Enrichment and Chemical Speciation of Vanadium and Cobalt in Stone Coal Combustion Products in Ankang, Shanxi Province, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Proximate Analysis
2.3. Simulated Combustion Experiment
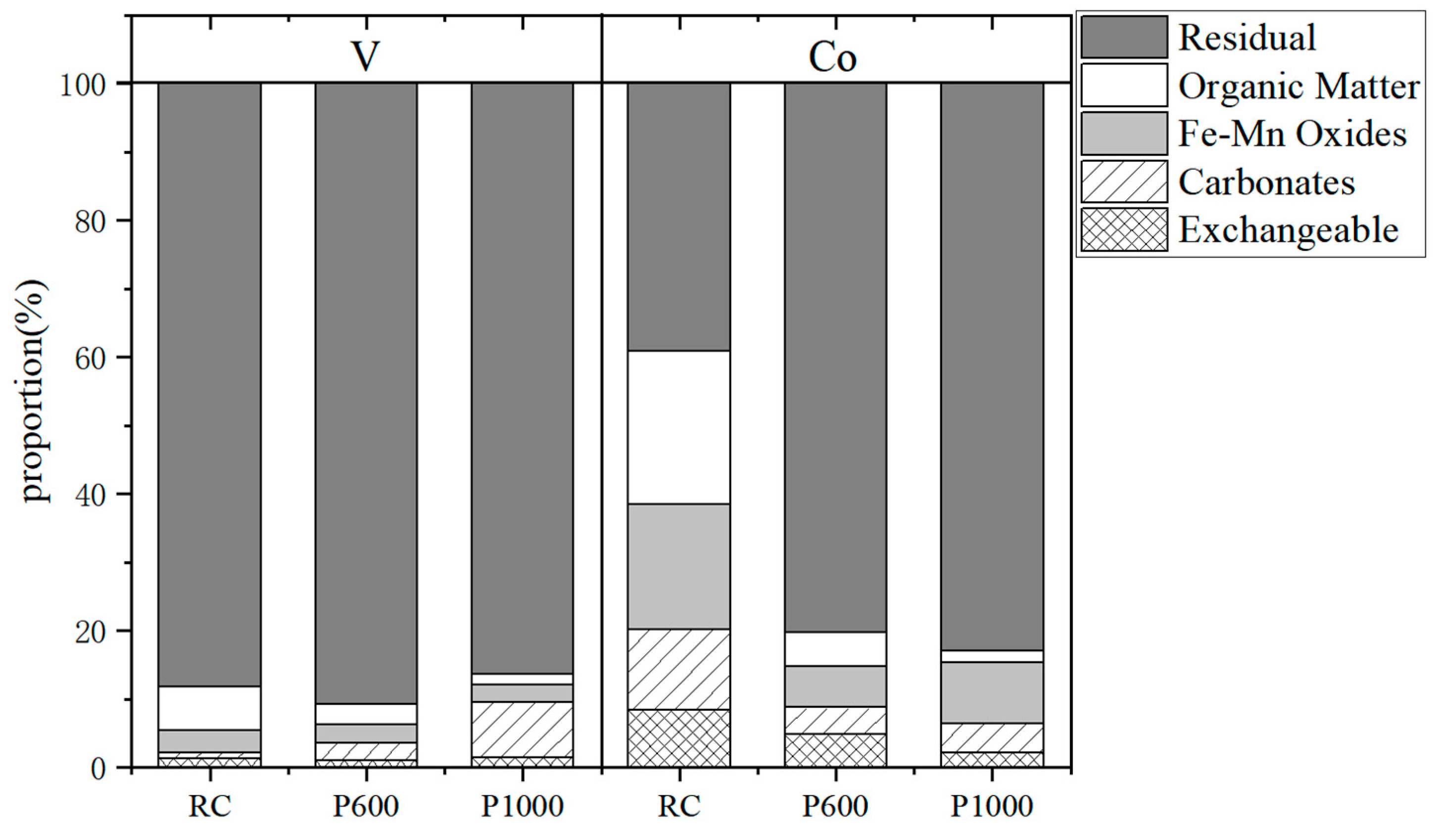
2.4. Sequential Chemical Extraction Procedure
2.5. Experimental Analysis
3. Results and Discussion
3.1. Proximate Analysis and Chemical Composition of Raw Stone Coal
3.2. Vanadium and Cobalt in Stone Coal
3.3. Enrichment of Vanadium in Stone Coal Combustion Products
3.4. Chemical Speciation of V and Co in Stone Coal and Its Combustion Products
3.5. Mineral Compositions of Stone Coal and Its Combustion Products
3.6. Potential Impact of Stone Coal Combustion on Surrounding Environment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Li, X.; Liu, J.; Zhang, L.; Chen, J.; Feng, X. Stone coal as a potential atmospheric mercury source in Da-Ba-Shan mountain areas, China. Int. J. Coal Geol. 2019, 206, 21–30. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Bao, S.-X.; Liu, T.; Chen, T.-J.; Huang, J. The technology of extracting vanadium from stone coal in China: History, current status and future prospects. Hydrometallurgy 2011, 109, 116–124. [Google Scholar] [CrossRef]
- Liu, G.Q.; Liu, K.; Gao, Y.K.; Chen, G. Co-Pyrolysis Kinetics Analysis of Stone Coal and Biomass for Vanadium Extraction. Metalurgija 2018, 57, 239–241. [Google Scholar]
- Yan, B.J.; Wang, D.Y.; Wu, L.S.; Dong, Y.C. A novel approach for pre-concentrating vanadium from stone coal ore. Miner. Eng. 2018, 125, 231–238. [Google Scholar] [CrossRef]
- Dai, S.F.; Zheng, X.; Wang, X.B.; Finkelman, R.B.; Jiang, Y.F.; Ren, D.Y.; Yan, X.Y.; Zhou, Y.P. Stone coal in China: A review. Int. Geol. Rev. 2018, 60, 736–753. [Google Scholar] [CrossRef]
- Li, C.; Ma, H.; Xie, B.; Zhang, B.; Zhao, X.; Wang, M.; He, Z.; Li, W.; Chen, J. A Comparison of Mineralogical and Thermal Storage Characteristics for Two Types of Stone Coal. Minerals 2019, 9, 594. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wang, J. Situation and analysis of Vanadium Pentoxide Market at Home and Abroad. Min. Metall. 2007, 2, 47–51. [Google Scholar]
- Liu, X.; Zhang, Y.; Bao, S.; Bian, Y.; Ren, L. Process mineralogy of vanadium-bearing stone coal ore from Hubei Province. Nonferrous Met. Extr. Metall. 2015, 39, 934–940. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Zhang, G.F.; Feng, Q.M.; Yi-Ping, L.U.; Le-Ming, O.U.; Huang, S.J. Acid leaching of vanadium from roasted residue of stone coal. Chin. J. Nonferrous Met. Engl. 2010, 5, s107–s111. [Google Scholar] [CrossRef]
- Cunxiong, L.; Chang, W.; Zhigan, D.; Mingting, L.; Xingbing, L.; Gang, F. Recovery of vanadium from black shale. Chin. J. Nonferrous Met. Engl. 2010, 20, 127–131. [Google Scholar]
- Zhang, X.; Zhu, S.; Zhong, C.M.; CNNC. The determination and evaluation of radioactive nuclide of stone-like coal in an ore district. Uranium Min. Metall. 2016, 35, 55–60. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, G.; Qi, C.; Cheng, S.; Sun, R. Chemical speciation and combustion behavior of chromium (Cr) and vanadium (V) in coals. Fuel 2016, 184, 42–49. [Google Scholar]
- Ying, L.; Jida, Y.; Liang, Z.; Zongmei, W.; Zhiyong, W. The Survey of status Quo on Heavy-Metal Contamination in the Bone-Coal Mining and Utilization. Energy Environ. Prot. 2005, 2, 58–61. (In Chinese) [Google Scholar]
- Yang, H.R.; Jin, J.; Liu, D.Y.; Wang, Y.Z.; Zhao, B. The influence of vermiculite on the ash deposition formation process of Zhundong coal. Fuel 2018, 230, 89–97. [Google Scholar] [CrossRef]
- Wang, K.; Han, T.; Deng, J.; Zhang, Y. Comparison of combustion characteristics and kinetics of Jurassic and Carboniferous-Permian coals in China. Energy 2022, 254, 124315. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.F.; Zhang, G.J.; Guo, Y.F.; Zhang, J.; Li, G.Q. Pyrolysis characteristics and kinetics of two Chinese low-rank coals. J. Therm. Anal. Calorim. 2015, 122, 975–984. [Google Scholar] [CrossRef]
- Wang, K.; Gao, P.; Sun, W.L.; Fan, H.H.; He, Y.Z.; Han, T. Thermal Behavior of the Low-temperature Secondary Oxidation of Coal under Different Pre-oxidation Temperatures. Combust. Sci. Technol. 2022, 194, 1712–1729. [Google Scholar] [CrossRef]
- Xu, Y.L.; Wang, D.M.; Wang, L.Y.; Zhong, X.X.; Chu, T.X. Experimental research on inhibition performances of the sand-suspended colloid for coal spontaneous combustion. Saf. Sci. 2012, 50, 822–827. [Google Scholar] [CrossRef]
- Chandra, M.; Yu, D.W.; Tian, Q.H.; Guo, X.Y. Recovery of Cobalt from Secondary Resources: A Comprehensive Review. Miner. Processing Extr. Metall. Rev. 2022, 43, 679–700. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Banoo, S. Spectrophotometric method for determination of vanadium and its application to industrial, environmental, biological and soil samples. Talanta 1999, 48, 1085–1094. [Google Scholar] [CrossRef]
- Teng, Y.G.; Zhang, Q.Q.; Xiao, J.; Wang, J.S.; Jiao, X.D. Geochemical Speciation and Potential Ecological Risk of Vanadium in the Soil in the Panzhihua Park. J. Mineral. Petrol. 2008, 2, 102–106. [Google Scholar]
- Xie, H.K. Research Progress on Remediation of Heavy Metal Polluted Soil by Cobalt. Mod. Agric. Sci. Technol. 2013, 7, 222–223. [Google Scholar]
- Xiao-Yan, X.U.; Jing, E.D.; Tang, Q.H.; Min, H.E.; Qing-Qing, G.U.; Pei-Zhi, Y.U.; Xi-Mei, X.U.; Zhang, R.; Liu, Y.H. Emergency Treatment of Source Water Polluted by Cobalt. China Water Wastewater 2017, 23, 138–140. [Google Scholar]
- Zhang, C.; Niu, M.; Chu, F. Analysis on Occurrence Characteristics and Extraction Process of Vanadium in Stone Coal. Mod. Min. 2018, 7, 91–95. [Google Scholar]
- Mu, Y.W.; Zhang, S.H.; Zhao, Y.S.; Yang, Q.Q.; Fang, Y.W.; Li, Y.G.; Liu, J.T.; Wu, Z.J. Summary of Progress in Extracting Vanadium. In Proceedings of the 4th International Conference on Mechanics, Simulation and Control (ICMSC), Moscow, Russia, 21–22 June 2014; pp. 60–65. [Google Scholar]
- Han, L.T. Explaination on revision of GB 475—2008 Method for manual sampling of commercial coal. Goal Qual. Technol. 2009, 3, 14–16. [Google Scholar]
- Wurzbach, R.N. Sampling Methods Outlined in the American Society for Testing and Materials (ASTM) Standard D7718. Available online: https://greasethief.com/wp-content/uploads/2013/12/ASTM-Sampling-Methods.pdf (accessed on 23 June 2022).
- GB/T 215-2003; Determination of Forms of Sulfur in Coal. General Administration of Quality Supervision. Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2003.
- Finkelman, R.B. Modes of occurrence of potentially hazardous elements in coal: Levels of confidence. Fuel Processing Technol. 1994, 39, 21–34. [Google Scholar] [CrossRef]
- Tomschey, O.; Harman, M.; Blasko, D. Trace Element Distribution on the Pukanec Lignite Deposit. 1986. Available online: https://www.researchgate.net/publication/305040201 (accessed on 23 June 2022).
- Tessier, A.; Campbell, P.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Aceto, L.; Ingolfsdottir, A.; Sack, J. Resource bisimilarity and graded bisimilarity coincide. Inf. Processing Lett. 2010, 111, 68–76. [Google Scholar] [CrossRef]
- Bin, Z.Y. Progress of the Research on Extraction of Vanadium Pentoxide from Stone Coal and the Market of the V2O5. Hunan Nonferrous Met. 2006, 1, 16–20+74. [Google Scholar]
- Jiang, Y.H.; Yue, W.Z.; Ye, Z.Z. Characteristics, sedimentary environment and origin of the Lower Cambrian stone-like coal in southern China. Coal Geol. China 1994, 4, 26–31. [Google Scholar]
- Yu, X.U.; Wang, J.; Jingrong, W.U. Analysis on Status of Cobalt Resources with Its Import and Export in China. Min. Res. Dev. 2014, 5, 112–115, +132. [Google Scholar]
- Ketris, M.P.; Yudovich, Y.E. Estimations of Clarkes for Carbonaceous biolithes: World averages for trace element contents in black shales and coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- Dai, X.; Yong-Qian, W.U.; Jian-Hui, W.U.; Chen, T.Z.; Zhou, K.J. Study on Oxygen-enriched Air Roasting Process for Vanadium-bearing Stone Coal. Min. Metall. Eng. 2013, 2, 88–90+96. [Google Scholar]
- Yuan, L. Vanadium in Coal Mining Area: Distribution, Modes of Occurrence, and Environmental Behavior; University of Science and Technology of China: Heifei, China, 2018. [Google Scholar]
- Finkelman, R.B. Trace and Minor Elements in Coal. In Organic Geochemistry; Elsevier: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Raask, E. The mode of occurrence and concentration of trace elements in coal. Prog. Energy Combust. Sci. 1985, 11, 97–118. [Google Scholar] [CrossRef]
- Wedepohl, K.H. Trace Elements in Coal; Dalway, J.S., Ed.; Butterworth & Co. Publ.: Salem, NH, USA, 1991; Volume 55, pp. 927–928. [Google Scholar]
- Huimin, L.; Wei-Ping, P.; Yue, Z.; Chunbo, W. Volatilization of Arsenic During Coal Combustion Based on Isothermal Thermogravimetric Analysis at 600–1500 °C. Energy Fuels 2016, 30, 816. [Google Scholar]
- Dai, Z.; Min-ting, L. A Study on the Variation of V Element Existence in Stone Coal Before-After Combustion. Yunnan Geol. 2014, 8, 401–408. (In Chinese) [Google Scholar]
- Escalera, E.; Antti, M.L.; Oden, M.; Iop. Thermal treatment and phase formation in kaolinite and illite based clays from tropical regions of Bolivia. In Proceedings of the 6th EEIGM International Conference on Advanced Materials Research (AMR), European Sch Mat Engn (EEIGM), Nancy, France, 7–8 November 2011. [Google Scholar]
- Quan, T.; Liu, G.; Zhou, C.; Zhang, H.; Sun, R. Distribution of environmentally sensitive elements in residential soils near a coal-fired power plant: Potential risks to ecology and children’s health. Chemosphere 2013, 93, 2473–2479. [Google Scholar]
- Ren, D.; Xu, D.; Zhao, F. A preliminary study on the enrichment mechanism and occurrence of hazardous trace elements in the Tertiary lignite from the Shenbei coalfield, China. Int. J. Coal Geol. 2004, 57, 187–196. [Google Scholar] [CrossRef]
- Deyi, R.; Fenghua, Z.; Yuanquan, W.; Shaojin, Y. Distributions of minor and trace elements in Chinese coals. Int. J. Coal Geol. 1999, 40, 109–118. [Google Scholar]
- Tang, Q.; Liu, G.; Zhou, C.; Sun, R. Distribution of trace elements in feed coal and combustion residues from two coal-fired power plants at Huainan, Anhui, China. Fuel 2013, 107, 315–322. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Tao, L.; Chen, T.; Bao, S. Pre-concentration of vanadium from stone coal by gravity separation. Int. J. Miner. Processing 2013, 121, 1–5. [Google Scholar] [CrossRef]
- Mao-Lin, L.I.; Liu, P.; Wang, F.; Cui, R. Study on Process Mineralogy of a Stone Coal Vanadium Ore in Hubei. Conserv. Util. Miner. Resour. 2013, 3, 401–408. [Google Scholar]
- Luo, X.; Zhi-Guang, L.E. Research on the Occurrence State of Vanadium of Pingbian Vanadium Ore in Honghe County. Multipurp. Util. Miner. Resour. 2011, 6, 23–25+29. [Google Scholar]
- Poledniok, J.; Buhl, F. Speciation of vanadium in soil. Talanta 2003, 59, 1–8. [Google Scholar] [CrossRef]
- Peng, C.Y.; Korshin, G.V. Speciation of trace inorganic contaminants in corrosion scales and deposits formed in drinking water distribution systems. Water Res. 2011, 45, 5553–5563. [Google Scholar] [CrossRef] [PubMed]



| Mad (wt. %) | Ad (%) | Vdaf (wt. %) | FCd (wt. %) | St,d (%) | Ss,d (wt. %) | Sp,d (wt. %) | So,d (wt. %) | Qgr,ad (MJ/kg) |
|---|---|---|---|---|---|---|---|---|
| 2.13 | 38.96 | 4.66 | 51.41 | 1.82 | 0.03 | 1.76 | 0.03 | 5.53 |
| Element | C | O | Fe | F | Mg | Al | Si | K | V | Co |
|---|---|---|---|---|---|---|---|---|---|---|
| ω (%) | 37.04 | 32.92 | 4.09 | 1.99 | 4.63 | 3.55 | 12.00 | 3.34 | 0.45 | ND |
| V (μg·g−1) | Co (μg·g−1) | References | |
|---|---|---|---|
| Stone coal | 1548 | 3.49 | In this study |
| World coal | 25.0 | 5.1 | Ketris and Yudovich, 2009 [36] |
| Chinese coal | 51.81 | 7.08 | Dai et al., 2012 [37] |
| Liu Yuan, 2018 [38] | |||
| US coal | 22 | 6.10 | Finkelman, 1993 [39] |
| Australian coal | 25 | 4.00 | Raask, 1985 [40] |
| Swain, 1990 [41] |
| Samples | Ad/% | Cv/μg·g−1 | EFs (V) | CCo/μg·g−1 | EFs (Co) |
|---|---|---|---|---|---|
| RC | 39.16 | 1548 | - | 3.49 | °C |
| P500 | 69.52 | 1771 | 0.79 | 5.02 | 0.99 |
| P600 | 61.83 | 2183 | 0.87 | 5.57 | 0.98 |
| P700 | 57.46 | 2309 | 0.86 | 5.48 | 0.90 |
| P800 | 48.55 | 2589 | 0.81 | 6.55 | 0.91 |
| P900 | 40.02 | 3188 | 0.82 | 8.72 | 0.99 |
| P1000 | 38.21 | 3739 | 0.92 | 9.03 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, W.; Meng, Q.; Li, W.; Feng, Q. Enrichment and Chemical Speciation of Vanadium and Cobalt in Stone Coal Combustion Products in Ankang, Shanxi Province, China. Int. J. Environ. Res. Public Health 2022, 19, 9161. https://doi.org/10.3390/ijerph19159161
Cui W, Meng Q, Li W, Feng Q. Enrichment and Chemical Speciation of Vanadium and Cobalt in Stone Coal Combustion Products in Ankang, Shanxi Province, China. International Journal of Environmental Research and Public Health. 2022; 19(15):9161. https://doi.org/10.3390/ijerph19159161
Chicago/Turabian StyleCui, Wei, Qingjun Meng, Wenbo Li, and Qiyan Feng. 2022. "Enrichment and Chemical Speciation of Vanadium and Cobalt in Stone Coal Combustion Products in Ankang, Shanxi Province, China" International Journal of Environmental Research and Public Health 19, no. 15: 9161. https://doi.org/10.3390/ijerph19159161
APA StyleCui, W., Meng, Q., Li, W., & Feng, Q. (2022). Enrichment and Chemical Speciation of Vanadium and Cobalt in Stone Coal Combustion Products in Ankang, Shanxi Province, China. International Journal of Environmental Research and Public Health, 19(15), 9161. https://doi.org/10.3390/ijerph19159161





