Abstract
Atopic dermatitis (AD), chronic hand eczema (CHE), and urticaria are common inflammatory skin diseases among adolescents and associated with air quality. However, the synergistic effects of ambient air pollution and second-hand smoke (SHS) have been unclear. We conducted a cross-sectional study including 20,138 Chinese college students where dermatological examinations and a questionnaire survey were carried out. A generalized linear mixed model was applied for the association between individualized exposure of O3, CO, NO2, SO2, PM2.5, and PM10 and the prevalence of inflammatory skin diseases. Interactions between air pollutants and SHS were analyzed. As a result, CO, NO2, SO2, PM2.5, and PM10 were positively correlated with the prevalence of AD, CHE, and urticaria. Higher frequency of SHS exposure contributed to increased probabilities of AD (p = 0.042), CHE (p < 0.001), and urticaria (p = 0.002). Of note, CO (OR: 2.57 (1.16–5.69) in third quartile) and NO2 (OR: 2.38 (1.07–5.27) in third quartile) had positive interactions with SHS for AD, and PM2.5 synergized with SHS for CHE (OR: 2.25 (1.22–4.15) for second quartile). Subgroup analyses agreed with the synergistic results. In conclusion, SHS and ambient air pollution are both associated with inflammatory skin diseases, and they have a synergistic effect on the prevalence of AD and CHE.
1. Introduction
Atopic dermatitis (AD), chronic hand eczema (CHE), and urticaria are among the most common inflammatory skin diseases in adolescents. These diseases heavily impair quality of life for patients and impose financial burdens for the society [1,2]. AD and CHE are atopic diseases characterized by intense pruritus and eczematous lesions [3]. Urticaria is pruritic wheal and/or angioedema which can be induced by certain triggering factors [4]. There is a strong link between atopic diseases and urticaria, and they share many similar risk factors [5,6]. It is known that the prevalence rates of atopic disease and skin allergy are higher in more industrialized and developed countries, and those in developing countries are relatively lower [7,8,9]. In addition to genetic factors, environmental determinants contribute to the epidemic variation and partially explain the etiology of atopic diseases.
Accumulating evidence has shown the association between ambient air pollution and atopic skin diseases. NO2, SO2, CO, or O3 were responsible for increased lifetime eczema or diagnosed AD over the last year [10,11,12,13]. PM2.5 and PM10 were associated with higher prevalence of eczema by some studies but protective factors in others [10,12,14], while some studies demonstrated that air pollution does not necessarily affect the development of eczema or AD [15,16,17]. Disruption of skin barrier integrity, immune-related responses, and alteration of gut microbiota have recently been proposed as the potential mechanisms of the association between air pollutants and atopic skin diseases [18,19,20]. The correlation between urticaria and air pollution has rarely been explored. An air quality health index based on O3, NO2, and PM2.5 was shown to be positively correlated with emergency department visits for urticaria [18]. Nonetheless, these studies failed to consider individualized metabolism, which influences the actual exposure levels of air pollutants. In addition, previous studies concentrated on younger age groups, mostly preschool children, but prevalence of atopic diseases varied among different ages; however, the effect of air pollution on adolescents is still vague [12,15,16].
Second-hand smoke (SHS) is a behavioral factor that threatens human health, and it is associated with up to 600,000 deaths in nonsmokers each year [21]. In China, smoking is not well controlled [22]. In public places including restaurants, hospitals, and universities, a smoke-free policy has not been implemented. A recent study by our team confirmed that SHS exposure was associated with atopic dermatitis and hand eczema in a dose–response manner, which coincides with a Korean study where SHS incursion into homes was related with allergic symptoms for children [23,24]. However, the relationship of SHS with urticaria was not addressed by previous studies. Furthermore, SHS has been combined with other factors to evaluate the interaction effect on development of allergic diseases. SHS can enhance the adverse health effect of antibiotic use on rhinitis and asthma [25]. However, the interactions between SHS and other ambient air pollutants in atopic diseases are still unclear.
In this study, we conducted a cross-sectional study including 20,138 Chinese college students, to investigate the association of individualized exposure to ambient air pollution with AD, CHE, and urticaria, as well as the interactions between SHS and air pollution. A generalized linear mixed model was applied as an appropriate model to fit the random effect of clusters (city district or county). Importantly, this study fills the gap in large-scale research regarding the prevalence of inflammatory skin diseases and air pollution in Chinese adolescents, as well as adds new knowledge on the synergistic effects between environmental factor and SHS in inflammatory skin diseases.
2. Materials and Methods
2.1. Study Population
The study was conducted in September and October 2018 on freshman students in five universities at Changsha City, where 20,138 students from all 34 provinces across China were enrolled. The geographical distribution of the students’ origin is shown in Supplementary Figure S1. With the consent to participate, students received an evaluation for dermatologic conditions. The disease diagnosis for each participant was determined by certificated and experienced dermatologists. The diagnostic criteria for AD, CHE, and urticaria included clinical manifestation, disease history, family history, and physical examinations, according to the guidelines from the American Academy of Dermatology [26]. All doctors were trained for the diagnostic criteria before the field survey. More than 80 investigators from different cooperating hospitals participated in the survey. For recurrent symptoms or lesions, only the current manifestation was considered. A questionnaire survey followed immediately, which included demographic information and lifestyle items including activities, sleep and bathing. The detailed questionnaire content relevant to this study is shown in Table S1. This study was approved by the medical ethics committee of Xiangya Hospital, Central South University.
2.2. Environmental Factors
Ambient air pollution levels of O3, CO, NO2, SO2, PM2.5, and PM10, as well as humidity and temperature, were obtained from the 6 year long high-resolution air quality dataset over China from 2013 to 2018 [27]. Of the 20,138 participants, 19,064 were linked to environmental data by city district or county code of the participants’ hometown, whereas the other 1074 had incomplete information of their hometown location. To fully consider individual differences, adjusted daily exposure to pollutants was estimated using the following procedure: (1) calculate the mean concentration of air pollutants for 2013 to 2018; (2) estimate basal metabolic rate (BMR) by individual age and sex according to a nationwide study among the Chinese population [28]; (3) estimate the metabolic equivalents (METs) for school time (assuming 40 h/week for all students), physical activities (self-reported type, frequency, and duration), and sleep (self-reported). The information on individual activities and sleep was obtained from the questionnaire survey, the details of which can be found in Table S1; (4) adjust BMR by METs; (5) estimate the METs-weighted respiratory rate; (6) estimate averagely daily exposure (mg/kg·day or μg/kg·day) to pollutants according to the 6 year mean concentrations and respiratory rate [3,29]. Consequently, the adjusted daily dose of each pollutant for each participant was calculated. Details can be found in our previous publication [30].
2.3. Covariates
For covariates, sex and family income were considered as demographic variables; SHS, bath frequency, and BMI were included as behavioral habit variables; humidity and temperature were treated as environmental covariates [21,29]. Information on sex (male/female), age (years), second-hand smoke (SHS) (never, <1 day/week, and ≥1 day/week), family annual income (<10,000, 10,000–29,999, 30,000–49,999, 50,000–99,999, 100,000–199,999, and >200,000 CNY/year), frequency of bathing (≤1/week, 2–4/week, 5–7/week, and ≥8/week) was obtained through the questionnaire survey mentioned above. The body mass index (BMI) was calculated as weight/squared height (kg/m2) obtained from physical examinations.
2.4. Statistical Analysis
Statistical analyses were performed using SPSS version 23 (IBM SPSS Statistics 23) and R version 4.0.4. Continuous data were presented as the mean ± standard deviation (SD) and compared using ANOVA for differences between groups, whereas categorical data were presented as the number (%) and compared using the chi-square test. Covariates were compared between case and control populations for AD, CHE, and urticaria. The adjusted daily dose of exposure to each pollutant was divided into quartiles according to 25th, 50th, and 75th percentiles to evaluate the odds ratio (OR) for diseases.
The generalized linear mixed model was applied to estimate the association between ambient air pollution and prevalence of AD, CHE, and urticaria, where city (the city from which the student comes) was treated as a random effect. The analysis consisted of unadjusted and adjusted models. The unadjusted model described the crude OR; in the adjusted model, OR was adjusted for demographic variables (sex and family income), behavioral habits (bath frequency and BMI), and environmental factors (humidity and temperature). The model was established using package “lme4” in R. Subgroup analysis was performed on the basis of the adjusted model. A depiction of the linear association between air pollutants and disease prevalence was further performed using package “ggplot2”.
To assess the synergistic effects between air pollutants and SHS for the prevalence of AD, CHE, and urticaria, the generalized linear mixed model was applied, with city as a random effect, and the model was adjusted for demographic, behavioral, and environmental variables.
3. Results
The study population included 19,064 college students (51.5% of male) with a mean age of 18.3 years. The general characteristics of the whole population are summarized in Table 1. Among these students, 481 (2.5%), 560 (2.9%), and 1332 (7.0%) were diagnosed with AD, CHE, and urticaria, respectively. The distribution of daily dose of exposure to O3, CO, NO2, SO2, PM2.5, and PM10 level is summarized for the total population and separately for case and control for AD, CHE, and urticaria in Table 2. The levels of each pollutant were divided into four quartiles to assess OR for diseases.

Table 1.
General information of total study population (n = 19,064).

Table 2.
Distribution of air pollution exposure for case and control populations.
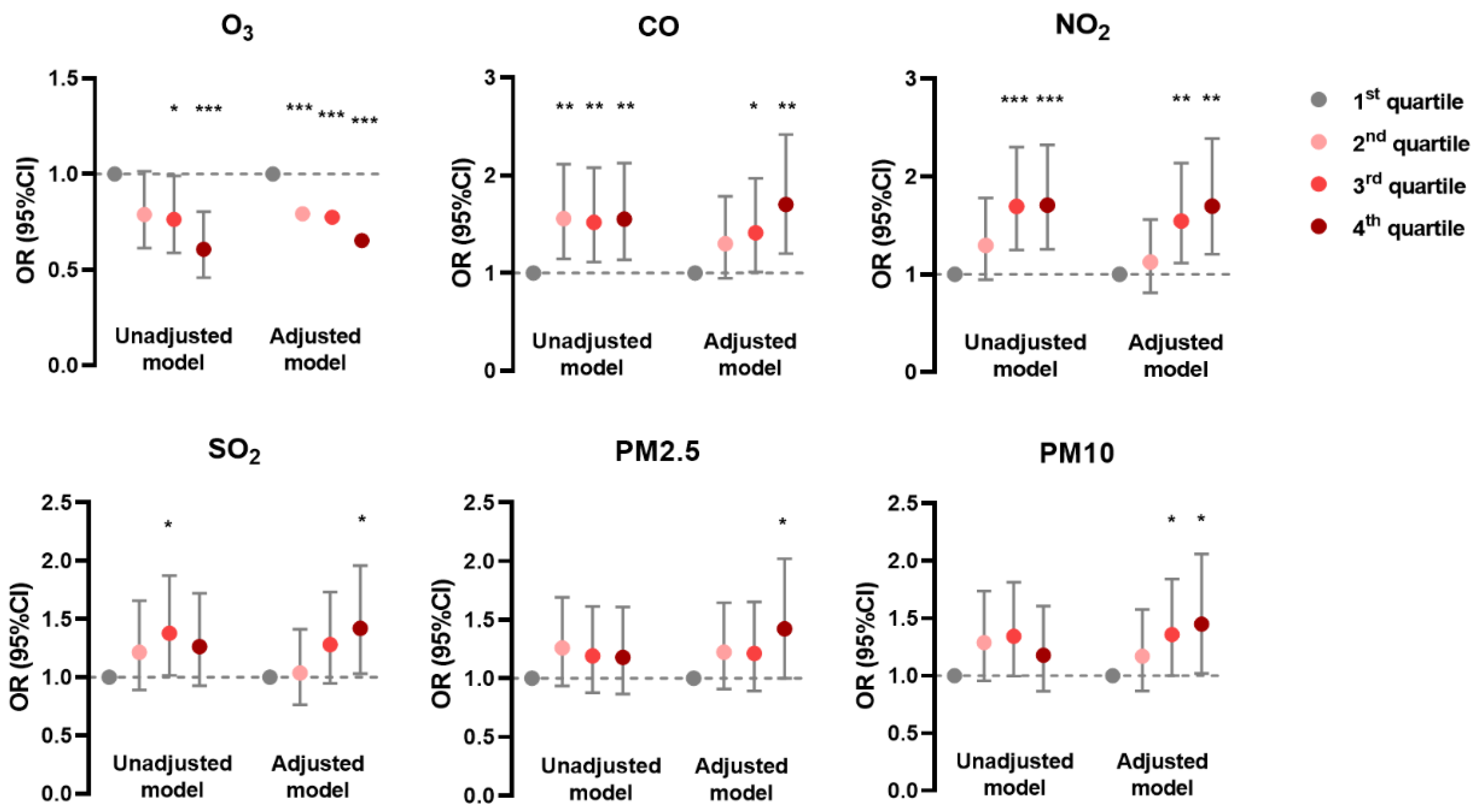
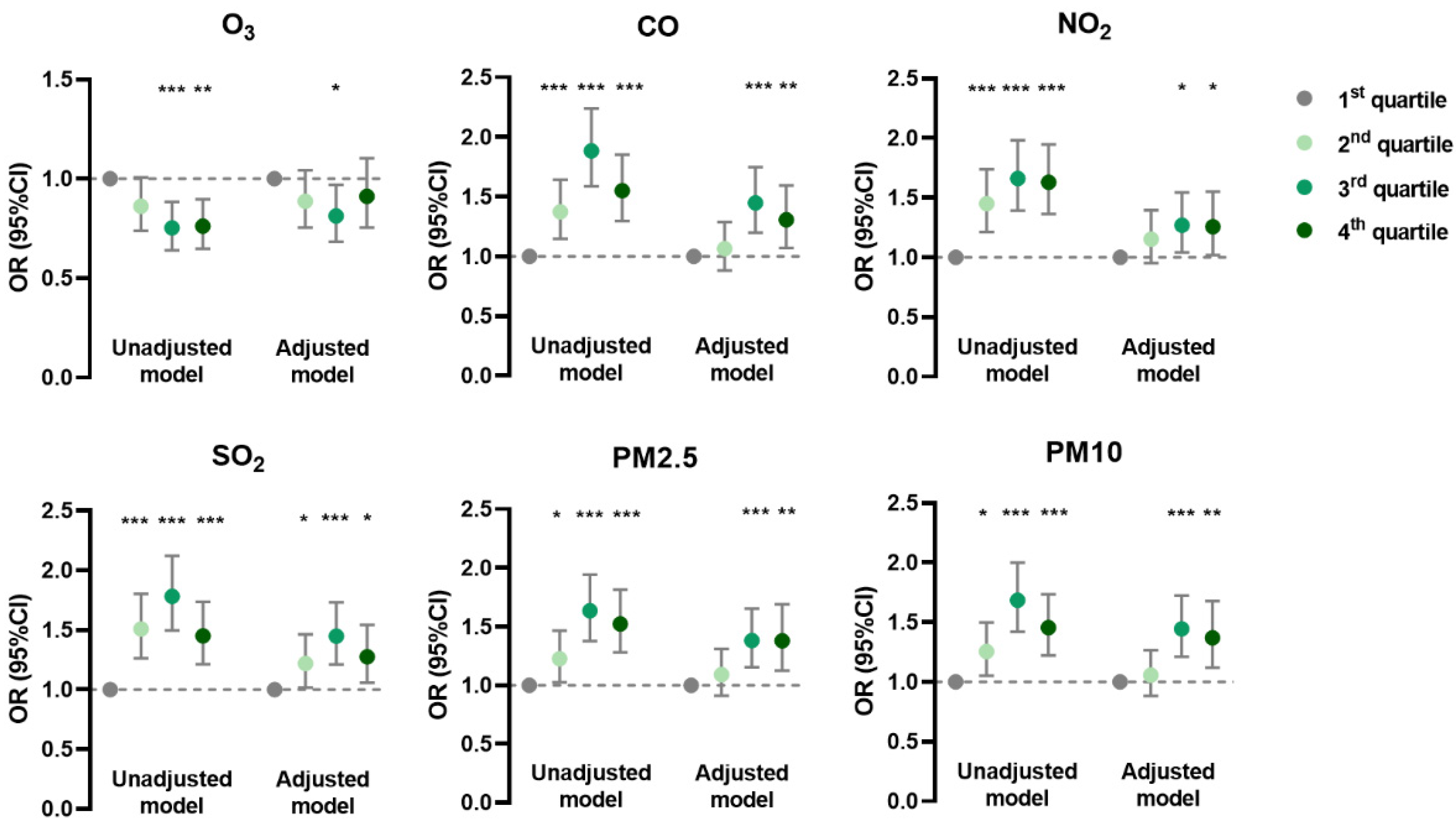
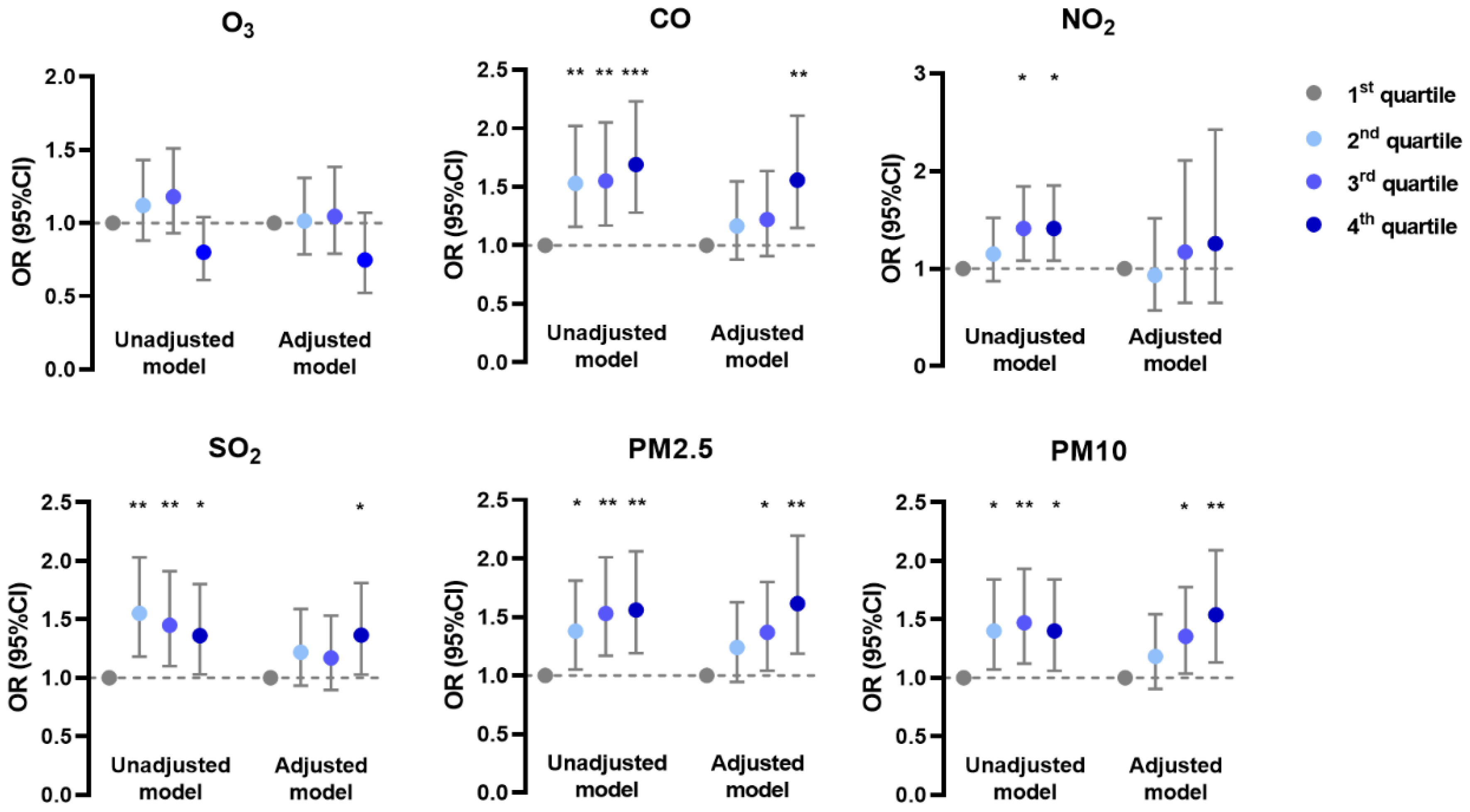
In the generalized linear mixed model, the prevalence of AD was negatively associated with O3 and positively associated with CO, NO2, and SO2 in unadjusted and adjusted models; it was positively associated with PM2.5 and PM10 mainly in the adjusted model (Figure 1). The prevalence of CHE had positive correlations with CO, SO2, PM2.5, and PM10 in both models, but with NO2 only in the unadjusted model (Figure 2). Meanwhile, the prevalence of urticaria increased with higher levels of CO, NO2, SO2, PM2.5, and PM10, but decreased with O3 in both models (Figure 3). Linear associations between air pollutants and the prevalence of AD, CHE, and urticaria are shown in Figures S2–S4.
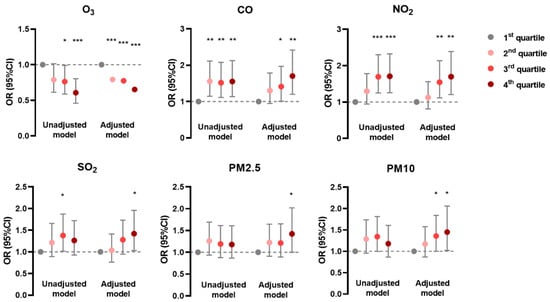
Figure 1.
Association between atopic dermatitis and adjusted daily dose of ambient air pollutants. The unadjusted model describes the crude OR; the adjusted model was adjusted for demographic variables, behavioral habits, and environmental factors. * 0.01 < p < 0.05; ** 0.001 < p < 0.01; *** p < 0.001.
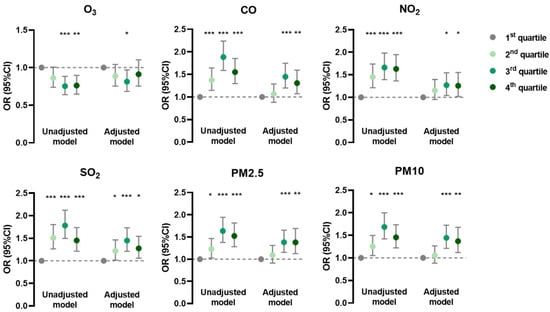
Figure 2.
Association between chronic hand eczema and adjusted daily dose of ambient air pollutants. The unadjusted model describes the crude OR; the adjusted model was adjusted for demographic variables, behavioral habits, and environmental factors. * 0.01 < p < 0.05; ** 0.001 < p < 0.01; *** p < 0.001.
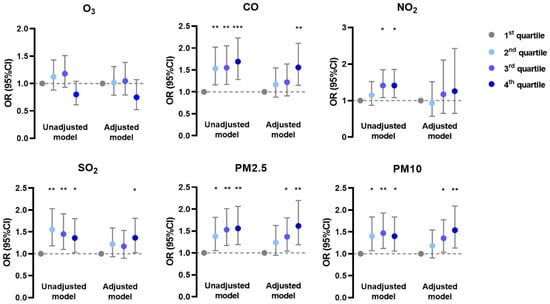
Figure 3.
Association between urticaria and adjusted daily dose of ambient air pollutants. The unadjusted model describes the crude OR; the adjusted model was adjusted for demographic variables, behavioral habits, and environmental factors. * 0.01 < p < 0.05; ** 0.001 < p < 0.01; *** p < 0.001.
The association of second-hand smoke (SHS) with AD, CHE, and urticaria was analyzed. Notably, a higher frequency of SHS exposure was positively correlated with the prevalence of all three diseases (Table 3). Other covariates including sex, family income, BMI, and bath frequency for AD, CHE, and urticaria are shown in Table S2.

Table 3.
Association of second-hand smoke with inflammatory skin diseases.
Furthermore, the synergistic effects between SHS and air pollution exposure were estimated. As the proportions of students with SHS exposure for <1 day/week and ≥1 day/week were relatively low, we combined these two groups as the SHS exposure population, which was compared to the group with no SHS exposure. As shown in Table 4, CO had a positive interaction with SHS for AD (OR (95% CI): 2.57 (1.16–5.69) for third quartile, p = 0.020), suggesting that students who were exposed to SHS, compared to those without SHS exposure, were more likely to have AD under higher exposure levels of CO. Therefore, SHS enhanced the adverse effects of CO for AD. Similarly, NO2 exposure synergized with SHS for AD (OR (95% CI): 2.38 (1.07–5.27) for third quartile, p = 0.032). Meanwhile, PM2.5 had an interaction with SHS for CHE (OR (95% CI): 2.25 (1.22–4.15) for second quartile, p = 0.009). However, NO2 had a negative interaction with SHS for urticaria (OR (95% CI): 0.65 (0.44–0.97) for fourth quartile, p = 0.034).

Table 4.
Interactions between second-hand smoke (SHS) and air pollution exposure (OR (95% CI)).
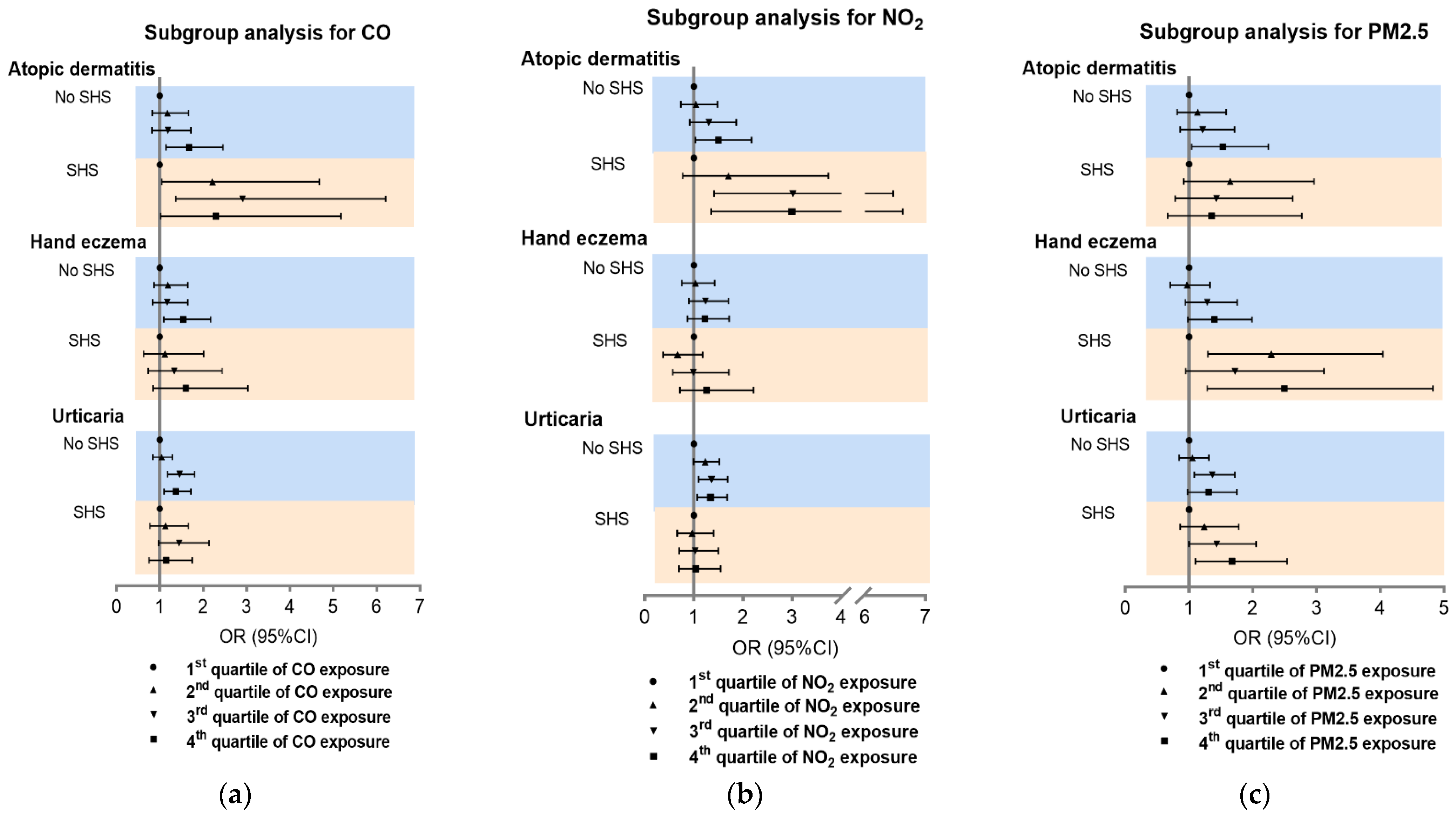
On the basis of the synergistic effects above, SHS subgroup analysis was carried out for the association of CO, NO2, and PM2.5 with inflammatory skin diseases. Consistent with the synergistic analysis results, the effects of CO and NO2 were greater on AD in students with SHS exposure than those without SHS (Figure 4a,b). Furthermore, PM2.5 had a more significant effect on CHE in students with SHS compared to those without SHS (Figure 4c). Subgroup analysis for O3, SO2, and PM10 showed a lower association of SHS with disease prevalence (Figure S5).
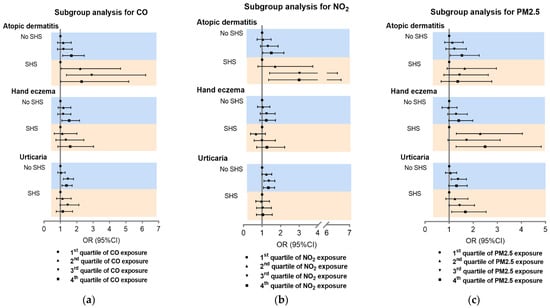
Figure 4.
Subgroup analysis of association between CO, NO2, and PM2.5 and inflammatory skin diseases. Subgroup analysis for CO (a), NO2 (b) and PM2.5 (c). SHS: second-hand smoke.
4. Discussion
In the present study, we found a significant correlation of AD and urticaria with O3, CO, NO2, SO2, PM2.5, and PM10, and of CHE with CO, SO2, PM2.5, and PM10. SHS contributed to a higher prevalence of AD, CHE, and urticaria. SHS had synergistic effects with NO2 and CO on AD, and with PM2.5 on CHE.
This is the first epidemic research showing the negative correlation between O3 and AD. A Korean study with elementary school children and a Belarusian study with 0–2 year old kids both showed that O3 level contributed to a high risk of AD [11,13]. Another cross-sectional study including over 1,000,000 children in Taiwan found no correlation between O3 and AD [17]. To our surprise, we found that O3 was negatively associated with AD prevalence. Interestingly, recent studies raised that O3 could potentially serve as a therapeutic regimen for AD. In a randomized controlled clinical trial, patients had a significant decrease in SCORAD scores and inflammatory cell infiltration in AD lesions after 3 day ozone therapy (ozone hydrotherapy followed by ozonated oil) [31]. Mechanistically, O3 has a bactericidal effect on Staphylococcus aureus (S. aureus), which accounts for 90% of the microbiome in AD lesions [31]. Another fundamental study revealed that ozonated oil decreased the Th2-dominant cytokines response and increased IL-10 expression, thereby suppressing inflammation in AD murine model [32]. However, whether O3 interferes with the original development and protects against the onset of AD is still unknown.
We found that PM2.5 and PM10 were both positively associated with AD. Particulate matter has been shown to be related with not only respiratory diseases (COPD and asthma) but also lung cancer, mental disorders, and pregnancy outcomes [33,34,35,36,37]. The correlation between particulate matter and atopic dermatitis is inconsistent. A France study showed that a higher level of PM10 increased the risk of both lifetime AD and AD in the past year [38]. PM2.5 increased the risk of lifetime AD by a Germany study with a population aged over 70 [14], while a US study showed that PM2.5 and PM10 were negatively associated with AD [10]. Results from our study agreed with the positive association. Research on the mechanism behind this association has been limited. In a mouse model study, particulate matter exposure upregulated the expression of SPRR2D, S100A9, STFA3, CHIL1, DBP, and IL1B, which are responsible for skin barrier integrity and immune response [18]. Another group revealed that skin inflammation induced by ambient particulate is attributable to oxidative stress or programmed cell death [19].
Other pollutants including NO2, SO2, and CO were all positively associated with AD in our study. The association between NO2 and AD was consistent a previous study with over 90,000 individuals aged 0–17 years in the US and a case–control study with 0–2 year old children in Belarus [10,13]. The association of CO with AD was less frequently reported, but our result agrees with the Belarusian study mentioned above [13]. Interestingly, another study from Shanghai, China, showed that SO2 was not associated with AD [12]. The difference could derive from the different sample sizes and age groups, as their study was performed on 3358 preschool children, while ours included 19,064 adolescents. In addition, only the fourth quartile of SO2 exposure level was significantly correlated with AD in our study, suggesting that the association was not necessarily distinct.
Chronic hand eczema was found to be positively associated higher levels of CO, SO2, PM2.5, and PM10 in our study. Although there is sufficient research on ambient air pollution and AD, studies on hand eczema are rarely reported. A cross-sectional study in southern Sweden showed that living within 100 m of a road with more than 10 cars per minute was associated with hand eczema during the last 12 months; however, the classification of air pollution source was not specified [39]. Therefore, our study adds new knowledge on the correlation between ambient air pollution and chronic hand eczema. It was observed that particulate matter, especially diesel exhaust particles (DEPs), could change the composition and function of the gut microbiota [20]. Furthermore, there was a varied abundance of specific bacterial genera in children who developed IgE-associated allergic disease [40]. This suggests a potential contributing role of particulate matter in the development of allergic diseases via an altered gut microbiota. Moreover, the immune tolerance through the generation of peripheral antigen experienced Treg cells could be related to the mechanism [41,42].
A significant association between CO, SO2, PM2.5, and PM10 and urticaria was demonstrated in our results. This is consistent with the previous finding that positive results were observed between air quality health index (AQHI) and odds ratio for emergency department visits for urticaria in Canada [43]. Although AQHI in their study was based on O3, NO2, and PM2.5, they did not specify the association between each air pollutant and visits for urticaria. Another study from the US showed that the ground-level O3 was positively associated with angioedema [44], while our results showed that the third quartile of adjusted O3 level was negatively associated with the prevalence of urticaria. The effect of O3 on urticaria still needs further exploration.
In this study, we found that SHS was positively correlated with inflammatory skin diseases. Smoking has been identified as a risk factor for AD and CHE, but the association of exposure to SHS with urticaria is barely understood. Our results add more evidence that SHS is hazardous to the health of nonsmoker adolescents. Previous studies showed that SHS induced IL-1β, IL-6, IL-13, and TNF-α secretion from the nasal cavity or saliva, as well as increased CD4+ T cells and IgE in the periphery, thus promoting inflammatory processes and immunological responses [45,46,47]. The association between SHS and inflammatory skin diseases could be due to a similar mechanism, but this still needs confirmation.
Furthermore, a positive interaction between SHS and air pollution has been found. SHS is a source of indoor particulate matter, while air pollution is an outdoor environmental factor. Exposure to both indoor and outdoor air pollution might have a synergistic effect. Recently, SHS was shown to break the homeostasis of cholesterol and bile acid metabolism, as well as change the composition of gut microbiota [48]. Combined with the finding that ambient air pollution, especially particulate matter, also changed the composition and function of the gut microbiota, we might speculate that SHS and ambient air pollution synergistically affect the prevalence of skin inflammatory diseases via mechanisms involving gut microbiota [20].
This study had several strengths. First, we used individualized exposure level for all pollutants, with the consideration that differences in age, sex, and lifestyle including activities and sleep influenced the metabolism and the actual exposure level for individuals. Second, instead of inquiring self-reported symptoms or diagnosis recalled by the participants, we evaluated our participants for dermatological conditions by physicians, thus avoiding recall bias. Third, this study found for the first time a positive interaction between ambient air pollution and SHS, providing evidence for better control of air quality and SHS.
This study had some limitations. First, we assumed that participants were exposed to only an outdoor environment and did not take indoor air quality into consideration. However, indoor particular matter and gas pollution levels are influenced by ventilation and outdoor levels; hence, the indoor air quality is not completely independent of outdoor factors [49,50]. Additionally, the actual indoor pollution levels for all participants are not feasible to obtain. Second, no causation can be concluded from the study since it had a cross-sectional design. However, we retrospectively collected data on air pollution during the last 6 years and diagnosed the skin diseases at the timepoint of research. To some extent, this might suggest that the diseases developed after the air pollution exposure.
5. Conclusions
In conclusion, ambient air pollution (NO2, SO2, CO, PM2.5, and PM10) and second-hand smoke exposure are associated with a higher prevalence of atopic dermatitis, chronic hand eczema, and urticaria among Chinese adolescents. Second-hand smoke interacts with NO2 and CO to enhance the adverse effect on atopic dermatitis, and it also synergizes with PM2.5 to increase the possibility of chronic hand eczema.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijerph191610011/s1: Figure S1. Geographical distribution of 20,138 students; Figure S2. Linear association between atopic dermatitis prevalence and adjusted daily dose of ambient air pollutants and environmental factors; Figure S3. Linear association between chronic hand eczema prevalence and adjusted daily dose of ambient air pollutants and environmental factors; Figure S4. Linear association between urticaria prevalence and adjusted daily dose of ambient air pollutants and environmental factors; Figure S5. Subgroup analysis of association between O3, SO2, PM10, and inflammatory skin diseases; Table S1. Questionnaire content relevant to the study; Table S2. Covariates between case and control populations for inflammatory skin diseases.
Author Contributions
M.L. and Y.X. analyzed the data and drafted the manuscript. M.S. and X.C. designed the study. B.Z. and S.L. analyzed the data. J.S. and J.L. were study site coordinators. M.S., X.C. and M.L. obtained the funding. All authors participated in the field survey and data collection, critically revised the manuscript, and gave final approval to the version submitted for publication. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Project of Intelligent Management Software for Multimodal Medical Big Data for New Generation Information Technology, Ministry of Industry and Information Technology of People’s Republic of China (TC210804V), the National Key Research and Development Project of China “Precision Medicine Initiative” (#2016YFC0900802), the Program of Introducing Talents of Discipline to Universities (111 Project, #B20017), the Natural Science Foundation of Hunan Province (No.2021JJ41004), the China Postdoctoral Science Foundation (2020M682595), and the Medical and Health Research Project of China Aerospace Science and Industry Corporation (2020-LCYL-025). The funders did not participate in this study.
Institutional Review Board Statement
This study was conducted according to the guidelines laid down in the Declaration of Helsinki. All procedures involving study participants were approved by the institutional research ethics boards of Xiangya Hospital, Central South University, China (#201709993).
Informed Consent Statement
Written informed consent was obtained from all participants or their custodians if aged under 18 before the investigation.
Data Availability Statement
Data are available on request from the authors.
Acknowledgments
The authors would like to thank the following dermatologists and investigators who participated in the field survey (in order of family name): Central South University: Lei Cai, Duling Cao, Qin Cao, Chao Chen, Liping Chen, Menglin Chen, Mengting Chen, Xiang Chen, Qing Deng, Xin Gao, Yihuan Gong, Jia Guo, Yeye Guo, Rui Hu, Xin Hu, Chuchu Huang, Huining Huang, Kai Huang, Xiaoyan Huang, Yuzhou Huang, Danrong Jing, Xinwei Kuang, Li Lei, Jia Li, Jiaorui Li, Jie Li, Keke Li, Peiyao Li, Yajia Li, Yayun Li, Yangfan Li, Dan Liu, Dihui Liu, Fangfen Liu, Nian Liu, Panoan Liu, Runqiu Liu, Hui Lu, Wenhua Lu, Yan Luo, Zhongling Luo, Manyun Mao, Mengping Mao, Yuyan Ouyang, Shiyao Pei, Qunshi Qin, Ke Sha, Lirong Tan, Ling Tang, Ni Tang, Yan Tang, Ben Wang, Yaling Wang, Tianhao Wu, Yun Xie, Siyu Yan, Sha Yan, Bei Yan, Xizhao Yang, Lin Ye, Hu Yuan, Taolin Yu, Yan Yuan, Yi Yu, Rui Zhai, Jianghua Zhang, Jianglin Zhang, Mi Zhang, Xingyu Zhang, Zhibao Zhang, Yaqian Zhao, Kuangbiao Zhong, Lei Zhou, Youyou Zhou, Zhe Zhou, and Susi Zhu; Huazhong University of Science and Technology: Xiangjie An, Siqi Da, Yaqi Dong, Yangxue Fu, Lixie Gao, Han Han, Biling Jiang, Jiajia Lan, Jun Li, Xiaonan Li, Yan Li, Liquan Liu, Yuchen Lou, Pu Meng, Yingli Nie, Gong Rao, Shanshan Sha, Xingyu Su, Huinan Suo, Juan Tao, Rongying Wang, Jun Xie, Yuanxiang Yi, Jia Zhang, Qiao Zhang, Li Zhu, and Yanming Zhu; Xiamen University: Zhiming Cai, Lina Chen, Xiaozhu Fu, Hongjun Jiang, Guihua Luo, Shijun Shan, Xiaohui Wang, Jianbing Xiahou, and Binxiang Zheng; People’s Hospital of Xinjiang Uygur Autonomous Region: Jianxia Chen, Xiaomin Chen, Xinqi Chen, Li Dai, Yanyan Feng, Fanhe Jiang, Lan Jin, Xiaojing Kang, Qingyu Ma, Qun Shi, Hongbo Tang, Fang Wang, Zhen Wang, Xiujuan Wu, Kunjie Zhang, and Yu Zhang; Xinjiang Medical University: Huagui Li, Jianguang Li, and Lei Shi; Inner Mongolia Medical University: Wei Wang, Bin Wu, Rina Wu, Hongjun Xing, and Baogui Yang.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Drucker, A.M.; Wang, A.R.; Li, W.-Q.; Sevetson, E.; Block, J.K.; Qureshi, A.A. The Burden of Atopic Dermatitis: Summary of a Report for the National Eczema Association. J. Investig. Dermatol. 2017, 137, 26–30. [Google Scholar]
- Goncalo, M.; Gimenéz-Arnau, A.; Al-Ahmad, M.; Ben-Shoshan, M.; Bernstein, J.; Ensina, L.; Fomina, D.; Galvàn, C.; Godse, K.; Grattan, C.; et al. The global burden of chronic urticaria for the patient and society. Br. J. Dermatol. 2021, 184, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Wallach, D.; Taieb, A. Atopic dermatitis/atopic eczema. Chem. Immunol. Allergy 2014, 100, 81–96. [Google Scholar] [PubMed]
- Zuberbier, T.; Aberer, W.; Asero, R.; Bindslev-Jensen, C.; Brzoza, Z.; Canonica, G.W.; Church, M.K.; Ensina, L.F.; Gimenéz-Arnau, A.; Godse, K.; et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy 2018, 73, 1393–1414. [Google Scholar] [CrossRef]
- Rosman, Y.; Hershko, A.Y.; Meir-Shafrir, K.; Kedem, R.; Lachover-Roth, I.; Mekori, Y.A.; Confino-Cohen, R. Characterization of chronic urticaria and associated conditions in a large population of adolescents. J. Am. Acad. Dermatol. 2019, 81, 129–135. [Google Scholar] [CrossRef]
- Wollenberg, A.; Feichtner, K. Atopic dermatitis and skin allergies—Update and outlook. Allergy 2013, 68, 1509–1519. [Google Scholar] [CrossRef]
- Sears, M.R. Trends in the prevalence of asthma. Chest 2014, 145, 219–225. [Google Scholar] [CrossRef]
- Beasley, R. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet 1998, 351, 1225–1232. [Google Scholar] [CrossRef]
- Tsai, T.F.; Rajagopalan, M.; Chu, C.-Y.; Encarnacion, L.; Gerber, R.A.; Santos-Estrella, P.; Llamado, L.J.Q.; Tallman, A.M. Burden of atopic dermatitis in Asia. J. Dermatol. 2019, 46, 825–834. [Google Scholar] [CrossRef]
- Kathuria, P.; Silverberg, J.I. Association of pollution and climate with atopic eczema in US children. Pediatr. Allergy Immunol. 2016, 27, 478–485. [Google Scholar] [CrossRef]
- Kim, H.-H.; Lee, C.-S.; Jeon, J.-M.; Yu, S.-D.; Park, J.-H.; Shin, D.C.; Lim, Y.-W. Analysis of the association between air pollution and allergic diseases exposure from nearby sources of ambient air pollution within elementary school zones in four Korean cities. Environ. Sci. Pollut. Res. Int. 2013, 20, 4831–4846. [Google Scholar] [CrossRef]
- Liu, W.; Cai, J.; Huang, C.; Hu, Y.; Fu, Q.; Zou, Z.; Sun, C.; Shen, L.; Wang, X.; Pan, J.; et al. Associations of gestational and early life exposures to ambient air pollution with childhood atopic eczema in Shanghai, China. Sci. Total Environ. 2016, 572, 34–42. [Google Scholar] [CrossRef]
- Belugina, I.N.; Yagovdik, N.Z.; Bekugina, O.S.; Belugin, S.N. Outdoor environment, ozone, radionuclide-associated aerosols and incidences of infantile eczema in Minsk, Belarus. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1977–1985. [Google Scholar] [CrossRef]
- Schnass, W.; Hüls, A.; Vierkötter, A.; Krämer, U.; Krutmann, J.; Schikowski, T. Traffic-related air pollution and eczema in the elderly: Findings from the SALIA cohort. Int. J. Hyg. Environ. Health 2018, 221, 861–867. [Google Scholar] [CrossRef]
- Brauer, M.; Hoek, G.; Smit, H.A.; De Jongste, J.C.; Gerritsen, J.; Postma, D.S.; Kerkhof, M.; Brunekreef, B. Air pollution and development of asthma, allergy and infections in a birth cohort. Eur. Respir. J. 2007, 29, 879–888. [Google Scholar] [CrossRef]
- Kramer, U.; Sugiri, D.; Ranft, U.; Krutmann, J.; von Berg, A.; Berdel, D.; Behrendt, H.; Kuhlbusch, T.; Hochadel, M.; Wichmann, H.-E.; et al. Eczema, respiratory allergies, and traffic-related air pollution in birth cohorts from small-town areas. J. Dermatol. Sci. 2009, 56, 99–105. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Su, H.-J.; Sheu, H.-M.; Yu, H.-S.; Guo, Y.L. Traffic-related air pollution, climate, and prevalence of eczema in Taiwanese school children. J. Investig. Dermatol. 2008, 128, 2412–2420. [Google Scholar] [CrossRef]
- Woo, Y.R.; Park, S.-Y.; Choi, K.; Hong, E.S.; Kim, S.; Kim, H.S. Air Pollution and Atopic Dermatitis (AD): The Impact of Particulate Matter (PM10) on an AD Mouse-Model. Int. J. Mol. Sci. 2020, 21, 6079. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.-M.; Liao, L.-Y.; Li, L.; Yi, F.; Meng, H.; He, Y.-F.; Guo, M.-M. Skin inflammation induced by ambient particulate matter in China. Sci. Total. Environ. 2019, 682, 364–373. [Google Scholar] [CrossRef]
- Van den Brule, S.; Rappe, M.; Ambroise, J.; Bouzin, C.; Dessy, C.; Paquot, A.; Muccioli, G.G.; Lison, D. Diesel exhaust particles alter the profile and function of the gut microbiota upon subchronic oral administration in mice. Part Fibre Toxicol. 2021, 18, 7. [Google Scholar] [CrossRef]
- Oberg, M.; Jaakkola, M.S.; Woodward, A.; Peruga, A.; Prüss-Ustün, A. Worldwide burden of disease from exposure to second-hand smoke: A retrospective analysis of data from 192 countries. Lancet 2011, 377, 139–146. [Google Scholar] [CrossRef]
- Reitsma, M.B.; Flor, L.S.; Mullany, E.C.; Gupta, V.; Hay, S.I.; Gakidou, E. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and initiation among young people in 204 countries and territories, 1990–2019. Lancet Public Health 2021, 6, e472–e481. [Google Scholar] [CrossRef]
- Lee, A.; Lee, S.Y.; Lee, K.-S. Association of secondhand smoke exposure with allergic multimorbidity in Korean adolescents. Sci. Rep. 2020, 10, 16409. [Google Scholar] [CrossRef]
- Kim, J.; Lee, E.; Lee, K.; Kim, K. Relationships Between Secondhand Smoke Incursion and Wheeze, Rhinitis, and Eczema Symptoms in Children Living in Homes without Smokers in Multi-Unit Housing. Nicotine Tob. Res. 2019, 21, 424–429. [Google Scholar] [CrossRef]
- Norback, D.; Lu, C.; Zhang, Y.; Li, B.; Zhao, Z.; Huang, C.; Zhang, X.; Qian, H.; Sun, Y.; Wang, J.; et al. Sources of indoor particulate matter (PM) and outdoor air pollution in China in relation to asthma, wheeze, rhinitis and eczema among pre-school children: Synergistic effects between antibiotics use and PM10 and second hand smoke. Environ. Int. 2019, 125, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Eichenfield, L.F.; Tom, W.L.; Chamlin, S.L.; Feldman, S.R.; Hanifin, J.M.; Simpson, E.L.; Berger, T.G.; Bergman, J.N.; Cohen, D.E.; Cooper, K.D.; et al. Guidelines of care for the management of atopic dermatitis: Section 1. Diagnosis and assessment of atopic dermatitis. J. Am. Acad. Dermatol. 2014, 70, 338–351. [Google Scholar] [CrossRef]
- Kong, L.; Tang, X.; Zhu, J.; Wang, Z.; Li, J.; Wu, H.; Wu, Q.; Chen, H.; Zhu, L.; Wang, W.; et al. A Six-Year Long High-Resolution Air Quality Reanalysis Dataset over China from 2013 to 2018 (Monthly and Annual Version); Science Data Bank: Beijing, China, 2020; Volume 1. [Google Scholar]
- Liu, P.; Wang, B.B.; Zhao, X.G.; Duan, X.L.; Huang, N.; Chen, Y.T.; Wang, L.M. Research on inhalation rate of Chinese adults. J. Environ. Health 2014, 31, 953–956. [Google Scholar]
- Ministry of Environmental Protection, P.R.C. Technical Guideline for Population Exposure Assessment of Environmental Pollutant; National Standard for Environmental Protection HJ 875-2017; Ministry of Environmental Protection, P.R.China: Beijing, China, 2017. [Google Scholar]
- Shen, M.; Gu, X.; Li, S.; Yu, Y.; Zou, B.; Chen, X. Exposure to black carbon is associated with symptoms of depression: A retrospective cohort study in college students. Environ. Int. 2021, 157, 106870. [Google Scholar] [CrossRef]
- Zeng, J.; Dou, J.; Gao, L.; Xiang, Y.; Huang, J.; Ding, S.; Chen, J.; Zeng, Q.; Luo, Z.; Tan, W.; et al. Topical ozone therapy restores microbiome diversity in atopic dermatitis. Int. Immunopharmacol. 2020, 80, 106191. [Google Scholar] [CrossRef]
- Lu, J.; Chen, M.; Gao, L.; Cheng, Q.; Xiang, Y.; Huang, J.; Wu, K.; Li, M. A preliminary study on topical ozonated oil in the therapeutic management of atopic dermatitis in murine. J. Dermatol. Treat 2018, 29, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Mengersen, K.; Kimlin, M.; Zhou, M.; Tong, S.; Fang, L.; Wang, B.; Hu, W. Lung cancer and particulate pollution: A critical review of spatial and temporal analysis evidence. Environ. Res. 2018, 164, 585–596. [Google Scholar] [CrossRef]
- Zhang, S.; Li, G.; Tian, L.; Guo, Q.; Pan, X. Short-term exposure to air pollution and morbidity of COPD and asthma in East Asian area: A systematic review and meta-analysis. Environ. Res. 2016, 148, 15–23. [Google Scholar] [CrossRef]
- Bloemsma, L.D.; Hoek, G.; Smit, L.A.M. Panel studies of air pollution in patients with COPD: Systematic review and meta-analysis. Environ. Res. 2016, 151, 458–468. [Google Scholar] [CrossRef]
- Dutheil, F.; Comptour, A.; Morlon, R.; Mermillod, M.; Pereira, B.; Baker, J.S.; Charkhabi, M.; Clinchamps, M.; Bourdel, N. Autism spectrum disorder and air pollution: A systematic review and meta-analysis. Environ. Pollut. 2021, 278, 116856. [Google Scholar] [CrossRef]
- Klepac, P.; Locatelli, I.; Korošec, S.; Künzli, N.; Kukec, A. Ambient air pollution and pregnancy outcomes: A comprehensive review and identification of environmental public health challenges. Environ. Res. 2018, 167, 144–159. [Google Scholar] [CrossRef]
- Penard-Morand, C.; Raherison, C.; Charpin, D.; Kopferschmitt, C.; Lavaud, F.; Caillaud, D.; Annesi-Maesano, I. Long-term exposure to close-proximity air pollution and asthma and allergies in urban children. Eur. Respir. J. 2010, 36, 33–40. [Google Scholar] [CrossRef]
- Lindgren, A.; Stroh, E.; Nihlén, U.; Montnémery, P.; Axmon, A.; Jakobsson, K. Traffic exposure associated with allergic asthma and allergic rhinitis in adults. A cross-sectional study in southern Sweden. Int. J. Health Geogr. 2009, 8, 25. [Google Scholar] [CrossRef]
- Sjodin, K.S.; Hammarström, M.-L.; Rydén, P.; Sjödin, A.; Hernell, O.; Engstrand, L.; West, C.E. Temporal and long-term gut microbiota variation in allergic disease: A prospective study from infancy to school age. Allergy 2019, 74, 176–185. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef]
- Round, J.L.; Lee, S.M.; Li, J.; Tran, G.; Jabri, B.; Chatila, T.A.; Mazmanian, S.K. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 2011, 332, 974–977. [Google Scholar] [CrossRef]
- Kousha, T.; Valacchi, G. The air quality health index and emergency department visits for urticaria in Windsor, Canada. J. Toxicol. Environ. Health A 2015, 78, 524–533. [Google Scholar] [CrossRef]
- Kedarisetty, S.; Jones, E.; Tint, D.; Soliman, A.M.S. Air Pollution and Angioedema. Otolaryngol. Head Neck Surg. 2019, 161, 431–438. [Google Scholar] [CrossRef]
- Riis, J.L.; Granger, D.A.; DiPietro, J.A.; Bandeen-Roche, K.; Johnson, S.B. Salivary cytokines as a minimally-invasive measure of immune functioning in young children: Correlates of individual differences and sensitivity to laboratory stress. Dev. Psychobiol. 2015, 57, 153–167. [Google Scholar] [CrossRef]
- Mahabee-Gittens, E.M.; Merianos, A.L.; Fulkerson, P.C.; Stone, L.; Matt, G.E. The Association of Environmental Tobacco Smoke Exposure and Inflammatory Markers in Hospitalized Children. Int. J. Environ. Res. Public. Health 2019, 16, 4625. [Google Scholar] [CrossRef]
- Yao, T.-C.; Chang, S.-W.; Hua, M.-C.; Liao, S.-L.; Tsai, M.-H.; Lai, S.-H.; Tseng, Y.-L.; Yeh, K.-W.; Tsai, H.-J.; Huang, J.-L.; et al. Tobacco smoke exposure and multiplexed immunoglobulin E sensitization in children: A population-based study. Allergy 2016, 71, 90–98. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, C.; Lei, Z.; Rong, H.; Yu, S.; Wu, H.; Yang, L.; Lei, Y.; Liu, W.; Nie, Y.; et al. Cigarette smoking exposure breaks the homeostasis of cholesterol and bile acid metabolism and induces gut microbiota dysbiosis in mice with different diets. Toxicology 2021, 450, 152678. [Google Scholar] [CrossRef]
- Cincinelli, A.; Martellini, T. Indoor Air Quality and Health. Int. J. Environ. Res. Public Health 2017, 14, 1286. [Google Scholar] [CrossRef]
- Vardoulakis, S.; Giagloglou, E.; Steinle, S.; Davis, A.; Sleeuwenhoek, A.; Galea, K.S.; Dixon, K.; Crawford, J.O. Indoor Exposure to Selected Air Pollutants in the Home Environment: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 8972. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).