A Therapeutic Approach Using the Combined Application of Virtual Reality with Robotics for the Treatment of Patients with Spinal Cord Injury: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Selection Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Data Synthesis
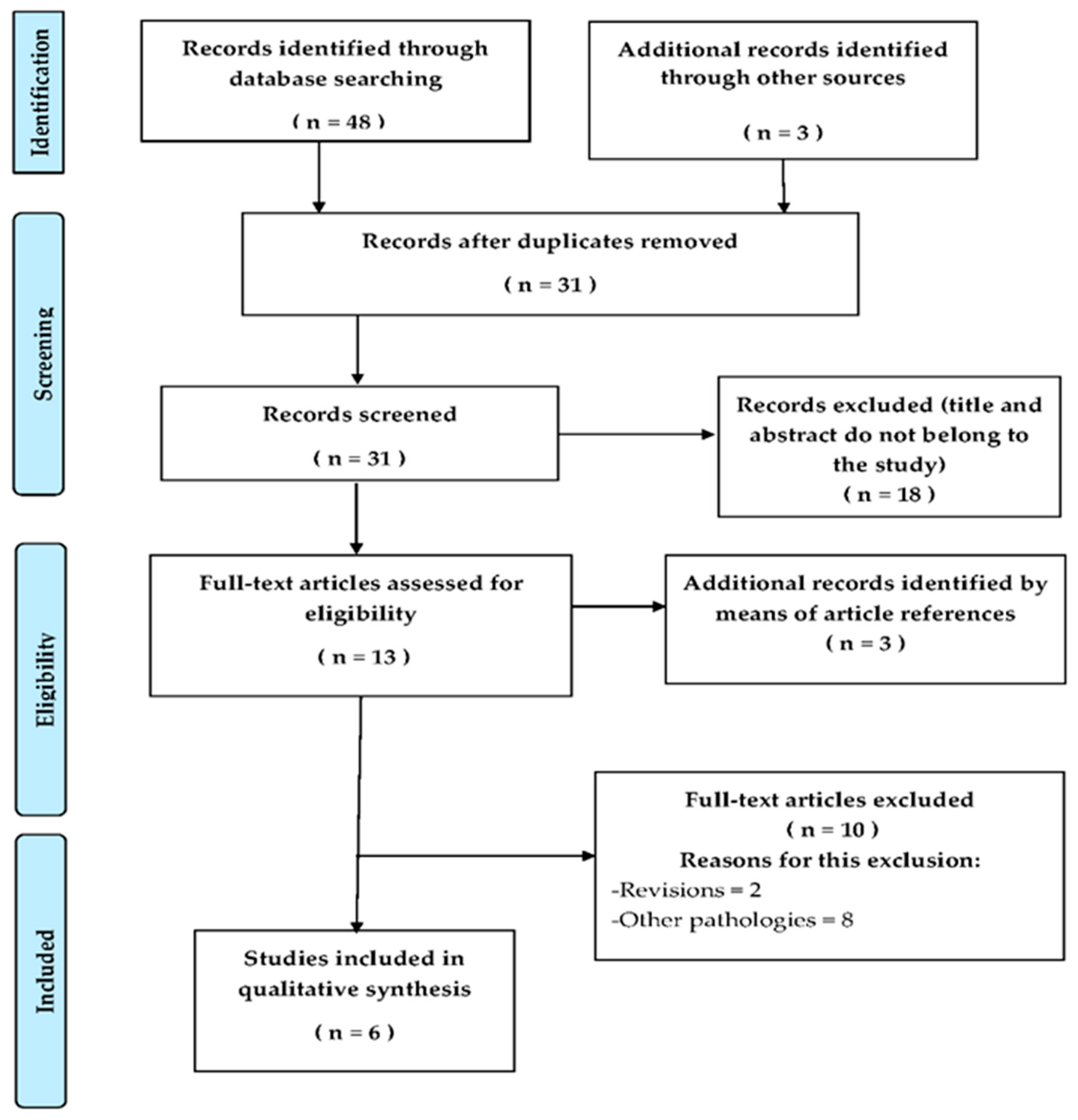
3. Results
3.1. Summary of the Main Results
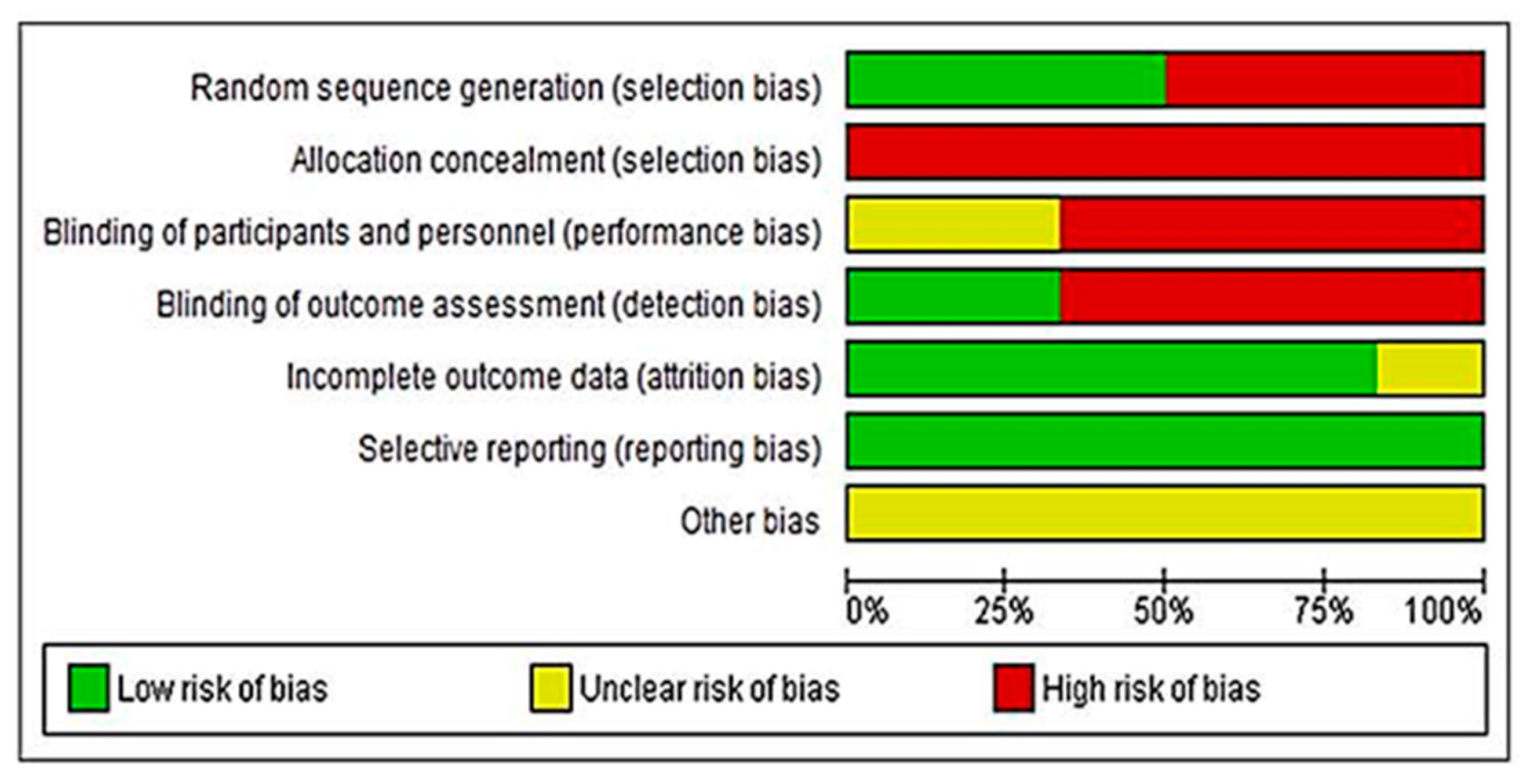
3.2. Asessment of the Risk of Bias and Methodological Quality of the Studies Included in the Review
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
References
- Galeiras Vázquez, R.; Ferreiro Velasco, M.E.; Mourelo Fariña, M.; Montoto Marqués, A.; de la Barrera, S.S. Actualización en lesión medular aguda postraumática. Parte 1. Med. Intensiva 2017, 41, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Huete-García, A.; Díaz-Velázquez, E. Análisis Sobre Lesión Medular en España. Toledo: Federación Nacional Aspaym. 2012. Available online: http://hdl.handle.net/11181/5510 (accessed on 17 October 2021).
- Strassburguer Lona, K.; Hernández Porras, S.; Barquín Santos, E. Lesión Medular: Guía para manejo integral del paciente con LM crónica. Fed. Nac. Aspaym Madr. 2014, 1, 1–161. [Google Scholar]
- Esclarín de Ruz, A. Capítulo 2. Lesión medular. In Análisis Sobre la Lesión Medular en España. Informe de Resultados; Huete García, A., Díaz Velázquez, E., Eds.; Federación Nacional Aspaym: Madrid, Spain, 2012; pp. 11–21. [Google Scholar]
- Puyuelo-Quintana, G.; Gil-Agudo, Á.M.; Cano-de la Cuerda, R. Effectiveness of the Lokomat robotic gait training system in patients with incomplete spinal cord injury. A systematic review. Rehabilitacion 2017, 51, 182–190. [Google Scholar] [CrossRef]
- Alcaraz, M.A.; Mazaira, J. Epidemiología de la lesión medular. In Lesión Medular Enfoque Multidisciplinario; Esclarín, A., Ed.; Editorial Médica Panamericana: Madrid, Spain, 2010; pp. 11–17. [Google Scholar]
- Oliva Jiménez, A. Terapia Ocupacional: Posicionamiento, adaptaciones y ayudas técnicas según el nivel de lesión. In Lesión Medular Enfoque Multidisciplinario; Esclarín, A., Ed.; Editorial Médica Panamericana: Madrid, Spain, 2010; pp. 53–60. [Google Scholar]
- Bickenbach, J.; Officer, A.; Shakespeare, T.; Von Groote, P. (Eds.) International Perspectives on Spinal Cord Injury; World Health Organization: Geneva, Switzerland, 2013.
- Dietz, V. Spinal Cord Lesion: Effects of and perspectives for treatment. Neural Plast. 2001, 8, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Khurana, M.; Walia, S.; Noohu, M.M. Study on the Effectiveness of Virtual Reality Game-Based Training on Balance and Functional Performance in Individuals with Paraplegia. Top. Spinal Cord Inj. Rehabil. 2017, 23, 263–270. [Google Scholar] [CrossRef]
- Dimbwadyo-Terrer, I.; Gil-Agudo, A.; Segura-Fragoso, A.; de los Reyes-Guzmán, A.; Trincado-Alonso, F.; Piazza, S.; Polonio-López, B. Effectiveness of the Virtual Reality System Toyra on Upper Limb Function in People with Tetraplegia: A Pilot Randomized Clinical Trial. Biomed Res. Int. 2016, 2016, 6397828. [Google Scholar] [CrossRef] [Green Version]
- Henao-Lema, C.P.; Pérez-Parra, J.E. Lesiones medulares y discapacidad: Revisión bibliográfica. Aquichan 2010, 10, 157–172. [Google Scholar] [CrossRef] [Green Version]
- Garraway, S.M.; Huie, J.R. Spinal Plasticity and Behavior: BDNF-Induced Neuromodulation in Uninjured and Injured Spinal Cord. Neural Plast. 2016, 2016, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.C.; Knikou, M. A Review on Locomotor Training after Spinal Cord Injury: Reorganization of Spinal Neuronal Circuits and Recovery of Motor Function. Neural Plast. 2016, 2016, 1–20. [Google Scholar] [CrossRef]
- Michele Basso, D.; Hansen, C.N. Biological Basis of Exercise-based Treatments: Spinal Cord Injury. PM&R 2011, 3, S73–S77. [Google Scholar]
- Mat Rosly, M.; Halaki, M.; Mat Rosly, H.; Cuesta, V.; Hasnan, N.; Davis, G.M.; Husain, R. Exergaming for Individuals with Spinal Cord Injury: A Pilot Study. Games Health J. 2017, 6, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Soriano, J.; Taylor, J. Espasticidad después de la lesión medular. Fisioterapia 2010, 32, 89–98. [Google Scholar] [CrossRef]
- Weber, L.M.; Stein, J. The use of robots in stroke rehabilitation. A narrative review. NeuroRehabilitation 2018, 43, 99–110. [Google Scholar] [CrossRef] [Green Version]
- Esposito, M.; Ruberto, M.; Gimigliano, F.; Marotta, R.; Gallai, B.; Parisi, L.; Marianna Lavano, S.; Roccella, M.; Carotenuto, M. Effectiveness and safety of Nintendo Wii Fit PlusTM training in children with migraine without aura: A preliminary study. Neuropsychiatr. Dis. Treat. 2013, 9, 1803–1810. [Google Scholar] [CrossRef] [Green Version]
- Henderson, A.; Korner-Bitensky, N.; Levin, M. Virtual reality in stroke rehabilitation: A systematic review of its effectiveness for upper limb motor recovery. Top. Stroke Rehabil. 2007, 14, 52–61. [Google Scholar] [CrossRef]
- Putrino, D. Telerehabilitation and emerging virtual reality approaches to stroke rehabilitation. Curr. Opin. Neurol. 2014, 27, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Gil Agudo, A. Nuevas tecnologías en neurorrehabilitación aplicadas al tratamiento del paciente con lesión medular. Medicine 2019, 12, 4437–4445. [Google Scholar] [CrossRef]
- Hillman, M. Rehabilitation robotics from past to present-A historical perspective. In Advances in Rehabilitation Robotics; Bien, Z.Z., Stefanov, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 25–44. [Google Scholar]
- Imam, B.; Jarus, T. Virtual reality rehabilitation from social cognitive and motor learning theoretical perspectives in stroke population. Rehabil. Res. Pract. 2014, 2014, 594540. [Google Scholar] [CrossRef] [PubMed]
- Sin, H.; Lee, G. Additional virtual reality training using Xbox kinect in stroke survivors with hemiplegia. Am. J. Phys. Med. Rehabil. 2013, 92, 871–880. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, S.H.; Kim, C.S.; Jung, J.H.; You, J.H. Use of virtual reality to enhance balance and ambulation in chronic stroke: A double-blind, randomized controlled study. Am. J. Phys. Med. Rehabil. 2016, 88, 693–701. [Google Scholar] [CrossRef]
- Dominguez-Tellez, P.; Moral-Munoz, J.A.; Casado-Fernandez, E.; Salazar, A.; Lucena-Anton, D. Effects of virtual reality on balance and gait in stroke: A systematic review and meta-analysis. Rev. Neurol. 2019, 69, 223–234. [Google Scholar] [PubMed]
- Booth, A.T.C.; Buizer, A.I.; Meyns, P.; Oude Lansink, I.L.B.; Steenbrink, F.; Van der Krogt, M.M. The efficacy of functional gait training in children and young adults with cerebral palsy: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2018, 60, 866–883. [Google Scholar] [CrossRef] [PubMed]
- Johansen, T.; Strøm, V.; Simic, J.; Rike, P.-O. Effectiveness of training with motion-controlled comercial video games for hand and arm function in people with cerebral palsy: A systematic review and meta-analysis. J. Rehabil. Med. 2020, 52, jrm00012. [Google Scholar] [CrossRef] [Green Version]
- Feng, H.; Li, C.; Liu, J.; Wang, L.; Ma, J.; Li, G.; Gan, L.; Shang, X.; Wu, Z. Virtual reality rehabilitation versus conventional physical therapy for improving balance and gait in parkinson’s disease patients: A randomized controlled trial. Med. Sci. Monit. 2019, 25, 4186–4192. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Sunzi, K.; Dai, F.; Liu, X.; Wang, Y.; Zhang, B.; He, L.; Ju, M. Effects of virtual reality rehabilitation training on gait and balance in patients with Parkinson’s disease: A systematic review. Clin. Med. 2020, 9, 2861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Verdú, M.; Ferreira-Sánchez, M.R.; Cano-De-La-Cuerda, R.; Jiménez-Antona, C. Efficacy of virtual reality on balance and gait in multiple sclerosis. Systematic review of randomized controlled trials. Rev. Neurol. 2019, 68, 357–368. [Google Scholar]
- Norouzi, E.; Gerber, M.; Pühse, U.; Vaezmosavi, M.; Brand, S. Combined virtual reality and physical training improved the bimanual coordination of women with multiple sclerosis. Neuropsychol. Rehabil. 2021, 31, 552–569. [Google Scholar] [CrossRef]
- Maggio, M.G.; Russo, M.; Cuzzola, M.F.; Destro, M.; La Rosa, G.; Molonia, F.; Bramanti, P.; Lombardo, G.; De Luca, R.; Salvatore Calabrò, R. Virtual reality in multiple sclerosis rehabilitation: A review on cognitive and motor outcomes. J. Clin. Neurosci. 2019, 65, 106–111. [Google Scholar] [CrossRef]
- Hutton, B.; Catalá-López, F.; Moher, D. The PRISMA statement extension for systematic reviews incorporating network meta-analysis: PRISMA-NMA. Med. Clin. 2016, 147, 262–266. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic’, J.; Schulz, K.F.; Weeks, L.; Sterne, J.C.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Eng, J.J.; Teasell, R.W.; Miller, W.C.; Wolfe, D.L.; Townson, A.F.; Aubut, J.A.; Abramson, C.; Hsieh, J.T.; Connoly, S.; Konnyu, K. Spinal cord injury rehabilitation evidence: Method of the SCIRE systematic review. Top. Spinal Cord Inj. Rehabil. 2007, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moseley, A.M.; Herbert, R.D.; Sherrington, C.; Maher, C.G. Evidence for physiotherapy practice: A survey of the Physiotherapy Evidence Database (PEDro). Aust. J. Physiother. 2002, 48, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Calabrò, R.S.; Naro, A.; Leo, A.; Bramanti, P. Usefulness of robotic gait training plus neuromodulation in chronic spinal cord injury: A case report. J. Spinal Cord Med. 2017, 40, 118–121. [Google Scholar] [CrossRef] [Green Version]
- Casadio, M.; Pressman, A.; Acosta, S.; Danziger, Z.; Fishbach, A.; Mussa-Ivaldi, F.A.; Muir, K.; Tseng, H.; Chen, D. Body machine interface: Remapping motor skills after spinal cord injury. In Proceedings of the IEEE International Conference on Rehabilitation Robotics, Zurich, Switzerland, 29 June–1 July 2011; pp. 1–6. [Google Scholar]
- Dimbwadyo-Terrer, I.; Trincado-Alonso, F.; De Los Reyes-Guzmán, A.; Aznar, M.A.; Alcubilla, C.; Pérez-Nombela, S.; Del Alma-Espinosa, A.; Polonio-López, B.; Gil-Agudo, Á. Upper limb rehabilitation after spinal cord injury: A treatment based on a data glove and an immersive virtual reality environment. Disabil. Rehabil. Assist. Technol. 2016, 11, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewski, J.; Chong, S.L.; Galea, M.; Prochazka, A. In-Home Tele-Rehabilitation Improves Tetraplegic Hand Function. Neurorehabilit. Neural Repair 2011, 25, 412–422. [Google Scholar] [CrossRef]
- Prochazka, A.; Kowalczewski, J. A Fully Automated, Quantitative Test of Upper Limb Function. J. Mot. Behav. 2015, 47, 19–28. [Google Scholar] [CrossRef]
- Tidoni, E.; Abu-Alqumsan, M.; Leonardis, D.; Kapeller, C.; Fusco, G.; Guger, C.; Hintermuller, C.; Peer, A.; Frisoli, A.; Tecchia, F.; et al. Local and Remote Cooperation With Virtual and Robotic Agents: A P300 BCI Study in Healthy and People Living With Spinal Cord Injury. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1622–1632. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, K.J.; Cen, S.Y. Model of Disablement and Recovery: Knowledge Translation in Rehabilitation Research and Practice. Phys. Ther. 2011, 91, 1892–1904. [Google Scholar] [CrossRef]
- Kumru, H.; Benito, J.; Murillo, N.; Valls-Sole, J.; Valles, M.; Lopez-Blazquez, R.; Costa, U.; Tormos, J.M.; Pascual-Leone, A.; Vidal, J. Effects of high-frequency repetitive transcranial magnetic stimulation on motor and gait improvement in incomplete spinal cord injury patients. Neurorehabilit. Neural Repair 2013, 27, 421–429. [Google Scholar] [CrossRef]
- Belci, M.; Catley, M.; Husain, M.; Frankel, H.L.; Davey, N.J. Magnetic brain stimulation can improve clinical outcome in incomplete spinal cord injured patients. Spinal Cord 2004, 42, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Curt, A.; Van Hedel, H.J.; Klaus, D.; Dietz, V.; EM-SCI Study Group. Recovery from a spinal cord injury: Significance of compensation, neural plasticity, and repair. J. Neurotrauma 2008, 25, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Mak, J.N.; Wolpaw, J.R. Clinical applications of brain computer interfaces: Current state and future prospects. IEEE Rev. Biomed Eng. 2009, 2, 187–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salisbury, S.K.; Choy, N.L.; Nitz, J. Shoulder pain, range of motion, and functional motor skills after acute tetraplegia. Arch. Phys. Med. Rehabil. 2003, 84, 1480–1485. [Google Scholar] [CrossRef]
- Ballinger, D.A.; Rintala, D.H.; Hart, K.A. The relation of shoulder pain and range-of-motion problems to functional limitations, disability, and perceived health of men with spinal cord injury: A multifaceted longitudinal study. Arch. Phys. Med. Rehabil. 2000, 81, 1575–1581. [Google Scholar] [CrossRef]
- Kantak, S.S.; Sullivan, K.J.; Fisher, B.E.; Knowlton, B.J.; Winstein, C.J. Neural substrates of motor memory consolidation depend on practice structure. Nat. Neurosci. 2010, 13, 923–925. [Google Scholar] [CrossRef]
- Leonardis, D.; Frisoli, A.; Solazzi, M.; Bergamasco, M. Illusory perception of arm movement induced by visuo-proprioceptive sensory stimulation and controlled by motor imagery. In Proceedings of the 2012 IEEE Haptics Symposium, Vancouver, BC, Canada, 4–7 March 2012; pp. 421–424. [Google Scholar]
- Ramos-Murguialday, A.; Schürholz, M.; Caggiano, V.; Wildgruber, M.; Caria, A.; Hammer, E.M.; Halder, S.; Birbaumer, N. Proprioceptive Feedback and Brain Computer Interface (BCI) Based Neuroprostheses. PLoS ONE 2012, 7, e47048. [Google Scholar] [CrossRef]
- Yao, L.; Meng, J.; Sheng, X.; Zhang, D.; Zhu, X. A novel calibration and task guidance framework for motor imagery BCI via a tendon vibration induced sensation with kinesthesia illusion. J. Neural Eng. 2015, 12, 016005. [Google Scholar] [CrossRef]
| Study Country | Participants (n) | Age (years) Mean ± SD (Range) | Sex F/M | ASIA Grade | Level of Injury | Time after Onset Injury (Months) |
|---|---|---|---|---|---|---|
| Calabrò et al., 2016 [40] (Messina) Italy | n = 1 | 31 | M | C | Incomplete T10 | 20 |
| Casadio et al., 2011 [41] (Chicago) USA | n = 14 CG: 8, IG: 6 | CG: (21–35) IG: 40.17 ± 3.53 | CG: 1F/7M IG: 6M | IG: A (3) C (3) | Complete cervical: C4 (1), C5 (1), C6 (1) Incomplete cervical: C3-C4 (1), C4 (2) | IG: 50.83 |
| Dimbwadyo-Terreret al., 2016 [42] (Toledo) Spain | n = 9 CG:3, IG: 6 | CG: 44.17 ± 22.29 IG: 54.3 ± 9.86 | CG: 1F/2M IG: 1F/5M | CG: A (3) IG: A (5) D (1) | CG: complete thoracic: T4 (2), T6 (1) IG: complete thoracic: T4 (5) incomplete cervical: C4 (1) | CG: 5 ± 1 IG: 5.83 ± 2.99 |
| Kowalczewski et al., 2011 [43] (Alberta) Canada | n = 13 GC: 7, IG: 6 | 35.92 ± 11.96 | 6F/7M | A-B | Complete cervical: C5 (5)-C6 (4)-C7 (4) | 3.62 ± 2.12 |
| Prochazka y Kowalczewski,2015 [44] (Alberta) Canada | n = 13. CG: ND IG: ND | (24–56) | ND | A-B | Complete cervical: C5-C6 | ND |
| Tidoni et al., 2016 [45] (Rome) Italy | n = 13 CG: 10, IG: 3 | CG: 29.33 ± 2.87 (24–32) IG: 28 ±5.19 (22–31) | CG: 4F/6M IG: 3M | A-B-D | Complete cervical: C4 (1), C4-C5 (1) Incomplete cervical: C6 (1) | IG: 88.67 |
| Study SCIRE-PEDro Scores | Group Interventions | Intensity | Session Duration | Intervention Duration | Outcome | Measuring Instrument | Results |
|---|---|---|---|---|---|---|---|
| Calabrò et al., 2016 [40] Case report Pre-post test Level 5 | IG: Lokomat Pro with motivating feedback in a virtual environment (non-immersive VR) IG (rTMS): Lokomat Pro (non-immersive VR), + repetitive transcranial magnetic stimulation | 5 times/week | 40 min | 8 weeks | Lower limbs: strength and rigidity in flexion/extension of hip and knee. | ASIA, LEMS RMT, MEP CCT, MUNE rigidity, strength, DGF | IG: slight improvement in kinetic parameters (reduces rigidity in knee and hip). No significant changes in clinical or electrophysiological parameters. IG (rTMS): improvement in ASIA (C to D), LEMS (3 to 9) scores, statistically significant reduction of hip and knee stiffness, device guidance force, BWS (61 ± 6% to 57 ± 3%), increase in hip flexion–extension force, MEP amplitude, MUNE, and speed (1.5 ± 0.3 Km/h to 1.7 ± 0.2 Km/h) |
| Casadio et al., 2011 [41] Controlled clinical assay. Pre-post test Level 4 | CG and IG: VR games, consisting of a virtual board (non immersive VR) and simulated conduction (immersive VR), combined with technologies capturing movement (BMI) | 2–3 times/week | 45 min | 3 weeks | Upper limb: ROM of shoulder. Isometric strength of shoulder in 3 directions. | MMT and normal scale with scoring from 0 to 5 for ROM | MMT improves for all individuals: F (1.5) = 10; p = 0.02. Significant correlations between shoulder muscle force in the upper, forward and backward directions and scapular elevation, shoulder protraction and retraction (R = 0.55 p = 0.0073, R = 0.72 p = 0.0012, R = 0.75 p < 0.0001, respectively). Five out of six subjects improved total isometric force. |
| Dimbwadyo-Terrer et al., 2016 [42] Clinical assay randomized pilot study. Level 1 | CG: CTP IG: immersive VR system + CyberTouch glove. Two session types: one of reaching and throwing movements, and the other only reaching ones. One type per day was performed. Same CTP as CG. | 2 times/week | 30 min | 2 weeks | Upperlimb: motor (muscle strength and self-management, co-ordination and fine motor control. | Functional state: MB, BI, SCIM. NHPT and JHFT scales. Time taken to complete the items. | No significant differences were found in the outcomes between groups, although MB was higher IG. The SCIM scale improved in both groups: >11 points in IG, and >4 points in SCIM self-care for IG (improved skills, coordination and fine movements of the fingers) IG needed shorter time for NHPT. |
| Kowalczewski et al., 2011 [43] Randomized controlled clinical assay. Level 1 | CG: CPT+ 1 month’s rest + ReJoyce with video games (non immersive VR) that mimic the ADL. IG: ReJoyce with videogames that mimic the ADL. + 1 month’s rest. +CPT. | 5 times/week | 60 min | 6 weeks | Upperlimb: functionality for ADL and ROM. | ARAT RAHFT | IG improved more than CG according to ARAT (13.0% ± 9.8% and 4.0% ± 9.6%, respectively=). |
| Prochazka y Kowalczewski 2015 [44] Randomized controlled clinical assay Level 1 | CG: telesupervised CPT. IG: FES + sessions telesupervised with ReJoyce (non immersive VR). | 6 times/week | 60 min | 6 weeks | Upperlimb: functionality and ROM. Validity of RAHFT, ARAT and FMA. | RAHFT ARAT FMA | RAHFT is better for studying functionality and FMA for the ROM. Effect sizes of IC group: RAHFT (0.64 ± 3.6) ARAT (1.3 ± 6.3), FMA (1.5 ± 5.2) |
| Tidoni et al., 2016 [45] Post-test Level 4 | CG and IG: immersive VR of mathematical game with board and proprioceptive stimulator in the brachial biceps tendon with feedback from a video recorded with a robot. | 12 times/ND | 6 min | ND | Results of questionnaire on user’s experience, optimization calls, and information transfer rate. | UE OC ITR | Patient 1: lesser precision in the task than CG and higher OC and lower ITR (p < 0.022). Patient 2: only VR. UE, OC and ITR did not differ from the CG. Patient 3: did not differ from the CG in the robot scenario, although UE and ITR obtained lower scores in VR. |
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimbwadyo-Terrer et al., 2016 [42] | - | YES | NO | YES | NO | NO | NO | YES | YES | YES | YES | 6 |
| Kowalczewski et al., 2011 [43] | - | YES | NO | YES | NO | YES | NO | YES | YES | YES | YES | 7 |
| Prochazka y Kowalczewski, 2015 [44] | - | YES | NO | YES | NO | NO | YES | NO | YES | YES | YES | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Miguel-Rubio, A.; Muñoz-Pérez, L.; Alba-Rueda, A.; Arias-Avila, M.; Rodrigues-de-Souza, D.P. A Therapeutic Approach Using the Combined Application of Virtual Reality with Robotics for the Treatment of Patients with Spinal Cord Injury: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 8772. https://doi.org/10.3390/ijerph19148772
De Miguel-Rubio A, Muñoz-Pérez L, Alba-Rueda A, Arias-Avila M, Rodrigues-de-Souza DP. A Therapeutic Approach Using the Combined Application of Virtual Reality with Robotics for the Treatment of Patients with Spinal Cord Injury: A Systematic Review. International Journal of Environmental Research and Public Health. 2022; 19(14):8772. https://doi.org/10.3390/ijerph19148772
Chicago/Turabian StyleDe Miguel-Rubio, Amaranta, Lorena Muñoz-Pérez, Alvaro Alba-Rueda, Mariana Arias-Avila, and Daiana Priscila Rodrigues-de-Souza. 2022. "A Therapeutic Approach Using the Combined Application of Virtual Reality with Robotics for the Treatment of Patients with Spinal Cord Injury: A Systematic Review" International Journal of Environmental Research and Public Health 19, no. 14: 8772. https://doi.org/10.3390/ijerph19148772







