to protect public health and geoenvironment. Environ. Geotech. 2020, 40, 1–15. [Google Scholar] [CrossRef]
- O’Kelly, B.C. Sewage sludge to landfill: Some pertinent engineering properties. J. Air Waste Manag. Assoc. 2005, 55, 765–771. [Google Scholar] [CrossRef] [PubMed]
- O’Kelly, B.C.; Oettle, N.K.; Ramos, J.A. Geotechnical properties of compacted biosolids for monofill design, As-Samra, Jordan. Environ. Geotech. 2018, 7, 404–434. [Google Scholar] [CrossRef] [Green Version]
- Babatunde, A.O.; Zhao, Y.Q. Constructive approaches toward water treatment works sludge management: An international review of beneficial reuses. Critical Reviews in Environment. Sci. Technol. 2007, 37, 129–164. [Google Scholar] [CrossRef]
- Fei, X.; Zekkos, D.; Li, L.; Woods, R.; Sanford, L. Geo-characterization of lime water treatment sludge. Environ. Geotech. 2017, 4, 209–219. [Google Scholar] [CrossRef]
- O’Kelly, B.C. Effect of biodegradation on the consolidation properties of a dewatered municipal sewage sludge. J. Waste Manag. 2008, 28, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Shahin, M.S.; Sangani, M.M.M.; Faghihinezhad, M.; Baghdadi, M. Wastewater aerosols produced during flushing toilets, WWTPs, and irrigation with reclaimed municipal wastewater as indirect exposure to SARS-CoV-2. J. Environ. Chem. Eng. 2021, 9, 106201. [Google Scholar] [CrossRef]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef] [Green Version]
- Amoah, I.D.; Kumari, S.; Bux, F. Coronaviruses in wastewater processes: Source, fate and potential risks. Environ. Int. 2020, 143, 105962. [Google Scholar] [CrossRef]
- Dada, A.C.; Gyawali, P. Quantitative microbial risk assessment (QMRA) of occupational exposure to SARS-CoV-2 in wastewater treatment plants. Sci. Total Environ. 2021, 763, 142989. [Google Scholar] [CrossRef]
- Tellier, R.; Li, Y.; Cowling, B.J.; Tang, J.W. Recognition of aerosol transmission of infectious agents: A commentary. BMC Mean. 2019, 19, 1–9. [Google Scholar] [CrossRef]
- Adhikari, U.; Chabrelie, A.; Weir, M.; Boehnke, K.; McKenzie, E.; Ikner, L.; Mitchell, J. A case study evaluating the risk of infection from Middle Eastern Respiratory Syndrome Coronavirus (MERS-CoV) in a Hospital Setting through Bioaerosols. Risk Anal. 2019, 39, 2608–2624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Read, B.U. Minimum sizes of respiratory particles carrying sars-cov-2 and the possibility of aerosol generation. Int. J. Environ. Res. Public Health 2020, 17, 6960. [Google Scholar] [CrossRef]
- Brisolara, K.F.; Maal-Bared, R.; Sobsey, M.D.; Reimers, R.S.; Rubin, A.; Bastian, R.K.; Brown, S. Assessing and managing SARS-CoV-2 occupational health risk to workers handling residuals and biosolids. Sci. Total Environ. 2021, 774, 145732. [Google Scholar] [CrossRef]
- Zaneti, R.N.; Girardi, V.; Spilki, F.R.; Mena, K.; Westphalen, A.P.C.; da Costa Colares, E.R.; Etchepare, R.G. QMRA of SARS-CoV-2 for workers in wastewater treatment plants. MedRxiv 2020, 20116277. [Google Scholar] [CrossRef]
- Cruz-Cruz, C.; Rodríguez-Dozal, S.; Cortez-Lugo, M.; Ovilla-Muñoz, M.; Carnalla-Cortés, M.; Sánchez-Pájaro, A.; Schilmann, A. Quick review: Monitoring the presence and infectivity of the SARS-CoV-2 virus and others coronavirus in wastewater. Salud Publica Mex. 2021, 63, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Tenorio-Chávez, P.; Cerro-López, M.; Castro-Pastrana, L.I.; Ramírez-Rodrigues, M.M.; Orozco-Hernández, J.M.; Gómez-Oliván, L.M. Effects of effluent from a hospital in Mexico on the embryonic development of zebrafish, Danio rerio. Sci. Total Environ. 2020, 727, 138716. [Google Scholar] [CrossRef]
- Pérez-Alvarez, I.; Islas-Flores, H.; Gómez-Oliván, L.M.; Barceló, D.; De Alda, M.L.; Solsona, S.P.; Galar-Martínez, M. Determination of metals and pharmaceutical compounds released in hospital wastewater from Toluca, Mexico, and evaluation of their toxic impact. Environ. Pollut. 2018, 240, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Tabla-Vázquez, C.G.; Chávez-Mejía, A.C.; Ledesma, M.T.O.; Ramírez-Zamora, R.M. Wastewater Treatment in Mexico. In Water Resources of Mexico. World Water Resources; Springer: Cham, Switzerland, 2020; pp. 133–155. [Google Scholar] [CrossRef]
- National Water Comission. Numeragua México 2017. Available online: http://sina.conagua.gob.mx/publicaciones/Numeragua_2017.pdf (accessed on 26 December 2020).
- Mahlknecht, J.; González-Bravo, R.; Loge, F.J. Water-energy-food security: A Nexus perspective of the current situation in Latin America and the Caribbean. Energy 2020, 194, 116824. [Google Scholar] [CrossRef]
- Maya Rodríguez, J.M.; Pineda Pablos, N. Advances, stagnation and limitations of sanitation policy in Mexico 1998–2014. Entreciencias: Diálogos en la Sociedad del Conocimiento 2018, 6, 35–50. [Google Scholar] [CrossRef]
- Hernández-Salazar, A.B.; Moreno-Seceña, J.C.; Sandoval-Herazo, L.C. Industrial wastewater treatment in Mexico: An approach to its current situation and challenges to be addressed. Renderesu 2018, 2, 75–87. Available online: http://www.rinderesu.com/index.php/rinderesu/article/view/27/33 (accessed on 3 July 2021).
- Boehm, A.B.; Silverman, A.I.; Schriewer, A.; Goodwin, K. Systematic review and meta-analysis of decay rates of waterborne mammalian viruses and coliphages in surface waters. Water Res. 2019, 164, 114898. [Google Scholar] [CrossRef] [PubMed]
- Tennant, B.J.; Gaskell, R.M.; Gaskell, C.J. Studies on the survival of canine coronaviruses under different environmental conditions. Vet. Microbiol. 1994, 42, 255–259. [Google Scholar] [CrossRef]
- Nannou, C.; Ofrydopoulou, A.; Evgenidou, E.; Heath, D.; Heath, E.; Lambropoulou, D. Antiviral drugs in aquatic environment and wastewater treatment plants: A review on occurrence, fate, removal and ecotoxicity. Sci. Total Environ. 2020, 699, 134322. [Google Scholar] [CrossRef] [PubMed]
- Wurtzer, S.; Marechal, V.; Mouchel, J.-M.; Moulin, L.; Metis, U.M.R.; Atelier, Z. Quantitative detection of the time course of SARS-CoV-2 in Parisian wastewater correlates with confirmed cases of COVID-(2020). MedRxiv 2020, in press. [Google Scholar]
- Rimoldi, S.G.; Stefani, F.; Gigantiello, A.; Polesello, S.; Commander, F.; Miletus, D.; Maresca, M.; Longobardi, C.; Mancon, A.; Romeri, F.; et al. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020, 744, 140911. [Google Scholar] [CrossRef]
- Weidhaas, J.; Aanderud, Z.T.; Roper, D.K.; VanDerslice, J.; Gaddis, E.B.; Ostermiller, J.; Hoffman, K.; Jamal, R.; Heck, P.; Zhang, Y.; et al. Correlation of SARS-CoV-2 RNA in wastewater with COVID-19 disease burden in sewersheds. Sci. Total Environ. 2021, 775, 145790. [Google Scholar] [CrossRef]
- Jiménez, B.; Asano, T. Water Reuse: An International Survey of Current Practice, Problems and Needs; IWA Publishing: London, UK, 2015; Volume 7, Available online: http://hdl.handle.net/10045/118411 (accessed on 3 July 2021)ISBN 9781780401881. [CrossRef]
- Mazari, M.; Loyola, A. Water problems and politics. In Environmental Agenda 2018. Leticia Merino Pérez and Alejandro Velázquez Montes (Coords.). Seminar (SUSMAI). UNAM 2018. Available online: https://www.jornada.com.mx/2020/06/20/delcampo/articulos/fallas-estructurales.html (accessed on 3 July 2021).
- Amirian, E.S. Potential fecal transmission of SARS-CoV-2: Current evidence and implications for public health. Int. J. Infect. Dis. 2020, 95, 363–370. [Google Scholar] [CrossRef]
- Verbyla, M.E.; Mihelcic, J.R. A review of virus removal in wastewater treatment pond systems. Water Res. 2015, 71, 107–124. [Google Scholar] [CrossRef]
- Chaudhry, R.M.; Nelson, K.L.; Drewes, J.E. Mechanisms of pathogenic virus removal in a full-scale membrane bioreactor. Environ. Sci. Technol. 2015, 49, 2815–2822. [Google Scholar] [CrossRef]
- Kitagawa, H.; Nomura, T.; Nazmul, T.; Omori, K.; Shigemoto, N.; Sakaguchi, T.; Ohge, H. Effectiveness of 222-nm ultraviolet light on disinfecting SARS-CoV-2 surface contamination. Am. J. Infect. Control 2021, 49, 299–301. [Google Scholar] [CrossRef]
- Kumari, A.; Maurya, N.S.; Tiwari, B. Hospital wastewater treatment scenario around the globe. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 549–570. [Google Scholar] [CrossRef]
- Exner, M.; Kramer, A.; Lajoie, L.; Gebel, J.; Engelhart, S.; Hartemann, P. Prevention and control of waterborne infections associated with health care in healthcare settings. health. Am. J. Epidemiol. Infect. Control. 2005, 33, S26–S40. [Google Scholar] [CrossRef] [PubMed]
- González, M.I.; Chiroles, S. Safe use and microbiological risks of residual water for agriculture. Rev. Cubana Salud Pública 2011, 37, 61–73. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0864-34662011000100007 (accessed on 3 July 2021).
- Xiao, F.; Tang, M.; Zheng, X.; Liu, Y.; Li, X.; Shan, H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 2020, 158, 1831–1833. Available online: https://www.gastrojournal.org/article/S0016-5085(20)30282-1/fulltext (accessed on 3 July 2021). [CrossRef] [PubMed]
- Xiao, F.; Sun, J.; Xu, Y.; Li, F.; Huang, X.; Li, H.; Zhao, J. Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerg. Infect. Dis. 2020, 26, 1920. [Google Scholar] [CrossRef]
- Barreto Torrella, S. Covid-19 and wastewater. Cuba. J. Trop. Med. 2020, 72. Available online: http://www.revmedtropical.sld.cu/index.php/medtropical/article/view/563 (accessed on 3 July 2021).
- Roldan Torres, J.; Luengo Schreck, T. Challenges and opportunities in the water sector during and after the COVID-19 pandemic. Impluvim 2021, 14. Available online: http://www.agua.unam.mx/assets/pdfs/impluvium/numero14.pdf (accessed on 3 July 2021).
- La Rosa, G.; Bonadonna, L.; Lucentini, L.; Kenmoe, S.; Suffredini, E. Coronavirus in water environments: Occurrence, persistence and concentration methods-A scoping review. Water Res. 2020, 179, 115899. [Google Scholar] [CrossRef]
- Kitajima, M.; Ahmed, W.; Bibby, K.; Carducci, A.; Gerba, C.P.; Hamilton, K.A.; Rose, J.B. SARS-CoV-2 in wastewater: State of the knowledge and research needs. Sci. Total Environ. 2020, 739, 139076. [Google Scholar] [CrossRef]
- Daughton, C.G. Wastewater surveillance for population-wide Covid-19: The present and future. Sci. Total Environ. 2020, 736, 139631. [Google Scholar] [CrossRef]
- Aguiar-Oliveira, M.D.L.; Campos, A.; Matos, A.R.; Rigotto, C.; Sotero-Martins, A.; Teixeira, P.F.; Siqueira, M.M. Wastewater-Based Epidemiology (WBE) and Viral Detection in Polluted Surface Water: A Valuable Tool for COVID-19 Surveillance—A Brief Review. Int. J. Environ. Res. Public Health 2020, 17, 9251. [Google Scholar] [CrossRef]
- Basani, M. Wastewater: The Great Ally in the Fight against COVID-19. Back to the Source 2021. Available online: https://doi.org/10.22201/iingen.0718378xe.2020.13.3.77049 (accessed on 3 July 2021).
- Romero, C. COVID-19 reminders about water in Mexico. IAGUA 2020. Available online: https://www.iagua.es/blogs/claudia-elvira-romero-herrera/recordatorios-covid-19-agua-mexico-1 (accessed on 3 July 2021).
- Malenovská, H. Coronavirus persistence on a plastic carrier under refrigeration conditions and its reduction using wet wiping technique, with respect to food safety. Food Environ. Virol. 2020, 12, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, B.; Touret, F.; Gilles, M.; de Lamballerie, X.; Charrel, R. Prolonged infectivity of SARS-CoV-2 in fomites. Emerg. Infect. Dis. 2020, 26, 2256–2257. [Google Scholar] [CrossRef] [PubMed]
- Netz, R.R.; Eaton, W.A. Physics of virus transmission by speaking droplets. Proc. Natl. Acad. Sci. USA 2020, 117, 25209–25211. [Google Scholar] [CrossRef]
- Smith, S.H.; Somsen, G.A.; van Rijn, C.; Kooij, S.; van der Hoek, L.; Bem, R.A.; Bonn, D. Aerosol persistence in relation to possible transmission of SARS-CoV-2. Phys. Fluids 2020, 32, 107108. [Google Scholar] [CrossRef]
- Tang, S.; Mao, Y.; Jones, R.M.; Tan, Q.; Ji, J.S.; Li, N.; Shen, J.; Lv, Y.; Pan, L.; Ding, P.; et al. Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environ. Int. 2020, 144, 106039. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Du, G. COVID-19 may transmit through aerosol. Ir. J. Med. Sci. 2020. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Marchal, T.; Sperry, T.; Yi, H. Influence of wind and relative humidity on the social distancing effectiveness to prevent COVID-19 airborne transmission: A numerical study. J. Aerosol Sci. 2020, 147, 105585. [Google Scholar] [CrossRef]
- Lednicky, J.A.; Lauzardo, M.; Fan, Z.H.; Jutla, A.; Tilly, T.B.; Gangwar, M.; Usmani, M.; Shankar, S.N.; Mohamed, K.; Eiguren-Fernandez, A.; et al. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int. J. Infect. Dis. 2020, 100, 476–482. [Google Scholar] [CrossRef]
- Fears, A.C.; Klimstra, W.B.; Duprex, P.; Hartman, A.; Weaver, S.C.; Plante, K.S.; Mirchandani, D.; Plante, J.A.; Aguilar, P.V.; Fernández, D.; et al. Persistence of severe acute respiratory syndrome coronavirus 2 in aerosol suspensions. Emerg. Infect. Dis. 2020, 26, 2168–2171. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Morgan, B.; Donner, E.; Short, M.D. The COVID-19 pandemic: Considerations for the waste and wastewater services sector. Case Stud. Chem. Environ. Eng. 2020, 1, 100006. [Google Scholar] [CrossRef]
- Carosso, A.; Cosma, S.; Benedetto, C. Vaginal delivery in COVID-19 pregnant women: Anorectum as a potential alternative route of SARS-CoV-2 transmission. Am. J. Obstet. Gynecol. 2020, 223. [Google Scholar] [CrossRef] [PubMed]
- Carosso, A.; Cosma, S.; Borella, F.; Marozio, L.; Coscia, A.; Ghisetti, V.; di Perri, G.; Benedetto, C. Pre-labor anorectal swab for SARS-CoV-2 in COVID-19 pregnant patients: Is it time to think about it? Eur. J. Obstet. Gynecol. Play Biol. 2020, 249, 99. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Jin, M.; Bao, P.; Zhao, W.; Zhang, S. Clinical characteristics and results of semen tests among men with Coronavirus Disease 2019. JAMA Netw. Open 2020, 3, e208292. [Google Scholar] [CrossRef]
- Patrì, A.; Gallo, L.; Guarino, M.; Fabbrocini, G. Sexual transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): A new possible route of infection? J. Am. Acad. Dermatol. 2020, 82, e227. [Google Scholar] [CrossRef]
- Ali, M.; Zaid, M.; Saqib, M.A.N.; Ahmed, H.; Afzal, M.S. SARS-CoV-2 and the hidden carriers: Sewage, feline, and blood transfusion. J. Med. Virol. 2020, 92, 2291–2292. [Google Scholar] [CrossRef]
- di Maria, F.; Beccaloni, E.; Bonadonna, L.; Cini, C.; Confalonieri, E.; la Rosa, G.; Milana, M.R.; Testai, E.; Scaini, F. Minimization of spreading of SARS-CoV-2 via household waste produced by subjects affected by COVID-19 or in quarantine. Sci. Total Environ. 2020, 743, 140803. [Google Scholar] [CrossRef]
- Groß, R.; Conzelmann, C.; Müller, J.A.; Stenger, S.; Steinhart, K.; Kirchhoff, F.; Münch, J. Detection of SARS-CoV-2 in human breastmilk. Lancet 2020, 395, 1757–1758. [Google Scholar] [CrossRef]
- Franklin, A.B.; Bevins, S.N. Spillover of SARS-CoV-2 into novel wild hosts in North America: A conceptual model for perpetuation of the pathogen. Sci. Total Environ. 2020, 733, 139358. [Google Scholar] [CrossRef]
- Sit, T.H.C.; Brackman, C.J.; Ip, S.M.; Tam, K.W.; Law, P.Y.; To, E.M.; Yu, V.Y.T.; Sims, L.D.; Tsang, D.N.C.; Chu, D.K.W.; et al. Infection of dogs with SARS-CoV-2. Nature 2020, 586, 1–6. [Google Scholar] [CrossRef]
- Newman, A. First Reported Cases of SARS-CoV-2 Infection in Companion Animals. New York, March-April 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 710–713. [Google Scholar] [CrossRef]
- Oreshkova, N.; Molenaar, R.J.; Vreman, S.; Harders, F.; Munnink, B.B.O.; Honing, R.W.H.; Gerhards, N.; Tolsma, P.; Bouwstra, R.; Sikkema, R.S.; et al. SARS-CoV2 infection in farmed mink, Netherlands, April 2020 (provisional article). BioRxiv 2020, 25, 2001005. [Google Scholar] [CrossRef]
- Considerations Related to Public Health and Social Measures in the Workplace in the Context of COVID-19: Annex to Considerations Regarding Adjustments to Public Health and Social Measures in the Context of COVID-19, 10 May 2020; World Health Organization: Geneva, Switzerland, 2020; Available online: https://apps.who.int/iris/handle/10665/332084 (accessed on 26 February 2022).
- Government of Mexico. Technical Guidelines for Health Safety in the Work Environment. Technical Guidelines for Health Safety in the Work Environment, 25 February 2020. Available online: https://nuevanormalidad.gob.mx/ (accessed on 26 February 2022).
- Geller, C.; Varbanov, M.; Duval, R.E. Human Coronaviruses: Insights into Environmental Resistance and Its Influence on the Development of Novel Antiseptic Strategies. Virus 2012, 4, 3044–3068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, D.; Verma, S.; Verma, P.; Mahanty, B.; Dutta, K.; Daverey, A.; Arunachalam, K. SARS-CoV-2 in wastewater: Challenges for developing countries. Int. J. Hyg. Environ. Health 2021, 231, 113634. [Google Scholar] [CrossRef]
- Ahmed, W.; Bertsch, P.M.; Bibby, K.; Haramoto, E.; Hewitt, J.; Huygens, F.; Gyawali, P.; Korajkic, A.; Riddell, S.; Sherchan, S.P.; et al. Decay of SARS-CoV-2 and surrogate murine hepatitis virus RNA in untreated wastewater to inform application in wastewater-based epidemiology. Environ. Res. 2020, 191, 110092. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.A.; Yaniv, K.; Bar-Zeev, E.; Chaudhury, S.; Shagan, M.; Lakkakula, S.; Ronen, Z.; Kushmaro, A.; Nir, O. Tracking SARS-CoV-2 RNA through the wastewater treatment process. ACS EST Water 2021, 1, 1161–1167. [Google Scholar] [CrossRef]
- Arora, S.; Nag, A.; Sethi, J.; Rajvanshi, J.; Saxena, S.; Shrivastava, S.K.; Gupta, A.B. Sewage surveillance for the presence of SARS-CoV-2 genome as a useful wastewater based epidemiology (WBE) tracking tool in India. Water Sci. Technol. 2020, 82, 2823–2836. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, L.; Deng, Q.; Zhang, G.; Wu, K.; Ni, L.; Yang, Y.; Liu, B.; Wang, W.; Wei, C.; et al. The presence of SARS-CoV-2 RNA in the stool of patients with COVID-19. J. Med. Virol. 2020, 92, 833–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Xu, Y.; Gao, R.; Lu, R.; Han, K.; Wu, G.; Tan, W. Detection of SARS-CoV-2 in different types of clinical samples. JAMA 2020, 323, 1843–1844. [Google Scholar] [CrossRef] [Green Version]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virologic evaluation of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Guo, C.; Tang, L.; Hong, Z.; Zhou, J.; Dong, X.; Yin, H.; Xiao, Q.; Tang, Y.; Qu, X.; et al. Prolonged presence of SARS-CoV-2 viral RNA in fecal samples. Lancet Gastroenterol. Hepatol. 2020, 5, 434–435. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Zhu, B.; Liang, H.; Fang, C.; Gong, Y.; Guo, Q.; Sun, X.; Zhao, D.; Shen, J.; et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence of persistent fecal viral shedding. Nat. Med. 2020, 26, 502–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foladori, P.; Cutrupi, F.; Segata, N.; Manara, S.; Pinto, F.; Malpei, F.; Bruni, L.; La Rosa, G. SARS-CoV-2 from feces to wastewater treatment: What do we know? A review. Sci. Total Environ. 2020, 743, 140444. [Google Scholar] [CrossRef] [PubMed]
- Kozak, S.; Petterson, S.; McAlister, T.; Jennison, I.; Bagraith, S.; Roiko, A. Utility of QMRA to compare health risks associated with alternative urban sewer overflow management strategies. J. Environ. Manag. 2020, 262, 110309. [Google Scholar] [CrossRef] [PubMed]
- Carducci, A.; Donzelli, G.; Cioni, L.; Federigi, I.; Lombardi, R.; Verani, M. Quantitative microbial risk assessment for workers exposed to bioaerosol in wastewater treatment plants aimed at the choice and setup of safety measures. Int. J. Environ. Res. Public Health 2018, 15, 1490–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Economic Status | Country | Type of Water | Technique | >SARS-CoV-2 Concentration | Reference |
|---|---|---|---|---|---|
| Developed | Netherlands | Untreated wastewater | RT-qPCR | 26–1800 gc/mL. | [30] |
| Germany | Untreated wastewater | RT-qPCR | 30.0 and 20.0 gc/mL inflow. 3.0 and 20 gc/mL effluent. | [31] | |
| United States of America | Untreated wastewater | RT-qPCR | 57 to 303 gc/mL. | [27] | |
| Australia | Untreated wastewater | RT-qPCR | 1.9 to 12 gc/100 mL. | [32] | |
| France | Untreated wastewater | RT-qPCR | 106 eq/L gc/L. | [33] | |
| United Arab Emirates | Wastewater | RT-qPCR | Wastewater influents: 7.50 × 102 and 3.40 × 104 cg/L, Untreated wastewater: 7.50 × 102 to over 3.40 × 104 gc/L | [34] | |
| China | Untreated wastewater | RT-qPCR | (14.7 ± 2.2) × 103 and (7.5 ± 2.8) × 103 gc/L in the effluents. | [18] | |
| Japanese | Untreated wastewater | RT-qPCR | Influent (4.0 × 103–8.2 × 104 cg/L), treated wastewater (1.4 × 102–2.5 × 103 cg/L). | [35] | |
| Japanese | Untreated wastewater | RT-qPCR | 1.2 × 103–4.4 × 103 gc/L. | [36] | |
| United States America | Untreated wastewater | RT-qPCR | 3.0 × 104 gc/L. | [37] | |
| Emerging | Spain | Untreated wastewater | RT-qPCR | Of 5.22 and 5.99 log10 gc/L. | [38] |
| Spain | Untreated wastewater | RT-qPCR | 5.4 ± 0.2 log10 gc/L on average. | [38] | |
| Spain | Untreated wastewater | RT-qPCR | 9 gc/mL rising to more than 20 gc/mL. | [39] | |
| Israel | Untreated wastewater | RT-qPCR | Ct of 33 to 33.6. | [40] | |
| Italy | Untreated wastewater | RT-qPCR | 50% of the samples showed positive. | [41] | |
| Underdeveloped | Mexico | Untreated wastewater | RT-qPCR | From 0.12 to 4 and 0.37–73 gc/mL. | [42] |
| Turkey | Untreated wastewater | RT-qPCR | 1.17 × 104 y 4.02 × 104 gc/L. | [43] | |
| Ecuador | Urban streams with low sanitation | RT-qPCR | 2.84 × 105 to 3.19 × 106 and 2.07 × 105 to 2.23 × 106 gc/L. | [44] | |
| Ecuador | Lagoon systems | PCR | In GEN N1 36.44, GEN N2 38.99; GEN N1 36.80 GEN N2 38.72. | [28] |
| Economic Status | Confirmed Cases | World Ranking Confirmed Cases | Country | Cases Number/100,000 Inhabitants’ Ratio |
|---|---|---|---|---|
| Developed | 8,118,400 | 15 | Netherlands | 10,754 |
| 27,124,689 | 5 * | Germany | 4542 | |
| 85,007,630 | 1 * | United States of America | 10,577 | |
| 7,719,719 | 16 | Australia | 137 | |
| 29,114,200 | 4 * | France | 9286 | |
| 921,566 | 52 | United Arab Emirates | 6931 | |
| 4,127,625 | 29 | China | 8 | |
| 9,108,323 | 14 | Japan | 756 | |
| 12,551,142 | 11 | Spain | 9556 | |
| Emerging | 4,216,009 | 27 | Israel | 10,224 |
| 17,773,764 | 9 * | Italy | 7316 | |
| 5,843,190 | 21 | Mexico | 2219 | |
| Underdeveloped | 15,085,742 | 10 * | Turkey | 6872 |
| 891,064 | 56 | Ecuador | 2764 |
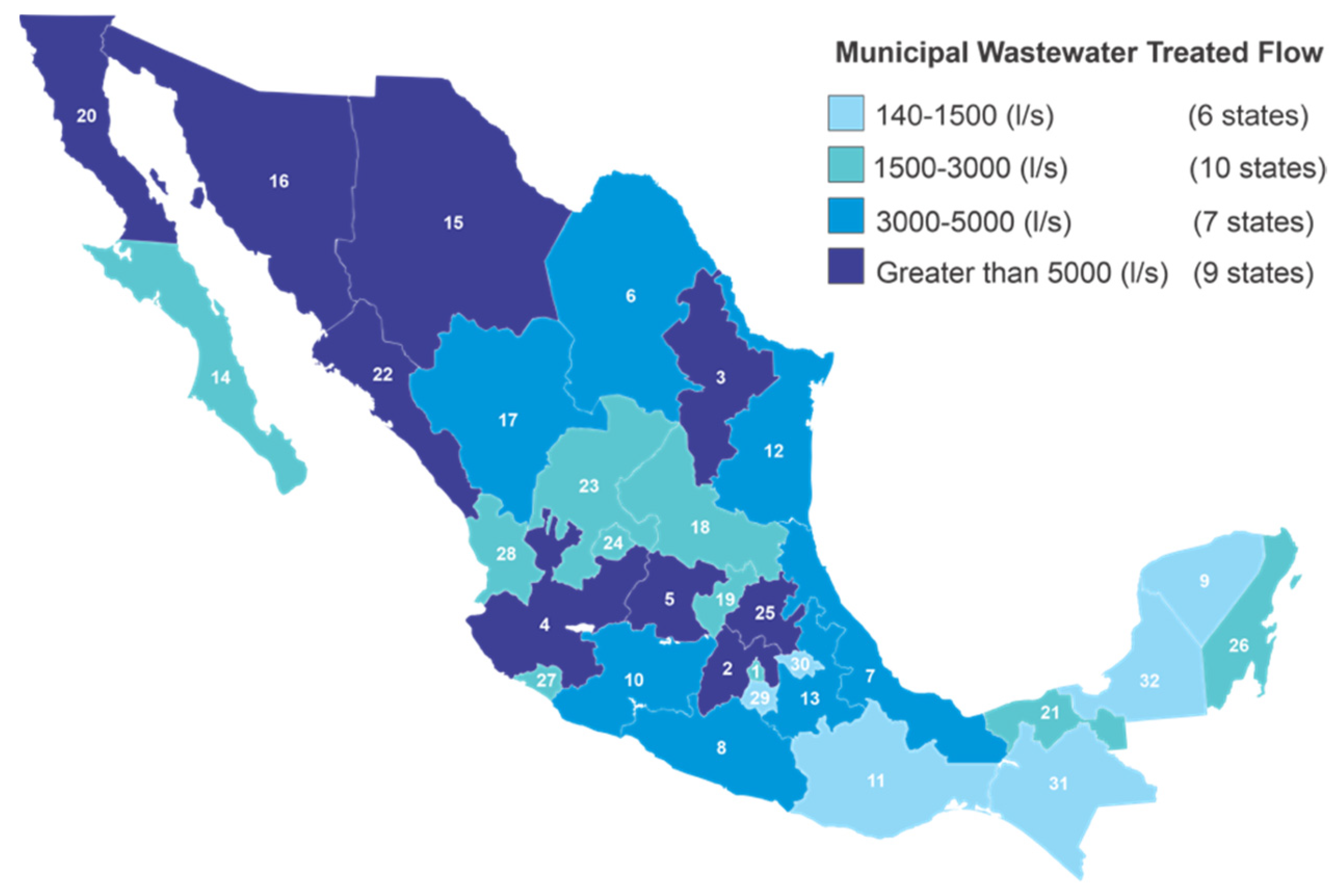
| No. | States | Accumulated Positive Cases | Estimated Assets | No. Plants | Installed Capacity (L−1/s) (to) | Treated Flow (L−1/s) (b) | % Treated (a)/(b) |
|---|---|---|---|---|---|---|---|
| 1 | Ciudad de México | 621,287 | 9156 | 29 | 5604.50 | 2451.50 | 43.74 |
| 2 | Estado de México | 237,961 | 2526 | 131 | 9744.70 | 6400.10 | 65.68 |
| 3 | Guanajuato | 129,001 | 774 | 64 | 7560.80 | 5221.20 | 69.06 |
| 4 | Nuevo León | 120,840 | 721 | 55 | 16,157.00 | 12,590.40 | 77.93 |
| 5 | Jalisco | 83,685 | 660 | 122 | 15,245.20 | 12,346.20 | 80.98 |
| 6 | Puebla | 80,504 | 974 | 85 | 3516.90 | 3592.50 | 102.15 |
| 7 | Sonora | 71,456 | 540 | 109 | 7394.10 | 6115.90 | 82.71 |
| 8 | Coahuila | 67,231 | 253 | 26 | 5680.00 | 4516.00 | 79.51 |
| 9 | Queretaro | 66,253 | 1072 | 51 | 2449.40 | 1892.40 | 77.26 |
| 10 | Tabasco | 62,195 | 885 | 99 | 2969.90 | 2665.00 | 89.73 |
| 11 | San Luis Potosi | 61,150 | 572 | 40 | 2572.70 | 2101.00 | 81.67 |
| 12 | Veracruz | 58,559 | 391 | 108 | 7014.80 | 4711.90 | 67.17 |
| 13 | Tamaulipas | 55,239 | 352 | 47 | 7369.20 | 4096.40 | 55.59 |
| 14 | Chihuahua | 48,596 | 1099 | 185 | 10,263.10 | 7031.70 | 68.51 |
| 15 | Baja California | 46,969 | 280 | Four. Five | 7882.60 | 5977.80 | 75.84 |
| 16 | Michoacan | 45,936 | 410 | 46 | 4145.50 | 3175.40 | 76.6 |
| 17 | Oaxaca | 44,639 | 373 | 76 | 1817.60 | 1291.20 | 71.04 |
| 18 | Guerrero | 38,373 | 500 | 67 | 4428.30 | 3755.50 | 84.81 |
| 19 | Hidalgo | 37,259 | 409 | 56 | 23,826.80 | 22,133.90 | 92.89 |
| 22 | Sinaloa | 36,821 | 406 | 279 | 6496.70 | 5837.20 | 89.85 |
| 21 | Yucatan | 35,856 | 576 | 28 | 448.70 | 231.50 | 51.59 |
| 22 | Durango | 32,765 | 355 | 220 | 4638.70 | 3496.10 | 75.37 |
| 23 | Morelos | 30,996 | 459 | 52 | 2769.70 | 1276.40 | 46.08 |
| 24 | Zacatecas | 29,300 | 252 | 65 | 2012.40 | 1616.00 | 80.3 |
| 25 | Baja California Sur | 29,081 | 616 | 31 | 2051.30 | 1626.50 | 79.29 |
| 26 | Aguascalientes | 25,341 | 273 | 135 | 4840.00 | 2982.70 | 61.63 |
| 27 | Quintana Roo | 21,783 | 405 | 31 | 2685.00 | 1780.20 | 66.3 |
| 28 | Tlaxcala | 18,954 | 197 | 55 | 1481.80 | 1049.60 | 70.83 |
| 29 | Nayarit | 114,283 | 137 | 70 | 3493.80 | 2510.30 | 71.85 |
| 30 | Colima | 7601 | 102 | 82 | 2434.90 | 1739.80 | 71.45 |
| 31 | Chiapas | 6574 | 99 | 3. 4 | 2001.20 | 1343.60 | 67.14 |
| 32 | Campeche | 6016 | 67 | 17 | 155.00 | 142.80 | 92.13 |
| 2,372,504 | 25,891 | 2540 | 181,152 | 137,699 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herazo, M.S.; Nani, G.; Zurita, F.; Nakase, C.; Zamora, S.; Herazo, L.C.S.; Betanzo-Torres, E.A. A Review of the Presence of SARS-CoV-2 in Wastewater: Transmission Risks in Mexico. Int. J. Environ. Res. Public Health 2022, 19, 8354. https://doi.org/10.3390/ijerph19148354
Herazo MS, Nani G, Zurita F, Nakase C, Zamora S, Herazo LCS, Betanzo-Torres EA. A Review of the Presence of SARS-CoV-2 in Wastewater: Transmission Risks in Mexico. International Journal of Environmental Research and Public Health. 2022; 19(14):8354. https://doi.org/10.3390/ijerph19148354
Chicago/Turabian StyleHerazo, Mayerlin Sandoval, Graciela Nani, Florentina Zurita, Carlos Nakase, Sergio Zamora, Luis Carlos Sandoval Herazo, and Erick Arturo Betanzo-Torres. 2022. "A Review of the Presence of SARS-CoV-2 in Wastewater: Transmission Risks in Mexico" International Journal of Environmental Research and Public Health 19, no. 14: 8354. https://doi.org/10.3390/ijerph19148354
APA StyleHerazo, M. S., Nani, G., Zurita, F., Nakase, C., Zamora, S., Herazo, L. C. S., & Betanzo-Torres, E. A. (2022). A Review of the Presence of SARS-CoV-2 in Wastewater: Transmission Risks in Mexico. International Journal of Environmental Research and Public Health, 19(14), 8354. https://doi.org/10.3390/ijerph19148354











