Spatiotemporal Pattern Evolution and Driving Factors of Brucellosis in China, 2003–2019
Abstract
:1. Background
2. Materials and Methods
2.1. Data and Sources
2.2. Statistical Methods
2.2.1. Spatiotemporal Scan Statistic
2.2.2. GeoDetector Method
Factor Detector
Interaction Detector
Risk Detector
3. Results
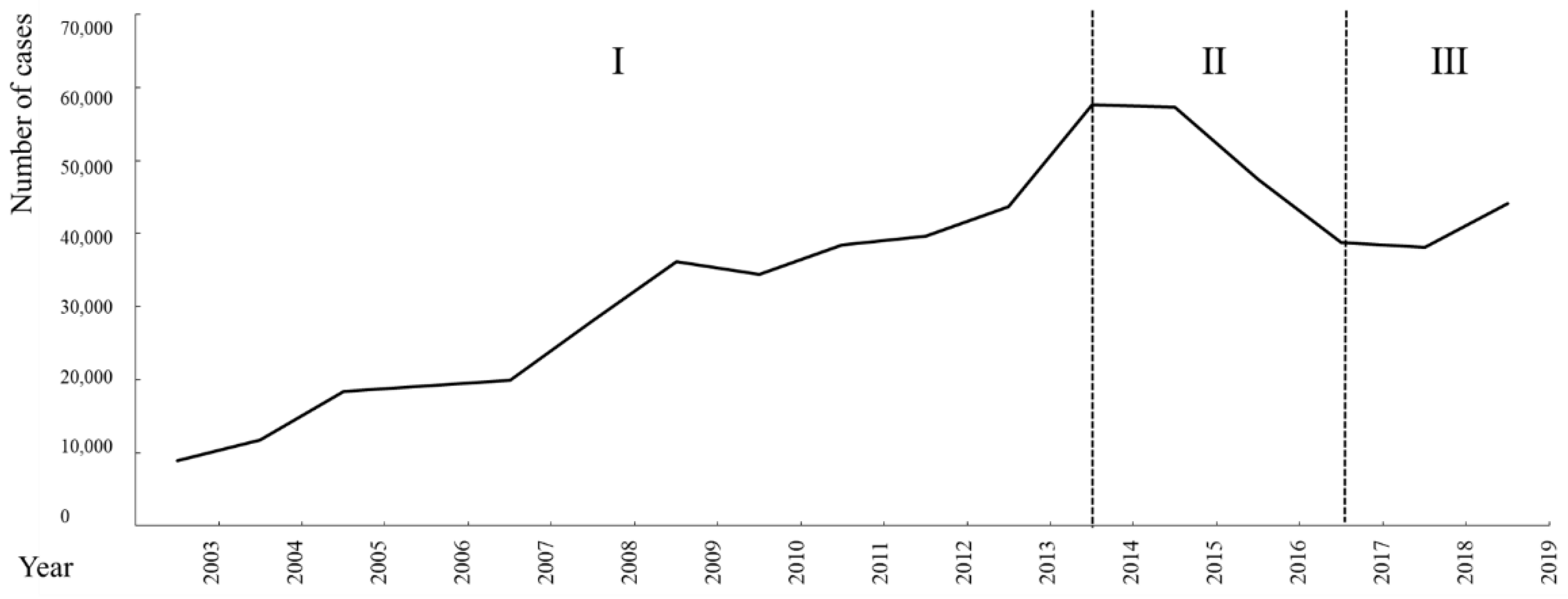
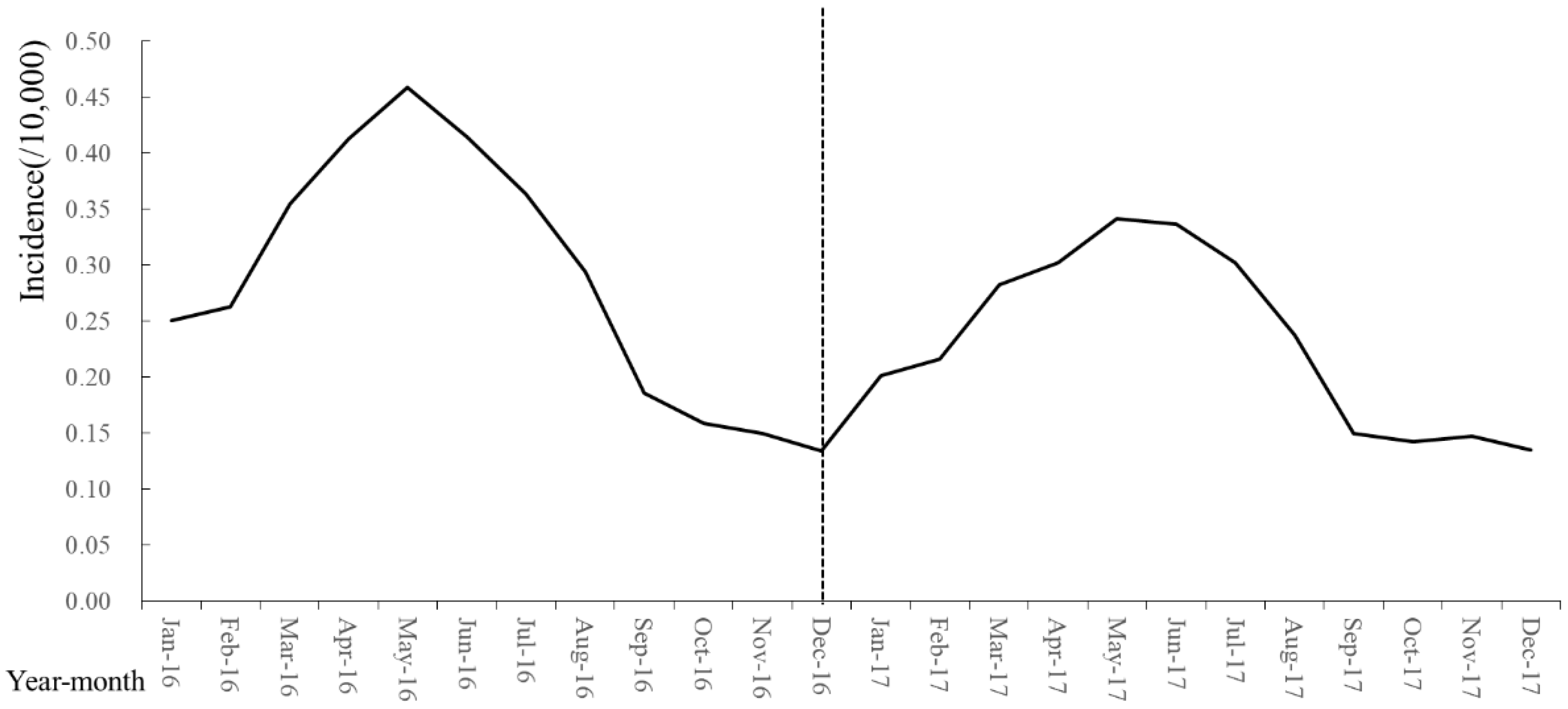
3.1. Trend Analysis of Brucellosis Epidemiological Data
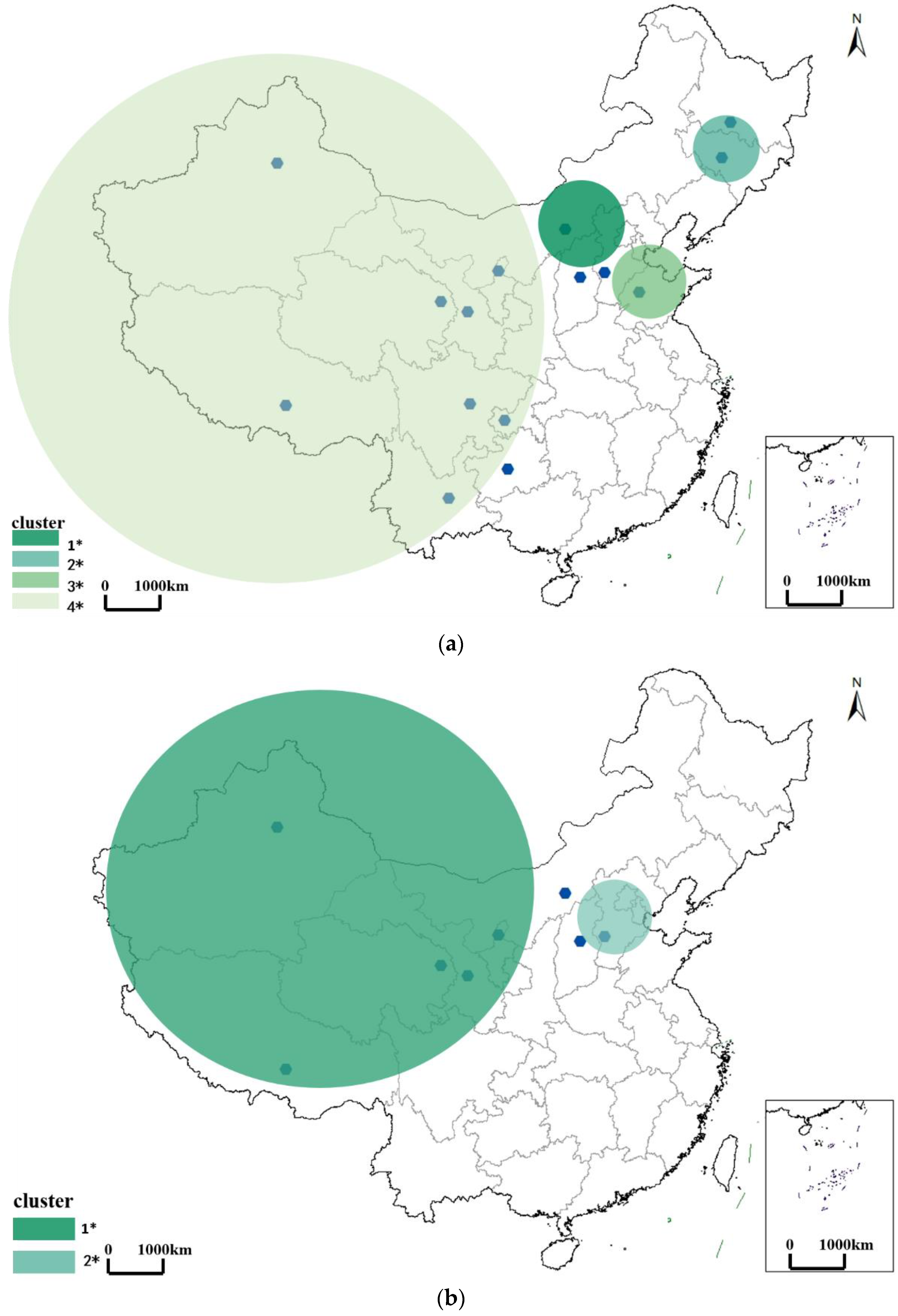
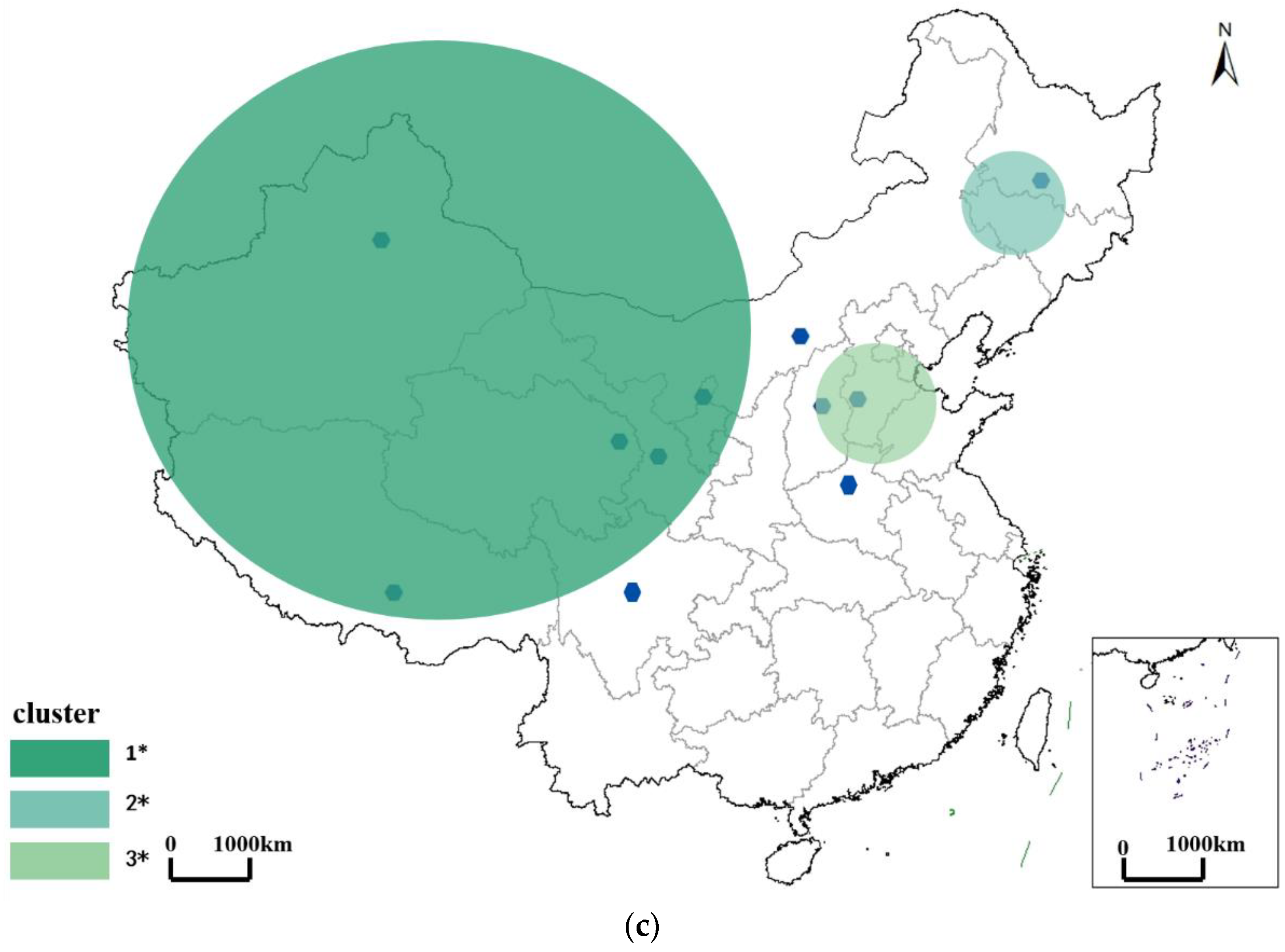
3.2. Spatiotemporal Pattern of Brucellosis
3.2.1. Spatiotemporal Pattern of Brucellosis in the Outbreak Period
3.2.2. Spatiotemporal Pattern of Brucellosis in the Mild Period
3.2.3. Spatiotemporal Pattern of Brucellosis in the Recurrence Period
3.3. Driving Factors for Spatial Heterogeneity of Brucellosis
3.3.1. Driving Effects of Potential Risk Factors on the Spatial Heterogeneity of Brucellosis
3.3.2. Analysis of Influence of Consumption and Production on Brucellosis Clusters (Areas in Most Likely Cluster in Recurrence Period)
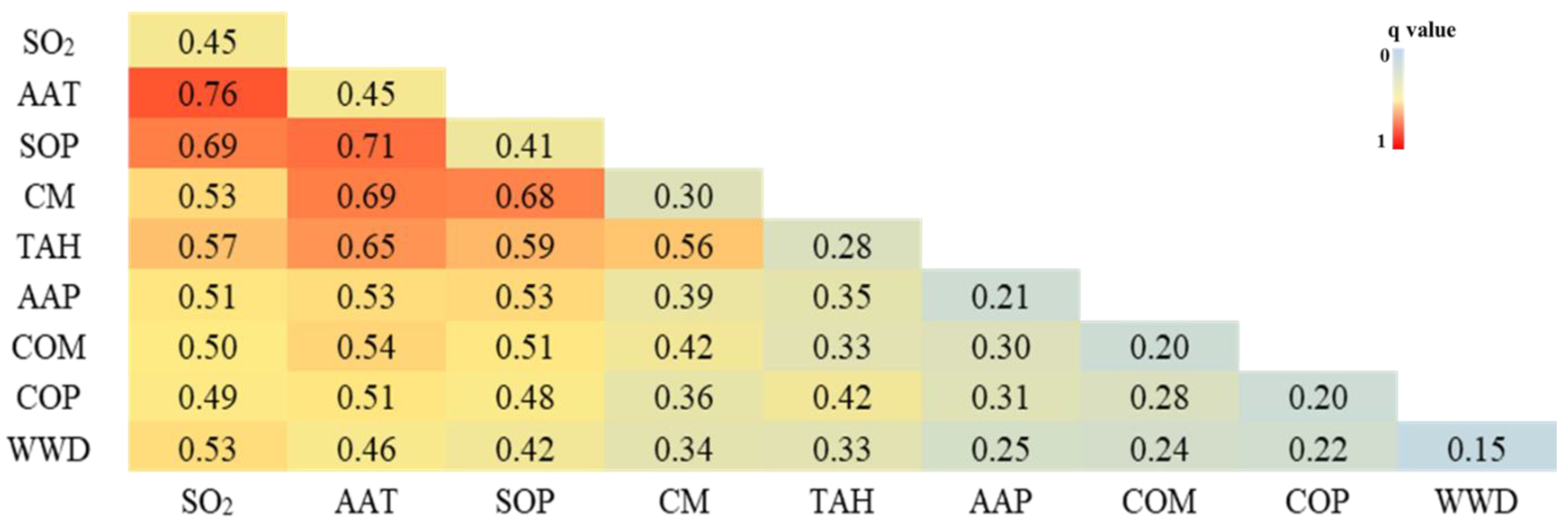
3.3.3. Interactions among Driving Factors
3.3.4. Favorable Conditions for Spread of Brucellosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corbel, M. Brucellosis: An Overview. Emerg. Infect. Dis. 1997, 3, 213–221. [Google Scholar] [CrossRef]
- Gul, S.; Khan, A. epidemiology and epizootology of brucellosis: A review. Pakistan Vet. J. 2007, 27, 145–151. [Google Scholar]
- Shang, D. 50 years of scientific research on brucellosis prevention and control in China. Chin. J. Epidemiol. 2020, 21, 56–58. [Google Scholar]
- Lai, S.; Chen, Q.; Li, Z. Human Brucellosis: An Ongoing Global Health Challenge. Chin. CDC Wkly. 2021, 3, 120–123. [Google Scholar]
- Wang, P.; Tu, W.; Chen, Q.; Wang, X.; Feng, Y.; Meng, L.; Wei, Y.; Xiang, N.; Shi, G. Risk assessment of public health emergencies requiring attention in Mainland China in May 2021. Dis. Surveill. 2021, 36, 403–405. [Google Scholar]
- Shi, Y.; Lai, S.; Chen, Q.; Mu, D.; Li, Y.; Li, X.; Yin, W.; Yu, H. Analysis of the epidemiological characteristics of human brucellosis in the north and south of China from 2015 to 2016. Chin. J. Epidemiol. 2017, 38, 435–440. [Google Scholar]
- Pakzad, R.; Pakzad, I.; Safiri, S.; Shirzadi, M.R.; Mohammadpour, M.; Behroozi, A.; Sullman, M.J.; Janati, A. Spatiotemporal analysis of brucellosis incidence in Iran from 2011 to 2014 using GIS. Int. J. Infect. Dis. 2018, 67, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Bourg, G.; O’Callaghan, D.; Boschiroli, M.L. The genomic structure of Brucella strains isolated from marine mammals gives clues to evolutionary history within the genus. Veter Microbiol. 2007, 125, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Wang, J.; Tang, N.; Miao, L.; Shen, Z. Research progress on the pathogenic mechanism and cellular immune mechanism of Brucella. Chin. J. Zoonoses. 2012, 28, 635–639. [Google Scholar]
- Ding, J.; Feng, Z. Application status and research progress of animal brucellosis vaccine. Chin. Bull. Life Sci. 2013, 25, 91–99. [Google Scholar]
- Alavi, S.M.; Alavi, L. Treatment of brucellosis: A systematic review of studies in recent twenty years. Casp. J. Intern. Med. 2013, 4, 636–641. [Google Scholar]
- Lemos, T.S.; Cequinel, J.C.; Costa, T.P.; Navarro, A.B.; Sprada, A.; Shibata, F.K.; Gondolfo, R.; Tuon, F.F. Outbreak of human brucellosis in Southern Brazil and historical review of data from 2009 to 2018. PLoS Negl. Trop. Dis. 2018, 12, e0006770. [Google Scholar] [CrossRef]
- Yang, W.; Chen, Q. Analysis on the epidemiological characteristics and temporal and spatial aggregation of brucellosis in Inner Mongolia and adjacent provinces from 2010 to 2014. Chin. J. Dis. Control Prev. 2017, 21, 30–34. [Google Scholar]
- Wang, Y.; Xu, C.; Zhang, S.; Wang, Z.; Zhu, Y.; Yuan, J. Temporal trends analysis of human brucellosis incidence in mainland China from 2004 to 2018. Sci. Rep. 2018, 8, 15901. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wu, H.; Lu, Q.; Ding, Z.; Lin, J. Study on the spatial distribution characteristics and influencing factors of human brucellosis in Zhejiang Province from 2010 to 2018. Chin. J. Zoonoses 2020, 36, 134–140. [Google Scholar]
- Amro, A.; Mansoor, B.; Hamarsheh, O.; Hjaija, D. Recent trends in human brucellosis in the West Bank, Palestine. Int. J. Infect. Dis. 2021, 106, 308–313. [Google Scholar] [CrossRef]
- Zhao, F.; An, M.; Bu, T.; Fan, W.; Yang, L.; Zhang, M. Study on the Temporal and Spatial Distribution Characteristics and Influencing Factors of Brucellosis in the Yellow River Basin. J. Henan Univ. 2020, 50, 505–514. [Google Scholar]
- Ron, L.; Benitez, W.; Speybroeck, N.; Ron, J.; Saegerman, C.; Berkvens, D.; Abatih, E. Spatio-temporal clusters of incident human brucellosis cases in Ecuador. Spat. Spatio Temporal Epidemiol. 2013, 5, 1–10. [Google Scholar] [CrossRef]
- Li, P.; Chen, Y.; Wu, D.; Liu, Y.; Guo, z.; Lu, J. The influence of socioeconomic factors on the prevalence of brucellosis. J. Trop. Med. 2020, 20, 465–468. [Google Scholar]
- Zhao, Y. Study on the Temporal and Spatial Distribution Characteristics of Human Brucellosis in Ningxia and Its Natural Environment Influencing Factors. Master’s Thesis, Ningxia Medical University, Ningxia, China, 2020. [Google Scholar] [CrossRef]
- Cárdenas, L.; Awada, L.; Tizzani, P.; Cáceres, P.; Casal, J. Characterization and evolution of countries affected by bovine brucellosis (1996–2014). Transbound. Emerg. Dis. 2019, 66, 1280–1290. [Google Scholar] [CrossRef] [PubMed]
- Kulldorff, M.; Huang, L.; Pickle, L.; Duczmal, L. An elliptic spatial scan statistic. Stat. Med. 2006, 25, 3929–3943. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, C. Geodetector: Principles and Prospects. Acta Geogr. Sin. 2017, 72, 116–134. [Google Scholar]
- Diagnostic criteria for Brucellosis. Dis. Surveill. 2009, 24, 781.
- Quintelas, C.; Mesquita, D.; Ferreira, E.C. Impact of industrial wastewater discharge on the environment and human health. In Biological Treatment of Industrial Wastewater; The Royal Society of Chemistry: London, UK, 2022; pp. 15–39. [Google Scholar] [CrossRef]
- Grassly, N.C.; Fraser, C. Seasonal infectious disease epidemiology. Proc. R. Soc. B: Boil. Sci. 2006, 273, 2541–2550. [Google Scholar] [CrossRef]
- Dadar, M.; Shahali, Y.; Whatmore, A.M. Human brucellosis caused by raw dairy products: A review on the occurrence, major risk factors and prevention. Int. J. Food Microbiol. 2019, 292, 39–47. [Google Scholar] [CrossRef]
- Song, Y.; Liu, Y.; Zhang, Y.; Wang, S. Spatial and temporal differentiation characteristics and influencing factors of hand, foot and mouth disease in China. Acta Geogr. Sin. 2022, 77, 574–588. [Google Scholar]
- Tang, X.; Zhou, H. Scanning statistics and its application in epidemiology. Chin. J. Health Stati. 2011, 28, 332–337. [Google Scholar]
- Shen, X.; Jia, Y.; He, J.; Zhen, E.; Qi, Y.; Huang, T. Epidemiological characteristics and spatiotemporal aggregation of hand-foot-mouth disease in Yunnan Province from 2009 to 2019. Chin. J. Public Health 2021, 37, 1267–1272. [Google Scholar]
- Xie, Z.; Qin, Y.; Li, Y.; Shen, W.; Zheng, Z.; Liu, S. Spatial and temporal differentiation of COVID-19 epidemic spread in mainland China and its influencing factors. Sci. Total Environ. 2020, 744, 140929. [Google Scholar] [CrossRef]
- Li, X. Study on the temporal and spatial distribution characteristics of Japanese encephalitis in Chinaand related geographical and climatic factors. Master’s Thesis, Chinese Center for Disease Control and Prevention, Beijing, China, 2014. [Google Scholar]
- Wei, S. Research on the Incidence Trend and Seasonality of Class B Notifiable Infectious Diseases in China. Master’s Thesis, Fudan University, Shanghai, China, 2013. [Google Scholar]
- Cui, M.; Yao, X.; Fang, H.; Zhang, Y.; Yang, D.; Pei, D. Spatial and temporal transmission differences between SARS and COVID- 19 and analysis of influence factors. Geo-Spat. Inf. Sci. 2021, 23, 1910–1923. [Google Scholar]
- Dadar, M.; Shahali, Y.; Fakhri, Y. A primary investigation of the relation between the incidence of brucellosis and climatic factors in Iran. Microb. Pathog. 2020, 139, 103858. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.; García-Díez, J.; Góis, J.; Rodrigues, J.; Coelho, A.C. Farm practices and risk factors which influence the high prevalence of brucellosis in small ruminant flocks in Northeast Portugal. Vet. Ital. 2019, 55, 355–362. [Google Scholar] [PubMed]
- Peng, C.; Li, Y.; Huang, D.; Guan, P. Spatiotemporal distribution of human brucellosis in mainland China from 2004 to 2017 and an analysis of social and environmental factors. Environ. Health Prev. Med. 2020, 25, 1–25. [Google Scholar] [CrossRef]
- Zhu, L.; Ren, H.; Ding, F.; Lu, L.; Wu, S.; Cui, C. Spatiotemporal variations and influencing factors of hemorrhagic fever with renal syndrome in Shaanxi Province. J. Geo-Info. Sci. 2020, 22, 1142–1152. [Google Scholar]
- Liu, B.; Hao, J.; He, K.; Cai, F.; Xue, Z.; Fan, Y.; Liu, Z.; Zhao, W. Research on designation of acid and SO2 pollution control areas and policy implementation. China Environ. Sci. 1998, 18, 1–8. [Google Scholar]
- He, J. Industrialization, Environment and Health: The Impacts of Industrial SO2 Emission on Public Health in China. Chin. J. Popul. Resour. Environ. 2008, 6, 14–24. [Google Scholar]
- Smits, H.L.; Kadri, S.M. Brucellosis in India: A deceptive infectious disease. Indian J. Med Res. 2005, 122, 375–384. [Google Scholar]
- Epstein, P.R. Climate Change and Emerging Infectious Diseases. Microbes Infect. 2001, 9, 747–754. [Google Scholar] [CrossRef]
- McMichael, A.J.; Haines, A.; Slooff, R.; Kovats, S. (Eds.) Climate Change and Human Health; World Health Organization: Geneva, Switzerland; World Meteorological Organization: Geneva, Switzerland; United Nations Environmental Program: Geneva, Switzerland, 1996. [Google Scholar]
- Liao, W.; Sun, J.; Yu, G.; Yong, W.; Tian, D.; Wang, X. Spatial distribution characteristics and correlations of human and livestock brucellosis in mainland China from 2006 to 2012. Chin. J. Public Health 2015, 31, 1289–1293. [Google Scholar]
- Ran, X.; Chen, X.; Wang, M.; Cheng, J.; Ni, H.; Zhang, X.-X.; Wen, X. Brucellosis seroprevalence in ovine and caprine flocks in China during 2000–2018: A systematic review and meta-analysis. BMC Veter Res. 2018, 14, 393. [Google Scholar] [CrossRef]
- Wang, L.; Yi, Y.; Chen, X.; Sun, C.; Zhang, M.; Zeng, F.; Zeng, Y.; He, D.; Deng, A. Investigation and analysis of a brucellosis outbreak in Guangdong Province in 2019. Dis. Surveill. 2020, 35, 167–171. [Google Scholar]
- Bamaiyi, P.H. Prevalence and risk factors of brucellosis in man and domestic animals: A review. Int. J. One Health 2016, 2, 29–34. [Google Scholar] [CrossRef]
| Type of Factor | Detection Factor | Measurement Units | Range of Time | Data Source |
|---|---|---|---|---|
| Environment | SO2 | tons (t) | 2003–2019 | Statistical Yearbook of each province in China |
| WWD | tons (t) | |||
| Meteorology | AAT | Celsius (°C) | 2003–2019 | China Meteorological Data Sharing Service System |
| AAP | mm | |||
| Socioeconomic | CM | kg | 2003–2019 | Statistical Yearbook of each province in China |
| TAH | Yuan | |||
| COM | kg | Chinese Health Statistics Yearbook | ||
| SOP | heads | Statistical Yearbook of each province in China | ||
| COP | heads |
| Description | Interaction |
|---|---|
| q(X1∩X2) < Min(q(X1), q(X2)) | Weakened, Nonlinear |
| q(X1∩X2) = q(X1) + q(X2) | Independent |
| q(X1∩X2) > Max(q(X1) + q(X2)) | Enhanced, Double factors |
| Center * | Radius ** | Areas *** | Number of Provinces | |
|---|---|---|---|---|
| Outbreak period | Inner Mongolia | 335.33 km | Inner Mongolia, Shanxi | 15 |
| Mild period | Xinjiang | 2000.09 km | Xinjiang, Qinghai, Tibet, Gansu, Ningxia, and Inner Mongolia | 8 |
| Recurrence period | Xinjiang | 2000.09 km | Qinghai, Tibet, Gansu, Ningxia, Inner Mongolia, and Sichuan | 11 |
| SO2 | AAT | SOP | CM | TAH | AAP | COM | COP | WWD | |
|---|---|---|---|---|---|---|---|---|---|
| 2003–2019 | 45.38 | 44.6 | 40.76 | 30.46 | 28.12 | 20.72 | 20.30 | 20.11 | 15.3 |
| Outbreak period | 39.29 | 35.13 | 40.4 | 27.98 | 24.66 | 20.79 | 19.05 | 21.36 | 12.05 |
| Mild period | 34.94 | 30.09 | 32.84 | 30.26 | 29.81 | 25.26 | 18.29 | 22.43 | 12.36 |
| Recurrence period | 47.18 | 28.9 | 42.43 | 49.65 | 35.47 | 22.77 | 24.75 | 23.59 | 16.55 |
| Areas in Cluster | Consumption | Production | ||||
|---|---|---|---|---|---|---|
| Pigs | Cattle | Sheep | Pigs | Cattle | Sheep | |
| Qinghai | 41.12 | 32.46 | 26.42 | 10.82 | 13.76 | 75.42 |
| Tibet | 23.32 | 58.00 | 18.68 | 3.15 | 28.47 | 68.38 |
| Gansu | 77.92 | 8.11 | 13.97 | 28.66 | 8.70 | 62.63 |
| Ningxia | 43.51 | 27.84 | 28.65 | 14.42 | 9.72 | 75.86 |
| Inner Mongolia | 57.58 | 14.05 | 28.36 | 11.09 | 4.83 | 84.08 |
| Sichuan | 94.27 | 4.49 | 1.24 | 74.35 | 3.50 | 22.15 |
| SO2 | WWD | AAT | AAP | CM | TAH | COM | SOP | COP | |
|---|---|---|---|---|---|---|---|---|---|
| /Million t | /Million t | /°C | /mm | /kg | /Billion Yuan | /kg | /Million Heads | /Million Heads | |
| 2003–2019 | 0.66–0.70 | 2444.04–3245.28 | 9.54–11.68 | 421.54–630.76 | 16.81–20.58 | 872.93–1256.43 | 12.99–17.26 | 63.28–72.40 | 2.93–3.51 |
| Outbreak period | 0.70–0.73 | 2245.28–3046.52 | 9.59–11.77 | 478.94–668.97 | 15.58–23.12 | 770.90–1147.42 | 11.69–14.91 | 64.21–78.96 | 3.21–3.57 |
| Mild period | 0.70–0.73 | 1065.10–207,136.33 | 9.59–11.77 | 417.53–623.77 | 16.89–21.54 | 1828.70–2548.30 | 13.37–16.59 | 58.46–70.09 | 2.09–2.61 |
| Recurrence period | 0.73–0.78 | 2071.36–3077.63 | 7.41–9.589 | 440.60–674.70 | 15.47–20.42 | 757.20–1239.60 | 17.52–20.79 | 64.01–73.71 | 2.91–3.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Deng, Y. Spatiotemporal Pattern Evolution and Driving Factors of Brucellosis in China, 2003–2019. Int. J. Environ. Res. Public Health 2022, 19, 10082. https://doi.org/10.3390/ijerph191610082
Xu L, Deng Y. Spatiotemporal Pattern Evolution and Driving Factors of Brucellosis in China, 2003–2019. International Journal of Environmental Research and Public Health. 2022; 19(16):10082. https://doi.org/10.3390/ijerph191610082
Chicago/Turabian StyleXu, Li, and Yijia Deng. 2022. "Spatiotemporal Pattern Evolution and Driving Factors of Brucellosis in China, 2003–2019" International Journal of Environmental Research and Public Health 19, no. 16: 10082. https://doi.org/10.3390/ijerph191610082





