Recurrent SARS-CoV-2 Serology Testing and Pandemic Anxiety: A Study of Pediatric Healthcare Workers
Abstract
:1. Introduction
2. Methods
2.1. Design and Participants
2.2. Procedures
2.3. Measures
2.4. Statistical Analysis
3. Results
3.1. Baseline Anxiety
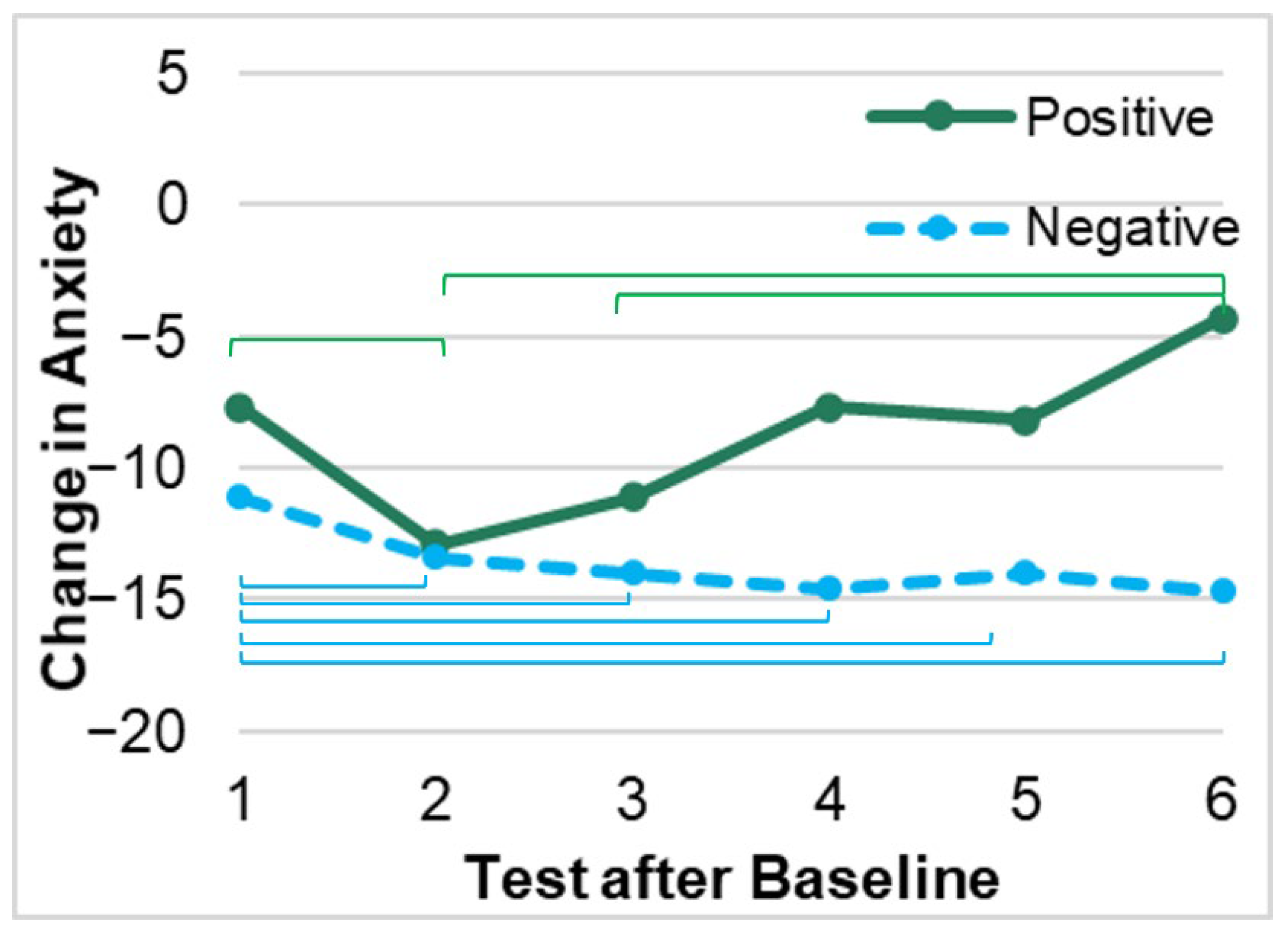
3.2. Changes in Anxiety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- COVID-19 Map—Johns Hopkins Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/map.html (accessed on 3 December 2021).
- Gaffney, A.; Himmelstein, D.U.; Woolhandler, S. COVID-19 and US Health Financing: Perils and Possibilities. Int. J. Health Serv. 2020, 50, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Armocida, B.; Formenti, B.; Ussai, S.; Palestra, F.; Missoni, E. The Italian health system and the COVID-19 challenge. Lancet Public Health 2020, 5, e253. [Google Scholar] [CrossRef]
- Twenge, J.M.; Joiner, T.E. Mental distress among U.S. adults during the COVID-19 pandemic. J. Clin. Psychol. 2020, 76, 2170–2182. [Google Scholar] [CrossRef] [PubMed]
- Kearney, A.; Hamel, L.; Brodie, M. Mental Health Impact of the COVID-19 Pandemic: An Update. Available online: https://www.kff.org/coronavirus-covid-19/poll-finding/mental-health-impact-of-the-covid-19-pandemic/ (accessed on 3 December 2021).
- Muller, A.E.; Hafstad, E.V.; Himmels, J.P.W.; Smedslunda, G.; Flottorp, S.; Stenslandb, S.Ø.; Stroobants, S.; Van de Velde, S.; Vist, G.E. The mental health impact of the covid-19 pandemic on healthcare workers, and interventions to help them: A rapid systematic review. Psychiatry Res. 2020, 293, 113441. [Google Scholar] [CrossRef] [PubMed]
- Batra, K.; Singh, T.P.; Sharma, M.; Batra, R.; Schvaneveldt, N. Investigating the Psychological Impact of COVID-19 among Healthcare Workers: A Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 9096. [Google Scholar] [CrossRef]
- Krishnamoorthy, Y.; Nagarajan, R.; Saya, G.K.; Menon, V. Prevalence of psychological morbidities among general population, healthcare workers and COVID-19 patients amidst the COVID-19 pandemic: A systematic review and meta-analysis. Psychiatry Res. 2020, 293, 113382. [Google Scholar] [CrossRef] [PubMed]
- D’Ettorre, G.; Ceccarelli, G.; Santinelli, L.; Vassalini, P.; Innocenti, G.P.; Alessandri, F.; Koukopoulos, A.E.; Russo, A.; d’Ettorre, G.; Tarsitani, L. Post-Traumatic Stress Symptoms in Healthcare Workers Dealing with the COVID-19 Pandemic: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 601. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.M.; Medak, A.J.; Baumann, B.M.; Lim, S.; Chinnock, B.; Frazier, R.; Cooper, R.J. Academic Emergency Medicine Physicians’ Anxiety Levels, Stressors, and Potential Stress Mitigation Measures During the Acceleration Phase of the COVID-19 Pandemic. Acad. Emerg. Med. 2020, 27, 700–707. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, S.X.; Looi, K.H.; Su, R.; Li, J. Perception of Health Conditions and Test Availability as Predictors of Adults’ Mental Health during the COVID-19 Pandemic: A Survey Study of Adults in Malaysia. Int. J. Environ. Res. Public Health 2020, 17, 5498. [Google Scholar] [CrossRef] [PubMed]
- Shanafelt, T.; Ripp, J.; Trockel, M. Understanding and Addressing Sources of Anxiety Among Health Care Professionals During the COVID-19 Pandemic. JAMA 2020, 323, 2133. [Google Scholar] [CrossRef] [PubMed]
- Park, P.; Simmons, R.K.; Prevost, A.T.; Griffin, S.J. Screening for type 2 diabetes is feasible, acceptable, but associated with increased short-term anxiety: A randomised controlled trial in British general practice. BMC Public Health 2008, 8, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaerlev, L.; Iachina, M.; Pedersen, J.H.; Green, A.; Nørgård, B.M. CT-Screening for lung cancer does not increase the use of anxiolytic or antidepressant medication. BMC Cancer 2012, 12, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, R.; Mills, N.; Sanford, E.; Graham, A.; Low, N.; Peters, T.J. Does population screening for Chlamydia trachomatisraise anxiety among those tested? Findings from a population based chlamydia screening study. BMC Public Health 2006, 6, 106. [Google Scholar] [CrossRef] [Green Version]
- Collins, R.E.; Lopez, L.M.; Marteau, T.M. Emotional impact of screening: A systematic review and meta-analysis. BMC Public Health 2011, 11, 603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chad-Friedman, E.; Coleman, S.; Traeger, L.N.; Pirl, W.F.; Goldman, R.; Atlas, S.J.; Park, E.R. Psychological distress associated with cancer screening: A systematic review: Cancer Screening-Associated Distress. Cancer 2017, 123, 3882–3894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woerner, A.C.; Gallagher, R.C.; Vockley, J.; Adhikari, A.N. The Use of Whole Genome and Exome Sequencing for Newborn Screening: Challenges and Opportunities for Population Health. Front. Pediatr. 2021, 9, 663752. [Google Scholar] [CrossRef] [PubMed]
- Ljubić, T.; Banovac, A.; Buljan, I.; Jerković, I.; Bašić, Ž.; Kružić, A.; Kolić, R.R.; Marušić, K.A.; Anđelinović, S. Effect of SARS-CoV-2 antibody screening on participants’ attitudes and behaviour: A study of industry workers in Split, Croatia. Public Health 2021, 191, 11–16. [Google Scholar] [CrossRef]
- Blake, H.; Corner, J.; Cirelli, C.; Hassard, J.; Briggs, L.; Daly, J.M.; Bennett, M.; Chappell, J.G.; Fairclough, L.; McClure, C.P.; et al. Perceptions and Experiences of the University of Nottingham Pilot SARS-CoV-2 Asymptomatic Testing Service: A Mixed-Methods Study. Int. J. Environ. Res. Public Health 2020, 18, 188. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.M.; Montoy, J.C.C.; Hoth, K.F.; Talan, D.A.; Harland, K.K.; Eyck, P.T.; Mower, W.; Krishnadasan, A.; Santibanez, S.; Mohr, N. Symptoms of Anxiety, Burnout, and PTSD and the Mitigation Effect of Serologic Testing in Emergency Department Personnel During the COVID-19 Pandemic. Ann. Emerg. Med. 2021, 78, 35–43.e2. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davey, H.M.; Barratt, A.L.; Butow, P.N.; Deeks, J.J. A one-item question with a Likert or Visual Analog Scale adequately measured current anxiety. J. Clin. Epidemiol. 2007, 60, 356–360. [Google Scholar] [CrossRef]
- Dutheil, F.; Marhar, F.; Boudet, G.; Perrier, C.; Naughton, G.; Chamoux, A.; Huguet, P.; Mermillod, M.; Saâdaoui, F.; Moustafa, F. Maximal tachycardia and high cardiac strain during night shifts of emergency physicians. Int. Arch. Occup. Environ. Health 2017, 90, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Dutheil, F.; Pereira, B.; Moustafa, F.; Naughton, G.; Lesage, F.-X.; Lambert, C. At-risk and intervention thresholds of occupational stress using a visual analogue scale. PLoS ONE 2017, 12, e0178948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesage, F.-X.; Berjot, S.; Deschamps, F. Clinical stress assessment using a visual analogue scale. Occup. Med. 2012, 62, 600–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.D.; Ferris, D.G. Measurement of subjective phenomena in primary care research: The Visual Analogue Scale. Fam. Pract. Res. J. 1993, 13, 15–24. [Google Scholar]
- Wewers, M.E.; Lowe, N.K. A critical review of visual analogue scales in the measurement of clinical phenomena. Res. Nurs. Health 1990, 13, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Rossi, V.; Pourtois, G. Transient state-dependent fluctuations in anxiety measured using STAI, POMS, PANAS or VAS: A comparative review. Anxiety Stress Coping 2012, 25, 603–645. [Google Scholar] [CrossRef] [Green Version]
- Williams, V.S.; Morlock, R.J.; Feltner, D. Psychometric evaluation of a visual analog scale for the assessment of anxiety. Health Qual. Life Outcomes 2010, 8, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danoff, J.R.; Goel, R.; Sutton, R.; Maltenfort, M.G.; Austin, M.S. How Much Pain Is Significant? Defining the Minimal Clinically Important Difference for the Visual Analog Scale for Pain After Total Joint Arthroplasty. J. Arthroplast. 2018, 33, S71–S75.e2. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Faravelli, C.; Alessandra Scarpato, M.; Castellini, G.; Lo Sauro, C. Gender differences in depression and anxiety: The role of age. Psychiatry Res. 2013, 210, 1301–1303. [Google Scholar] [CrossRef]
- Elbay, R.Y.; Kurtulmuş, A.; Arpacıoğlu, S.; Karadere, E. Depression, anxiety, stress levels of physicians and associated factors in Covid-19 pandemics. Psychiatry Res. 2020, 290, 113130. [Google Scholar] [CrossRef] [PubMed]
- Heyming, T.; Bacon, K.; Lara, B.; Knudsen-Robbins, C.; Tongol, A.; Sanger, T. SARS-CoV-2 Serology Testing in an Asymptomatic, At-Risk Population: Methods, Results, Pitfalls. Infect. Dis. Rep. 2021, 13, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Heyming, T.; Sanger, T.; Tongol, A.; Schomberg, J.; Bacon, K.; Lara, B. Provider Antibody Serology Study of Virus in the Emergency Room (PASSOVER) Study: Special Population COVID-19 Seroprevalence. West J. Emerg. Med. 2021, 22, 565. [Google Scholar] [CrossRef] [PubMed]
- Eborall, H.C.; Griffin, S.J.; Prevost, A.T.; Kinmonth, A.L.; French, D.P.; Sutton, S. Psychological impact of screening for type 2 diabetes: Controlled trial and comparative study embedded in the ADDITION (Cambridge) randomised controlled trial. BMJ 2007, 335, 486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Negative Sample (n = 307) | Positive Sample (n = 55) | |
|---|---|---|
| Median (IQR) | Median (IQR) | |
| Years of Experience | 10.00 (13.00) | 9.00 (14.00) |
| n (%) | n (%) | |
| Age | ||
| ≤30 years | 98 (31.9) | 20 (36.4) |
| 31–40 years | 98 (31.9) | 17 (30.9) |
| 41–50 years | 72 (23.5) | 13 (23.6) |
| ≥51 years | 39 (12.7) | 5 (9.1) |
| Gender | ||
| Female | 219 (71.3) | 38 (69.1) |
| Male | 88 (28.7) | 17 (30.9) |
| Race/Ethnicity | ||
| African American, Black | 5 (1.6) | 0 (0) |
| American Indian/Alaskan | 1 (0.3) | 0 (0) |
| Asian, Pacific Islander | 64 (20.8) | 8 (14.5) |
| Hispanic/Latino | 55 (17.9) | 13 (23.6) |
| White | 159 (51.8) | 30 (54.5) |
| Multi-Racial | 18 (5.9) | 2 (3.6) |
| Other | 5 (1.6) | 2 (3.6) |
| Department | ||
| Emergency Department | 120 (39.1) | 13 (23.6) |
| Operating Room Pre-Operative | 91 (29.6) | 17 (30.9) |
| Pediatric Intensive Care Unit | 60 (19.5) | 17 (30.9) |
| Transport | 36 (11.7) | 8 (14.5) |
| Position | ||
| Physician | 58 (18.9) | 11 (20.0) |
| Physician Assistant | 10 (3.3) | 0 (0) |
| Nurse Practitioner, Registered Nurse | 166 (54.1) | 28 (50.9) |
| Tech or Administrative | 73 (23.8) | 16 (29.1) |
| Baseline Anxiety Mean (SD) | p-Value | ||||
|---|---|---|---|---|---|
| Gender | Female | Male | 0.009 | ||
| 51.55 (24.93) a | 39.56 (27.29) b | ||||
| Job Position | Physician/ PA | Nurse | Technician | 0.003 | |
| 44.70 (25.40) a | 52.84 (25.07) b | 40.69 (27.28) a | |||
| Department | Emergency Department | Intensive Care Unit | Peri-Operative | Transport | 0.002 |
| 43.42 (26.73) a | 55.75 (23.39) b | 49.61 (26.12) a,b | 45.00 (26.52) a | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Martin, S.R.; Heyming, T.W.; Knudsen-Robbins, C.; Sanger, T.; Kain, Z.N. Recurrent SARS-CoV-2 Serology Testing and Pandemic Anxiety: A Study of Pediatric Healthcare Workers. Int. J. Environ. Res. Public Health 2022, 19, 9562. https://doi.org/10.3390/ijerph19159562
Li N, Martin SR, Heyming TW, Knudsen-Robbins C, Sanger T, Kain ZN. Recurrent SARS-CoV-2 Serology Testing and Pandemic Anxiety: A Study of Pediatric Healthcare Workers. International Journal of Environmental Research and Public Health. 2022; 19(15):9562. https://doi.org/10.3390/ijerph19159562
Chicago/Turabian StyleLi, Natasha, Sarah R. Martin, Theodore W. Heyming, Chloe Knudsen-Robbins, Terence Sanger, and Zeev N. Kain. 2022. "Recurrent SARS-CoV-2 Serology Testing and Pandemic Anxiety: A Study of Pediatric Healthcare Workers" International Journal of Environmental Research and Public Health 19, no. 15: 9562. https://doi.org/10.3390/ijerph19159562
APA StyleLi, N., Martin, S. R., Heyming, T. W., Knudsen-Robbins, C., Sanger, T., & Kain, Z. N. (2022). Recurrent SARS-CoV-2 Serology Testing and Pandemic Anxiety: A Study of Pediatric Healthcare Workers. International Journal of Environmental Research and Public Health, 19(15), 9562. https://doi.org/10.3390/ijerph19159562






