Impact of Environmental Radiation on the Incidence of Cancer and Birth Defects in Regions with High Natural Radioactivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Analyses
2.2. Calculation of Effective Dose
- CRn is the radon concentration in the air (Bq m−3);
- DCF is the dose conversion factor for inhaled 222Rn and equal to 9 nSv Bq−1 h−1 m3 [38];
- FRn is the attached fraction of Rn progeny and assumed as 0.8;
- OF is the occupation factor, and made equal to 0.4 for indoor and outdoor occupation;
- T is exposure time (8760 h y−1).
2.3. Public Health Data Analysis
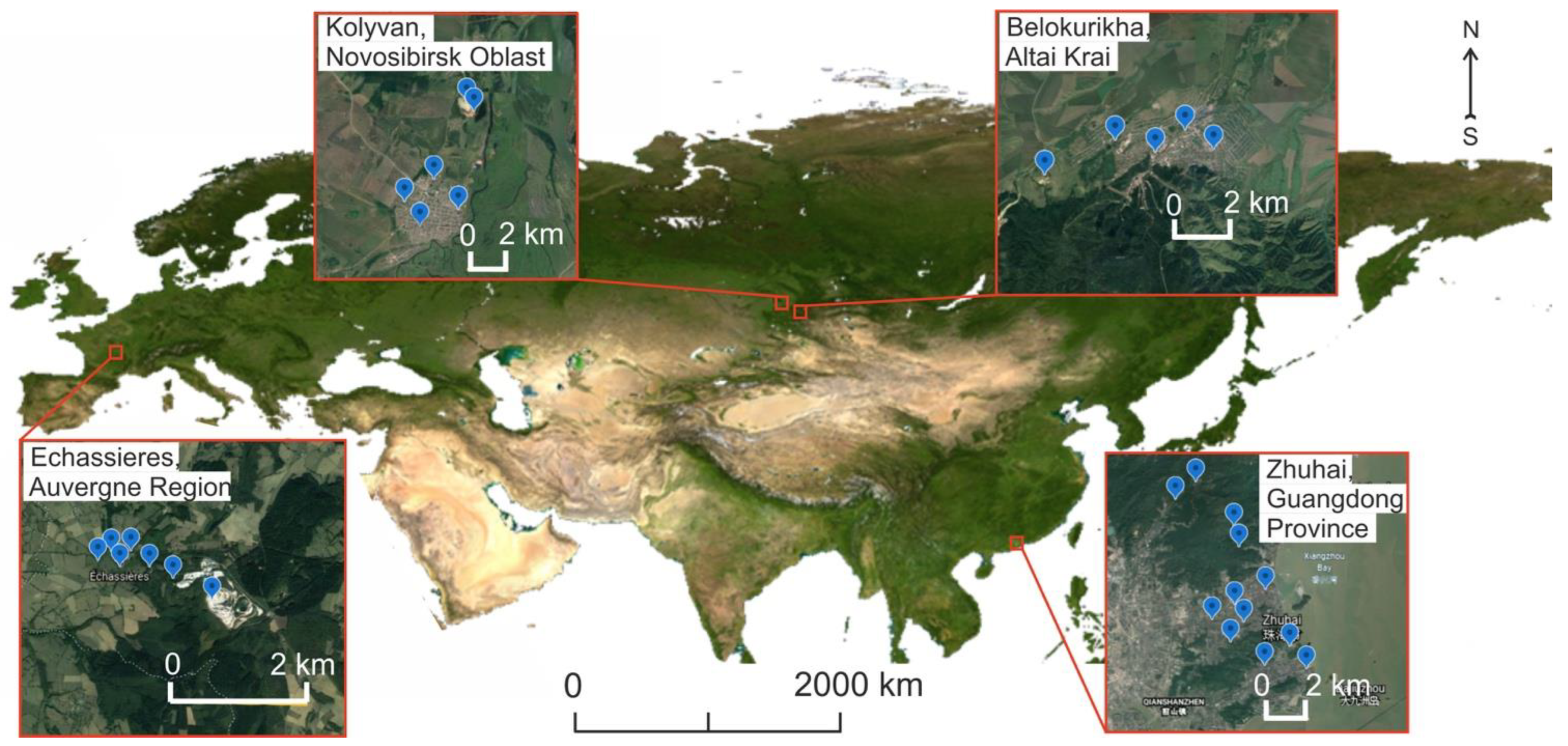
2.4. Description of the Geology of the Study Regions
2.4.1. Belokurikha, Altai Region, Russia
2.4.2. Kolyvan, Novosibirsk Region, Russia
2.4.3. Zhuhai, Guangdong Province, China
2.4.4. Echassières, Auvergne Region, France
3. Results
3.1. Geochemical Characteristics
3.2. Radiological Characteristics

3.3. Effective Doses
3.4. Analysis of Statistical Data on Disease Incidence Rates
3.4.1. Belokurikha, Altai Region, Russia
3.4.2. Kolyvan, Novosibirsk Region, Russia
3.4.3. Zhuhai, Guangdong Province, China
3.4.4. Echassières, Auvergne Region, France
3.5. Analysis of the Dependence of Morbidity Rates on Radioecological Parameters
4. Data Uncertainties and Limitations
5. Conclusions
- Finally, clarifying whether there is a need to implement radiation protection measures in HBRAs to protect the public health;
- Learning from epidemiological studies in HBRAs in order to extrapolate lessons into the planning of remediation measures of areas affected by human activities, such as uranium mining areas and areas contaminated by nuclear accidents;
- Learning more about the effects of low doses in order to apply knowledge in the radiological risk assessment of medical exposures and of prolonged radiation exposures during space missions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zlobina, A.N.; Rikhvanov, L.P.; Baranovskaya, N.V.; Wang, N.; Farkhutdinov, I.M. Distribution of Radioactive and Rare-Earth Elements in Ferralsols of the Guangdong Province (China). Eurasian. Soil. Sci. 2019, 52, 644–653. [Google Scholar] [CrossRef]
- Roslyakov, N.A.; Zhmodik, S.M.; Pakhomov, V.G. Natural radionuclides in the geological environment of the Novosibirsk region. Radioactivity and radioactive elements in the human habitat. In Proceedings of the IV International Conference, Tomsk, Russia, 13–19 December 2013; pp. 461–464. [Google Scholar]
- Wang, N.; Xiao, L.; Li, C.; Huang, Y.; Pei, S.; Liu, S.; Xie, F.; Cheng, Y. Determination of Radioactivity Level of 238U, 232Th and 40K in Surface Medium in Zhuhai City by in-situ Gamma-ray Spectrometry. J. Nucl. Sci. Technol. 2005, 42, 888–896. [Google Scholar] [CrossRef]
- Hendry, J.H.; Simon, S.L.; Wojcik, A.; Sohrabi, M.; Burkart, W.; Cardis, E.; Laurier, D.; Tirmarche, M.; Hayata, I. Human exposure to high natural background radiation: What can it teach us about radiation risks? J. Radiol. Prot. J. Soc. Radiol. Prot. 2009, 29, A29–A42. [Google Scholar] [CrossRef]
- Aliyu, A.S.; Ramli, A.T. The world’s high background natural radiation areas (HBNRAs) revisited: A broad overview of the dosimetric, epidemiological and radiobiological issues. Radiat. Meas. 2015, 73, 51–59. [Google Scholar] [CrossRef]
- UNSCEAR: United Nations. Sources and Effects of Ionizing Radiation; Volume I: Sources; Volume II: Effects. United Nations Scientific Committee on the Effects of Atomic Radiation, 2000 Report to the General Assembly, with scientific annexes; United Nations: New York, NY, USA, 2000.
- National Research Council. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation; National Research Council: Washington, DC, USA, 2006; 424p. [CrossRef]
- UNSCEAR. Sources and Effects of Ionizing Radiation. United Nations Scientific Committee on the Effects of Atomic Radiation, 1993 Report to the General Assembly, with Scientific Annexes; UNSCEAR 1993 Report, United Nations sales publication E.94.IX.2; United Nations: New York, NY, USA, 1993.
- UNSCEAR. Effects of Ionizing Radiation. Volume I: Report to the General Assembly, Scientific Annexes A and B. United Nations Scientific Committee on the Effects of Atomic Radiation; UNSCEAR 2006 Report, United Nations sales publication E.08.IX.6; United Nations: New York, NY, USA, 2008.
- UNSCEAR. Effects of Ionizing Radiation. Volume II: Scientific Annexes C, D and E. United Nations Scientific Committee on the Effects of Atomic Radiation; UNSCEAR 2006 Report, United Nations sales publication E.09.IX.5; United Nations: New York, NY, USA, 2009.
- UNSCEAR. Sources and Effects of Ionizing Radiation. Volume I: Sources: Report to the General Assembly, Scientific Annexes A and B. United Nations Scientific Committee on the Effects of Atomic Radiation; UNSCEAR 2008 Report, United Nations Sales Publication E.10.XI.3; United Nations: New York, NY, USA, 2010.
- UNSCEAR. Report of the United Nations Scientific Committee on the Effects of Atomic Radiation 2010. Fifty-Seventh Session, Includes Scientific Report: Summary of Low-Dose Radiation Effects on Health. United Nations Scientific Committee on the Effects of Atomic Radiation; UNSCEAR 2010 Report, United Nations Sales Publication M.II.IX.4; United Nations: New York, NY, USA, 2011.
- UNSCEAR. Sources, Effects and Risks of Ionizing Radiation UNSCEAR 2017. Report to the General Assembly Scientific Annexes A and B; United Nations Publication, No. E.18.IX.1; United Nations: New York, NY, USA, 2018.
- Sharma, N.; Singh, J. Radiological and Chemical Risk Assessment due to High Uranium Contents Observed in the Ground Waters of Mansa District (Malwa Region) of Punjab State, India: An Area of High Cancer Incidence. Expo. Health 2016, 8, 513–525. [Google Scholar] [CrossRef]
- Shrivastava, B.K. Elevated Uranium and Toxic Elements Concentration in Groundwater in Punjab State of India: Extent of the Problem and Risk Due to Consumption of Unsafe Drinking Water. Water Qual. Expo. Health 2015, 7, 407–421. [Google Scholar] [CrossRef]
- Hayata, I.; Wang, C.; Zhang, W.; Chen, D.; Minamihisamatsu, M.; Morishima, H.; Yuan, Y.; Wei, L.; Sugahara, T. Chromosome Translocation in Residents of the High Background Radiation Areas in Southern China. J. Radiat. Res. 2000, 41, 69–74. [Google Scholar] [CrossRef]
- Kochupillai, N.; Verma, I.C.; Grewal, M.S.; Ramalingaswami, V. Down’s syndrome and related abnormalities in an area of high background radiation in coastal Kerala. Nature 1976, 262, 60–61. [Google Scholar] [CrossRef]
- Bolviken, B. Ecological analysis: Nasopharyngeal carcinoma and multiple sclerosis versus radioactive elements. In Natural Ionizing Radiation and Health. Proceedings from a Symposium Held at the Norwegian Academy of Science and Letters, Oslo; The Norwegian Academy of Science and Letters: Oslo, Norway, 2001; pp. 126–134. [Google Scholar]
- Henshaw, D.L.; Allen, J.E. Is indoor radon linked to leukaemia in children and adults?—A review of the evidence. In Natural Ionizing Radiation and Health. Proceedings from a Symposium Held at the Norwegian Academy of Science and Letters, Oslo 6–7 June 2001; The Norwegian Academy of Science and Letters: Oslo, Norway, 2002. [Google Scholar]
- Ruano-Ravina, A.; Aragones, N.; Kelsey, K.T.; Perez-Rios, M.; Pineiro-Lamas, M.; Lopez-Abente, G.; Barros-Dios, J.M. Residential radon exposure and brain cancer: An ecological study in a radon prone area (Galicia, Spain). Sci. Rep. 2017, 7, 2–7. [Google Scholar]
- Rodriguez-Martinez, A.; Torres-Durin, M.; Barros-Dios, J.M.; Ruano-Ravina, A. Residential radon and small cell lung cancer. A systematic review. Cancer Lett. 2018, 426, 57–62. [Google Scholar] [CrossRef]
- Darby, S.; Hill, D.; Auvinen, A.; Barros-Dios, J.M.; Baysson, H.; Bochicchio, F.; Deo, H.; Falk, R.; Forastiere, F.; Hakama, M.; et al. Radon in homes and risk of lung cancer: Collaborative analysis of individual data from 13 European case-control studies. Br. Med. J. 2005, 330, 223–228. [Google Scholar] [CrossRef]
- Jaikrishan, G.; Sudheer, K.R.; Andrews, V.J.; Koya, P.K.M.; Madhusoodhanan, M.; Jagadeesan, C.K.; Seshadri, M. Study of stillbirth and major congenital anomaly among newborns in the high-level natural radiation areas of Kerala, India. J. Community Genet. 2013, 4, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, Z.; Goldberg, Z. Radiation-induced effects in unirradiated cells: A review and implications in cancer. Int. J. Oncol. 2002, 21, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyński, L.; Fornalski, K.W.; Feinendegen, L.E. The human cancer in high natural background radiation areas. Int. J. Low Radiat. 2015, 10, 143–154. [Google Scholar] [CrossRef]
- Geetha, A.C.; Sreedharan, H. Review on studies in high background radiation areas (HBRAs) of various parts of the world. Int. J. Adv. Res. Biol. Sci. 2016, 3, 163–169. [Google Scholar]
- Nambi, K.S.V.; Soman, S.D. Environmental radiation and cancer in India. Health Phys. 1987, 52, 653–657. [Google Scholar] [CrossRef]
- Wei, L.; Sugahara, T.; Tao, Z. High background radiation area. High Levels Nat. Radiat. 1997, 58–59, 63–66. [Google Scholar]
- David, E.; Wolfson, M.; Fraifeld, V.E. Background radiation impacts human longevity and cancer mortality: Reconsidering the linear no-threshold paradigm. Biogerontology 2021, 22, 189–195. [Google Scholar] [CrossRef]
- Ali, Y.F.; Cucinotta, F.A.; Ning-Ang, L.; Zhou, G. Cancer Risk of Low Dose Ionizing Radiation. Front. Phys. 2020, 8, 234. [Google Scholar] [CrossRef]
- Sohrabi, M. World high background natural radiation areas: Need to protect public from radiation exposure. Radiat. Meas. 2013, 50, 166–171. [Google Scholar] [CrossRef]
- Mortazavi, S.M.J.; Ghiassi-Nejad, M.; Karam, P.A.; Ikushima, T.; Niroomand-Rad, A.; Cameron, J.R. Cancer incidence in areas with elevated levels of natural radiation. Int. J. Low Radiat. 2006, 2, 20–27. [Google Scholar] [CrossRef]
- Hajo, Z.; Ferid, S. WHO Handbook on Indoor Radon: A Public Health Perspective; World Health Organization: Geneva, Switzerland, 2009; Available online: https://apps.who.int/iris/bitstream/handle/10665/44149/9789241547673_eng.pdf?sequence=1 (accessed on 7 May 2022).
- EU. European Council Directive 2013/59/Euratom on basic safety standards for protection against the dangers arising from exposure to ionising radiation and repealing Directives 89/618/Euratom, 90/641/Euratom, 96/29/Euratom, 97/43/Euratom and 2003/122/Euratom. Off. J. Eur. Union. 2013, 57, 1–73. [Google Scholar]
- Hygienic Standards SP 2.6.1.758-99; Radiation Safety Standards (NRB-99). Ministry of Health: Moscow, Russia, 1999.
- Carvalho, F.P.; Oliveira, J.M.; Malta, M. Intake of Radionuclides with the Diet in Uranium Mining Areas. Procedia Earth Planet. Sci. 2014, 8, 43–47. [Google Scholar] [CrossRef][Green Version]
- Van, D.N.; Dinh, T.D.; Duc, N.D.; Carvalho, F.P.; Van, T.D.; Hao, Q.N. Radiation exposure in a region with natural high background radiation originated from rare earth element deposits at Bat Xat district, Vietnam. Radiat. Environ. Biophys. 2022, 61, 309–324. [Google Scholar]
- IAEA. Radiation Protection and Safety of Radiation Sources: International Basic Safety Standards; International Atomic Energy Agency: Vienna, Austria, 2014; Volume 441. [Google Scholar]
- ICRP Statement on Radon International Commission on Radiological Protection; ICRP Ref 00/902/09; ICRP: Oxford, UK, 2009.
- Preston, R.J. The LNT model is the best we can do-today. J. Radiol. Prot. 2003, 23, 263. [Google Scholar] [CrossRef]
- State Standard 17.4.1.03–83; Nature Protection. Soil. General Requirements for Sampling. Standartinform Publisher: Moscow, Russia, 2008; 54p.
- Karelin, V.A. Identification of Radionuclides by Gamma-Spectrometry. Guidelines for Laboratory Work; TPU: Tomsk, Russia, 2012; 25p. [Google Scholar]
- MU 2.6.1.037-2015; Methodical Instructions. Determination of Average Annual Values of Equivalent Equilibrium Volume Activity of Radon Isotopes in Indoor Air by the Results of Measurements of Different Duration. FMBA Alfa-Print: Moscow, Russia, 2016; 48p.
- ISO 11665-11: 2016; Measurement of Radioactivity in the Environment—Air: Radon-222—Part 11: Test Method for Soil Gas with Sampling at Dept. ISO/TC 85/SC 2 Radiological Protection. International Organization for Standardization: Geneva, Switzerland, 2016; 25p.
- Paschenko, I. Report the State of Sanitary and Epidemiological Welfare of the Population in the Altai Region in 2016”. FBUZ “Center of Hygiene and Epidemiology in the Altai Region; RITTER: Moscow, Russia, 2017; 255p. [Google Scholar]
- Pashchenko, I.; Gubareva, T.; Ushakov, A.; Karpova, N.; Katunina, S. Social—Sanitary Passport on Congenital Malformations in Children (fetus) in the Altai Region (Based on Social and Hygienic Passport for 1997–2016); Newsletter RITTER: Barnaul, Russia, 2017; 129p. [Google Scholar]
- Popova, A. State Report “The State of Sanitary and Epidemiological Welfare of the Population in the Novosibirsk Region in 2014”; Alfa-Port: Novosibirsk, Russia, 2015; 226p. [Google Scholar]
- Meng, R.; Wei, K.; Xia, L.; Xu, Y.; Chen, W.; Zheng, R.; Lin, L. Cancer incidence and mortality in Guangdong province, 2012. Chin. J. Cancer Res. 2016, 28, 311–320. [Google Scholar] [CrossRef]
- Jemal, A.; Vineis, P.P.; Bray, F.; Torre, L.; Forman, D. The Cancer Atlas, 2nd ed.; American Cancer Society: Atlanta, GA, USA, 2014; 136p. [Google Scholar]
- Pépin, P. Le cancer en Auvergne. In Bulletin de Veille Sanitaire № 30; BVC: Clermont-Ferrand, France, 2015; 19p. [Google Scholar]
- Isfan, F.; Blouin, P.P.; Gembara, P.P.; Piguet, C.; Chazal, J.; Lumley, L.; Demeocq, F.; Kanold, J. Incidence et survie des cancers de l’enfant en Auvergne-Limousin, France, 1986–2003. Bull. Epidemiol. Hebd. 2007, 14, 16–119. [Google Scholar]
- Chatignoux, É.; Pépin, P. Atlas de la Mortalité par Cancer en Ile-de-France 2000–2007; Observatoire régional de santé d’Ile-de-France, BVC: Clermont-Ferrand, France, 2012; 140p. [Google Scholar]
- Demikova, N.S.; Lapina, A.S.; Podolnaya, M.A.; Kobrinsky, B.A. Dynamics of the frequency of congenital malformations in the Russian Federation (according to the Federal database of monitoring of the CP congenital malformations for 2006–2012). Russ. J. Perinatol. Pediatr. 2015, 2, 72–77. [Google Scholar]
- Kaprin, A.D.; Starinsky, V.V.; Petrova, G.V.; Herzen, P.A. Malignant Neoplasms in Russia in 2016 (Morbidity and Mortality); MNIO—Branch of FGBU “NMITS Radiology” of the Ministry of Health of Russia: Moscow, Russia, 2018; 250p. [Google Scholar]
- Hoffman, J.I.; Kaplan, S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002, 19, 1890–1900. [Google Scholar] [CrossRef]
- Salem, G.; Libbey, J. Atlas De La Sante En France. Volume 2. Comportements Et Maladies; John Libbey Eurotext: Montrouge, France, 2006; 220p. [Google Scholar]
- Tang, L.L.; Chen, W.Q.; Xue, W.Q. Global trends in incidence and mortality of nasopharyngeal carcinoma. Cancer Lett. 2016, 374, 22–30. [Google Scholar] [CrossRef]
- Ho, C.S. Beating “Guangdong cancer”: A review and update on nasopharyngeal cancer. Hong Kong Med. J. 2017, 23, 497–502. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qu, Y.; Liu, X.; Zhuang, J.; Chen, G.; Mai, J.; Guo, X.; Ou, Y.; Chen, J.; Gong, W.; Gao, X.; et al. Incidence of Congenital Heart Disease: The 9-Year Experience of the Guangdong Registry of Congenital Heart Disease, China. PLoS ONE 2016, 11, e0159257. [Google Scholar] [CrossRef]
- Chen, W.; Sun, K.; Zheng, R.; Zeng, H.; Zhang, S.; Xia, C.; Yang, Z.; Li, H.; Zou, X.; He, J. Cancer incidence and mortality in China, 2014. Chin. J. Cancer Res. 2018, 30, 1–12. [Google Scholar] [CrossRef]
- Ma, J.; Lei, Y.; Ye, T. Study on the ecological association between natural radioactivity and childhood leukemia in Guangdong province. China J. Epidemiol. 2008, 29, 343–345. [Google Scholar]
- Aksel, E.M.; Gorbacheva, I.A. Cancer morbidity and mortality of children in Russia and Former Soviet Republics in 2007. Vestnik RONC. N. N. Blokhin RAMS 2009, 20, 139–156. [Google Scholar]
- Tabakaeva, E.M. Petrological-Geochemical Criteria of Ore Content of Belokurikha Complex of Altai. Ph.D. Thesis, TPU Publisher, Tomsk, Russia, 2011. [Google Scholar]
- Kaznacheev, V.P.; Chernyavsky, E.F. Resort Belokurikha, 6th ed.; Publishing House SB RAS: Novosibirsk, Russia, 2011; 204p. [Google Scholar]
- Suslin, V.P. Atlas of Natural Radioactivity of the Territories of the Cities of Novosibirsk, Berdsk and Novosibirsk Region; Alfa-Port: Novosibirsk, Russia, 2017. [Google Scholar]
- Li, J.; Long, Y.; Lu, W. Discussion on the genesis of the ion-adsorption REE deposits in Jiangmen area, Guangdong Province. West-China Explor. Eng. 2005, 113, 101–103. [Google Scholar]
- Jin, Y.X.; Aijun, L.; Li, X.; Wu, Y.; Zhou, W.; Chen, Z. China’s ion-adsorption rare earth resources, mining consequences and preservation. Environ. Dev. 2013, 8, 131–136. [Google Scholar]
- Cuney, M.; Marignac, C.; Weisbrod, A. The Beauvoir Topaz-Lepidolite Albite Granite (Massif Central, France): The Disseminated Magmatic Sn-Li-Ta-Nb-Be Mineralization. Econ. Geol. 1992, 87, 1766–1794. [Google Scholar] [CrossRef]
- Grigoriev, N.A. Distribution of Chemical Elements in the Upper Part of the Continental Crust; Uro RAS: Yekaterinburg, Russia, 2009; 383p. [Google Scholar]
- Bowen, H.J.M. Trace Elements in Biochemistry; Academic Press: Cambridge, MA, USA, 1966; 248p. [Google Scholar]
- Zlobina, A.N.; Rikhvanov, L.P.; Baranovskaya, N.V.; Farkhutdinov, I.M.; Wang, N. Radioecological hazard for the population living in the regions with high radioactive granites. Bull. Tomsk. Polytech. Univ. Geo Assets Eng. 2019, 330, 111–125. [Google Scholar]
- Wang, N.; Peng, A.; Xiao, L.; Chu, X.; Yin, Y.; Qin, C.; Zheng, L. The level and distribution of 220Rn concentration in soil-gas in Guangdong province, China. Radiat. Prot. Dosim. 2012, 152, 204–209. [Google Scholar] [CrossRef]
- Pakhomov, V.G. Atlas of Radiation Situation in Novosibirsk; Funds GGP “Berezovgeologia”: Novosibirsk, Russia, 2003; 97p. [Google Scholar]
- Popov, Y.P. Zoning of Novosibirsk Region According to the Degree of Potential Danger of Radon and other Sources of Radiation on the Population; Funds GGP “Berezovgeologia”: Novosibirsk, Russia, 1994; 87p. [Google Scholar]
- Krupp, K.; Baskaran, M.; Brownlee, S.J. Radon emanation coefficients of several minerals: How they vary with physical and mineralogical properties. Am. Mineral. 2017, 102, 1375–1383. [Google Scholar] [CrossRef]
- Cinelli, G.; Capaccioni, B.; Hernández-Ceballos, M.A.; Mostacci, D.; Perghem, A.; Tositti, L. Radiological risk from thoron, a case study: The particularly radon-prone area of Bolsena, and the lesson learned. Radiat. Phys. Chem. 2015, 116, 381–385. [Google Scholar] [CrossRef]
- Chen, D.; Wang, X.; Guan, X. Radioelements concentration in groundwater and its effect on indoor radon in Zhuhai city. Environ. Chem. 2000, 19, 377–381. [Google Scholar]
- Billon, S.; Morin, S. French population exposure to radon, terrestrial gamma and cosmic rays. Radiat. Prot. Dosim. 2005, 113, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Balandovich, B.A.; Potseluev, N.Y.; Slutsky, A.R.; Swed, O.I. Hygienic assessment of radiation situation in the Altai territory and monitoring problem. Bull. Altai Sci. 2014, 2–3, 66–72. [Google Scholar]
- Kalinina, L. Practical Guidance “Engineering and Environmental Surveys for Construction”; Gosstroy of Russia, CPP GUP: Moscow, Russia, 2001; 20p. [Google Scholar]
- Diyun, C.; Xingbao, Y.; Ruiying, H. Indoor radon survey in indoor environments in Zhuhai city, China. Radiat. Meas. 2005, 39, 205–207. [Google Scholar] [CrossRef]
- Akiba, S.; Tokonami, S.; Bochicchio, F.; McLaughlin, J.; Tommasino, L.; Harley, N. Thoron: Its metrology, health effects and implications for radon epidemiology: A summary of roundtable discussions. Radiat. Prot. Dosim. 2010, 141, 477–481. [Google Scholar] [CrossRef]
- Tokonami, S. Characteristics of Thoron (220Rn) and Its Progeny in the Indoor Environment. Int. J. Environ. Res. Public Health 2020, 17, 8769. [Google Scholar] [CrossRef]
- World Health Organization. Nasopharynx. The Global Cancer Observatory—All Rights Reserved; International Agency for Research on Cancer: Lyon, France, 2019. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/4-Nasopharynx-fact-sheet.pdf (accessed on 7 May 2022).
- Carvalho, F.P.; Chambers, D.; Fesenko, S.; Moore, W.S.; Porcelli, D.; Vandenhove, H.; Yankovich, T. Environmental Pathways and Corresponding Models. In The Environmental Behaviour of Radium: Revised Edition; Technical Reports Series; International Atomic Energy Agency: Vienna, Austria, 2014; Volume 476, pp. 106–172. [Google Scholar]
- Carvalho, F.S.; Fernandes, S.; Fesenko, E.; Holm, B.; Howard, P.; Martin, M.; Phaneuf, D.; Porcelli, G.; Pröhl, J.; Twining. The Environmental Behaviour of Polonium; Technical Report Series; International Atomic Energy Agency: Vienna, Austria, 2017; Volume 484, p. 255. [Google Scholar]
- Carvalho, F.P.; Tufa, M.B.; Oliveira, J.M.; Malta, M. Radionuclides and Radiation Exposure in Tantalite Mining, Ethiopia. Arch. Env. Contam. Toxicol. 2021, 81, 648–659. [Google Scholar] [CrossRef]
- Nugraha, E.D.; Hosoda, M.; Kusdiana; Untara; Mellawati, J.; Nurokhim; Tamakuma, Y.; Ikram, A.; Syaifudin, M.; Yamada, R.; et al. Comprehensive exposure assessments from the viewpoint of health in a unique high natural background radiation area, Mamuju, Indonesia. Sci. Rep. 2021, 11, 14578. [Google Scholar] [CrossRef]


| Crystalline Granite Bedrock | Zone of Granite Disintegration | Granitic Sub-Soil | Clay | Soil | ||
|---|---|---|---|---|---|---|
| Belokurikha, Altai | U | 6.4 ± 0.5 | 9.7 ± 0.9 | 8.0 ± 0.8 | 11.4 ± 1.8 | 8.6 ± 0.9 |
| Th | 21.1 ± 3.8 | 36.5 ± 3.5 | 52.6 ± 4.4 | 58.8 ± 4.8 | 35.9 ± 3.8 | |
| Th/U | 3.3 | 4.4 | 6.5 | 5.1 | 4.2 | |
| Kolyvan, Novosibirsk | U | 9.6 ± 0.8 | 15.6 ± 1.3 | 10.2 ± 1.0 | 10.6 ± 1.1 | 4.9 ± 0.3 |
| Th | 34.0 ± 2.9 | 73.0 ± 5.9 | 47.1 ± 3.3 | 57.2 ± 4.6 | 14.9 ± 1.3 | |
| Th/U | 3.5 | 4.7 | 4.6 | 5.4 | 3.1 | |
| Zhuhai, Guangdong | U | 26.1 ± 3.1 | 12.4 ± 0.9 | 8.0 ± 0.3 | 8.5 ± 0.3 | 8.1 ± 0.7 |
| Th | 100 ± 9.5 | 50.4 ± 3.1 | 51.2 ± 4.6 | 53.4 ± 4.6 | 47.6 ± 2.7 | |
| Th/U | 3.8 | 4.1 | 6.4 | 6.2 | 5.9 | |
| Echassières, Auvergne | U | 18 [68] | n.d. | n.d. | n.d. | 6.5 ± 0.5 |
| Th | 1.7 [68] | n.d. | n.d. | n.d. | 4.6 ± 0.3 | |
| Th/U | 0.1 | n.d. | n.d. | n.d. | 0.7 | |
| Worldwide averages [69,70] | U | 3.9 | n.d. | n.d. | 4.3 | 1 |
| Th | 18 | n.d. | n.d. | 14 | 5 | |
| Th/U | 4.6 | n.d. | n.d. | 3.2 | 5 |
| Belokurikha, Altai | Kolyvan, Novosibirsk | Zhuhai, Guangdong | Echassières, Auvergne | |
|---|---|---|---|---|
| External dose, mSv y−1 | ||||
| Average | 3.1 | 4.4 | 1.6 | 1.8 |
| Min | 0.8 | 1.3 | 0.5 | 0.7 |
| Max | 3.5 | 5.3 | 2.6 | 2.2 |
| Standard deviation | 1.1 | 1.6 | 0.6 | 0.6 |
| Outdoor 222Rn Inhalation, mSv y−1 | ||||
| Average | 5.3 | 8.6 | 7.3 | 3.8 |
| Min | 2.2 | 2.3 | 0.5 | 0.4 |
| Max | 8.4 | 25.2 | 28.1 | 7.5 |
| Standard deviation | 1.9 | 3.2 | 2.7 | 1.1 |
| Indoor 222Rn Inhalation, mSv y−1 | ||||
| Average | 7.1 | 10.0 | 3.5 | n.d. |
| Min | 2.0 | 1.0 | 0.5 | n.d. |
| Max | 12.3 | 27.3 | 24.0 | n.d. |
| Standard deviation | 2.5 | 3.8 | 1.1 | n.d. |
| Effective dose, mSv y−1 (222Rn inhalation + External irradiation) | ||||
| Average | 15.5 | 23.0 | 12.4 | 5.6 |
| Min | 5.0 | 4.6 | 1.5 | 1.1 |
| Max | 24.2 | 57.8 | 54.7 | 9.7 |
| Standard deviation | 5.5 | 8.6 | 4.4 | 1.7 |
| Disease Incidence Rate per 100,000 Inhabitants (0/0000) | Belokurikha, Altai Region, Russia (2014–2017) | Kolyvan, Novosibirsk Region, Russia (2011–2016) | Guangdong Province, China (2008–2018) | Auvergne Region, France (2007–2015) | Russia Average Value (2007–2018) | Worldwide Standard 2007–2018 |
|---|---|---|---|---|---|---|
| Cancer incidence rate for all population | 451 | 420 | 250 [48] | 615 [50] | 408 [54,56] | 242 [49] |
| Cancer incidence rate for children aged 0–14 | 36 | 52 | 20 [48] | 15 [51] | 13 [54] | 13 [54] |
| Cancer incidence rate for adults aged 18 and over | 590 | 3334 | 591 [48] | n.d. | 2400 [54,56] | n.d. |
| Lung cancer incidence rate for both sexes | 33 | 352 | 45 [48] | 63 [50] | 41 [54] | 24 [49] |
| Nasopharyngeal carcinoma incidence rate for both sexes | 7 | 5 | 11–25 [48,58] | 4 [50] | 5 [54] | 1–2 [49,57] |
| Leukemia incidence rate for all age groups combined | 20 | 216 | 6 [60] | 17 [52] | 19 [54] | 14 [49] |
| Leukemia incidence rate for children aged 0–14 | 7 | 52 | 3.2 [61] | 4.2 [51] | 3.7 [62] | 3.8 [62] |
| Congenital malformations of the fetus (CMF) | 300 [46] | 897 | 374–1129 [59] | n.d. | ≈632 [53] | ≈600 [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zlobina, A.; Farkhutdinov, I.; Carvalho, F.P.; Wang, N.; Korotchenko, T.; Baranovskaya, N.; Farkhutdinov, A. Impact of Environmental Radiation on the Incidence of Cancer and Birth Defects in Regions with High Natural Radioactivity. Int. J. Environ. Res. Public Health 2022, 19, 8643. https://doi.org/10.3390/ijerph19148643
Zlobina A, Farkhutdinov I, Carvalho FP, Wang N, Korotchenko T, Baranovskaya N, Farkhutdinov A. Impact of Environmental Radiation on the Incidence of Cancer and Birth Defects in Regions with High Natural Radioactivity. International Journal of Environmental Research and Public Health. 2022; 19(14):8643. https://doi.org/10.3390/ijerph19148643
Chicago/Turabian StyleZlobina, Anastasia, Iskhak Farkhutdinov, Fernando P. Carvalho, Nanping Wang, Tatiana Korotchenko, Natalia Baranovskaya, and Anvar Farkhutdinov. 2022. "Impact of Environmental Radiation on the Incidence of Cancer and Birth Defects in Regions with High Natural Radioactivity" International Journal of Environmental Research and Public Health 19, no. 14: 8643. https://doi.org/10.3390/ijerph19148643
APA StyleZlobina, A., Farkhutdinov, I., Carvalho, F. P., Wang, N., Korotchenko, T., Baranovskaya, N., & Farkhutdinov, A. (2022). Impact of Environmental Radiation on the Incidence of Cancer and Birth Defects in Regions with High Natural Radioactivity. International Journal of Environmental Research and Public Health, 19(14), 8643. https://doi.org/10.3390/ijerph19148643







