Development of a Risk Score to Predict Sudden Infant Death Syndrome
Abstract
:1. Background
2. Methods
2.1. Study Population and Data Collection
2.2. Selection of Predictor Variables
2.3. Statistical Analysis
2.4. Derivation of SIDS Risk Score
3. Results
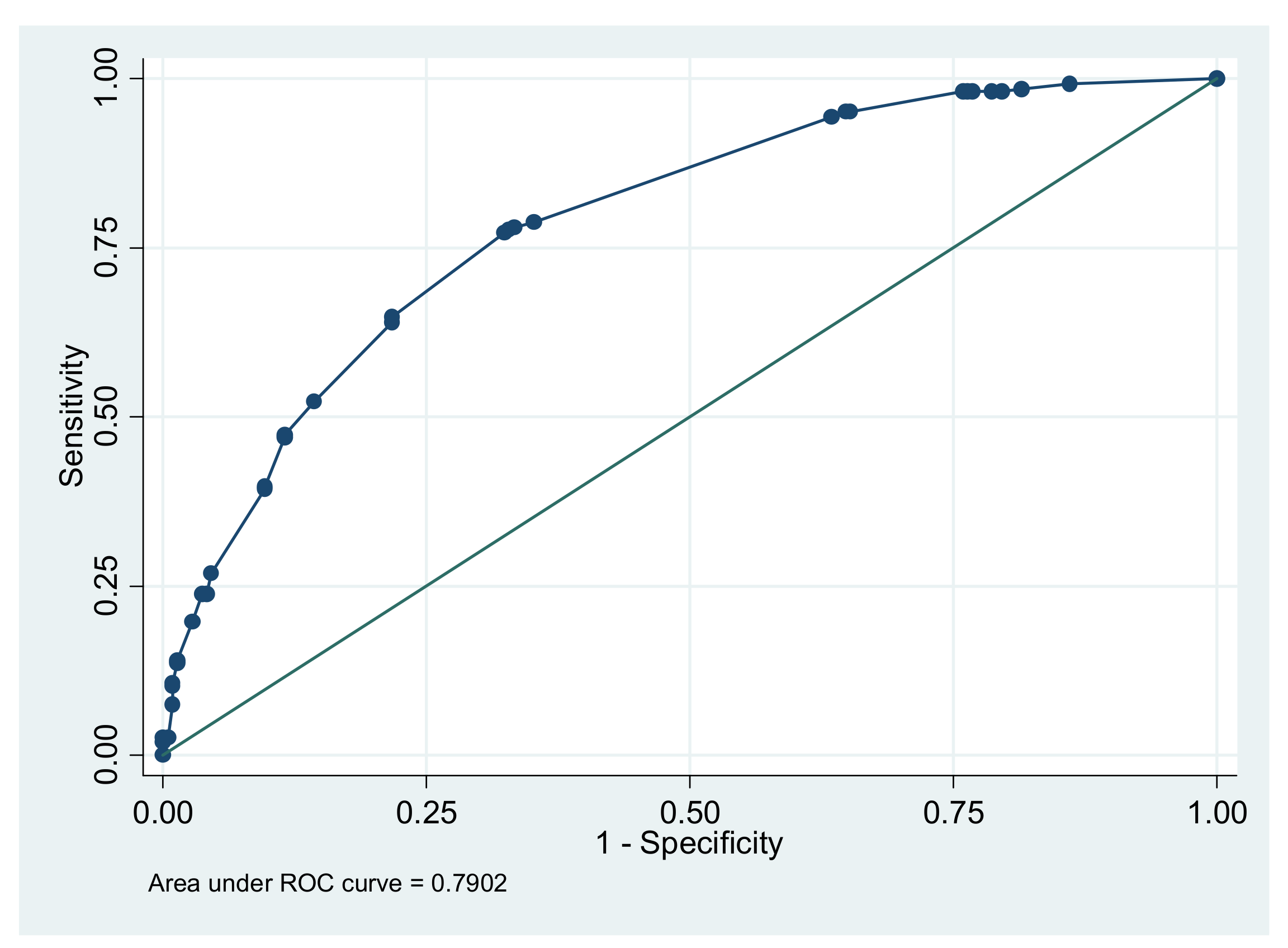
3.1. Our Risk Score Model
3.2. Performance of Our Risk Score Compared to Existing Risk Scores for SIDS
3.3. Post-Hoc Analysis
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Byard, R.W. Sudden Infant Death Syndrome: Definitions. In SIDS Sudden Infant and Early Childhood Death: The Past, the Present and the Future; Duncan, J.R., Byard, R.W., Eds.; University of Adelaide Press: Adelaide, Australia, 2018; ISBN 9781925261677. [Google Scholar]
- Kinney, H.C.; Richerson, G.B.; Dymecki, S.M.; Darnall, R.A.; Nattie, E.E. The Brainstem and Serotonin in the Sudden Infant Death Syndrome. Annu. Rev. Pathol. 2009, 4, 517–550. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.B.E.; Parks, S.E.; Camperlengo, L.; Cottengim, C.; Anderson, R.L.; Covington, T.M.; Shapiro-Mendoza, C.K. Death Scene Investigation and Autopsy Practices in Sudden Unexpected Infant Deaths. J. Pediatrics 2016, 174, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.R.; Byard, R.W. Sudden Infant Death Syndrome: An Overview. In SIDS Sudden Infant and Early Childhood Death: The Past, the Present and the Future; Duncan, J.R., Byard, R.W., Eds.; University of Adelaide Press: Adelaide, Australia, 2018; ISBN 9781925261677. [Google Scholar]
- Perrone, S.; Lembo, C.; Moretti, S.; Prezioso, G.; Buonocore, G.; Toscani, G.; Marinelli, F.; Nonnis-Marzano, F.; Esposito, S. Sudden Infant Death Syndrome: Beyond Risk Factors. Life 2021, 11, 184. [Google Scholar] [CrossRef] [PubMed]
- CDC. Data and Statistics for SIDS and SUID. Available online: https://www.cdc.gov/sids/data.htm (accessed on 26 July 2022).
- Moon, R.Y.; Carlin, R.F.; Hand, I. The Task Force on Sudden Infant Death Syndrome and the Committee on Fetus and Newborn Evidence Base for 2022 Updated Recommendations for a Safe Infant Sleeping Environment to Reduce the Risk of Sleep-Related Infant Deaths. Pediatrics 2022, 150, e2022057991. [Google Scholar] [CrossRef]
- Harrington, C.T.; Hafid, N.A.; Waters, K.A. Butyrylcholinesterase Is a Potential Biomarker for Sudden Infant Death Syndrome. eBioMedicine 2022, 80, 104041. [Google Scholar] [CrossRef]
- Klonoff-Cohen, H.; Edelstein, S.L. Bed Sharing and the Sudden Infant Death Syndrome. BMJ 1995, 311, 1269–1272. [Google Scholar] [CrossRef]
- Goldstein, R.D.; Trachtenberg, F.L.; Sens, M.A.; Harty, B.J.; Kinney, H.C. Overall Postneonatal Mortality and Rates of SIDS. Pediatrics 2016, 137, 1–10. [Google Scholar] [CrossRef]
- Fast Facts About SIDS|Safe to Sleep®. Available online: https://safetosleep.nichd.nih.gov/safesleepbasics/SIDS/fastfacts (accessed on 26 July 2022).
- Yamada, M.M.; Rosamilia, M.B.; Chiswell, K.E.; D’Ottavio, A.; Spears, T.; Osgood, C.; Miranda, M.L.; Forestieri, N.; Li, J.S.; Landstrom, A.P. Risk Factors for Sudden Infant Death in North Carolina. Front. Pediatrics 2021, 9, 770803. [Google Scholar] [CrossRef]
- The Office of Minority Health. Infant Mortality and African Americans. Available online: https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=23 (accessed on 26 July 2022).
- Matthews, T.J.; MacDorman, M.F.; Thoma, M.E. Infant Mortality Statistics From the 2013 Period Linked Birth/Infant Death Data Set. Natl. Vital Stat. Rep. 2015, 64, 1–30. [Google Scholar]
- Steer, J.; Annavarapu, S. The Genetics of Sudden Infant Death Syndrome. In Investigation of Sudden Infant Death Syndrome; Hauck, F.R., Scheimberg, I.B., Beckwith, J.B., Cohen, M.C., Eds.; Diagnostic Pediatric Pathology; Cambridge University Press: Cambridge, UK, 2019; pp. 159–171. ISBN 9781108185981. [Google Scholar]
- Filiano, J.J.; Kinney, H.C. A Perspective on Neuropathologic Findings in Victims of the Sudden Infant Death Syndrome: The Triple-Risk Model. Neonatology 1994, 65, 194–197. [Google Scholar] [CrossRef]
- Kim, H.; Pearson-Shaver, A.L. Sudden Infant Death Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Paterson, D.S. Serotonin Gene Variants Are Unlikely to Play a Significant Role in the Pathogenesis of the Sudden Infant Death Syndrome. Respir. Physiol. Neurobiol. 2013, 189, 301–314. [Google Scholar] [CrossRef]
- Moon, R.Y.; Hauck, F.R. Risk Factors and Theories. In SIDS Sudden Infant and Early Childhood Death: The Past, the Present and the Future; Duncan, J.R., Byard, R.W., Eds.; University of Adelaide Press: Adelaide, Australia, 2018; ISBN 9781925261677. [Google Scholar]
- Goodstein, M. Environmental Risk Factors for SIDS. In Investigation of Sudden Infant Death Syndrome; Hauck, F.R., Scheimberg, I.B., Beckwith, J.B., Cohen, M.C., Eds.; Diagnostic Pediatric Pathology; Cambridge University Press: Cambridge, UK, 2019; pp. 136–141. ISBN 9781108185981. [Google Scholar]
- Sidebotham, P.; Bates, F.; Ellis, C.; Lyus, L. Preventive Strategies for Sudden Infant Death Syndrome. In SIDS Sudden Infant and Early Childhood Death: The Past, the Present and the Future; Duncan, J.R., Byard, R.W., Eds.; University of Adelaide Press: Adelaide, Australia, 2018; ISBN 9781925261677. [Google Scholar]
- Protestos, C.D.; Carpenter, R.G.; McWeeny, P.M.; Emery, J.L. Obstetric and Perinatal Histories of Children Who Died Unexpectedly (Cot Death). Arch. Dis. Child. 1973, 48, 835–841. [Google Scholar] [CrossRef]
- Lewak, N.; van den Berg, B.J.; Beckwith, J.B. Sudden Infant Death Syndrome Risk Factors: Prospective Data Review. Clin. Pediatrics 1979, 18, 404–405. [Google Scholar] [CrossRef]
- Murphy, J.F.; Newcombe, R.G.; Sibert, J.R. The Epidemiology of Sudden Infant Death Syndrome. J. Epidemiol. Community Health 1982, 36, 17–21. [Google Scholar] [CrossRef]
- Golding, J.; Limerick, S.; Macfarlane, A. Sudden Infant Death: Patterns, Puzzles, and Problems; University of Washington Press: Seattle, WA, USA, 1985. [Google Scholar]
- Cameron, M.H.; Williams, A.L. Development and Testing of Scoring Systems for Predicting Infants with High-Risk of Sudden Infant Death Syndrome in Melbourne. Aust. Paediatr. J. 1986, 22 (Suppl. 1), 37–45. [Google Scholar]
- Nelson, E.A.; Williams, S.M.; Taylor, B.J.; Morris, B.; Ford, R.P.; Binney, V.M.; Wilson, C. Prediction of Possibly Preventable Death: A Case-Control Study of Postneonatal Mortality in Southern New Zealand. Paediatr. Perinat. Epidemiol. 1990, 4, 39–52. [Google Scholar] [CrossRef]
- Klonoff-Cohen, H.S.; Edelstein, S.L. A Case-Control Study of Routine and Death Scene Sleep Position and Sudden Infant Death Syndrome in Southern California. JAMA 1995, 273, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Klonoff-Cohen, H.S.; Edelstein, S.L.; Lefkowitz, E.S.; Srinivasan, I.P.; Kaegi, D.; Chang, J.C.; Wiley, K.J. The Effect of Passive Smoking and Tobacco Exposure through Breast Milk on Sudden Infant Death Syndrome. JAMA 1995, 273, 795–798. [Google Scholar] [CrossRef]
- Malloy, M.H. Size for Gestational Age at Birth: Impact on Risk for Sudden Infant Death and Other Causes of Death, USA 2002. Arch. Dis. Child Fetal Neonatal Ed. 2007, 92, F473–F478. [Google Scholar] [CrossRef]
- Office on Smoking and Health (US). The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General; Publications and Reports of the Surgeon General; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2006.
- Mitchell, E.A.; Ford, R.P.; Stewart, A.W.; Taylor, B.J.; Becroft, D.M.; Thompson, J.M.; Scragg, R.; Hassall, I.B.; Barry, D.M.; Allen, E.M. Smoking and the Sudden Infant Death Syndrome. Pediatrics 1993, 91, 893–896. [Google Scholar] [CrossRef]
- Blackburn, C.M.; Bonas, S.; Spencer, N.J.; Coe, C.J.; Dolan, A.; Moy, R. Parental Smoking and Passive Smoking in Infants: Fathers Matter Too. Health Educ. Res. 2005, 20, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Bailey, B.A.; Sokol, R.J. Prenatal Alcohol Exposure and Miscarriage, Stillbirth, Preterm Delivery, and Sudden Infant Death Syndrome. Alcohol Res. Health 2011, 34, 86–91. [Google Scholar] [PubMed]
- Ali, K.; Ahmed, N.; Greenough, A. Sudden Infant Death Syndrome (SIDS), Substance Misuse, and Smoking in Pregnancy. RRN 2012, 2, 95–101. [Google Scholar] [CrossRef]
- Kraus, J.F.; Greenland, S.; Bulterys, M. Risk Factors for Sudden Infant Death Syndrome in the US Collaborative Perinatal Project. Int. J. Epidemiol. 1989, 18, 113–120. [Google Scholar] [CrossRef]
- Mage, D.T.; Latorre, M.L.; Jenik, A.G.; Donner, E.M. An Acute Respiratory Infection of a Physiologically Anemic Infant Is a More Likely Cause of SIDS than Neurological Prematurity. Front. Neurol. 2016, 7, 129. [Google Scholar] [CrossRef]
- Thompson, J.M.D.; Tanabe, K.; Moon, R.Y.; Mitchell, E.A.; McGarvey, C.; Tappin, D.; Blair, P.S.; Hauck, F.R. Duration of Breastfeeding and Risk of SIDS: An Individual Participant Data Meta-Analysis. Pediatrics 2017, 140, e20171324. [Google Scholar] [CrossRef]
- Elhaik, E. A “Wear and Tear” Hypothesis to Explain Sudden Infant Death Syndrome. Front. Neurol. 2016, 7, 180. [Google Scholar] [CrossRef]
- Dwyer, T.; Ponsonby, A.L. SIDS Epidemiology and Incidence. Pediatric Ann. 1995, 24, 350–356. [Google Scholar] [CrossRef]
- Dwyer, T.; Ponsonby, A.-L.B.; Newman, N.M.; Gibbons, L.E. Prospective Cohort Study of Prone Sleeping Position and Sudden Infant Death Syndrome. Lancet 1991, 337, 1244–1247. [Google Scholar] [CrossRef]
- Vennemann, M.M.; Hense, H.-W.; Bajanowski, T.; Blair, P.S.; Complojer, C.; Moon, R.Y.; Kiechl-Kohlendorfer, U. Bed Sharing and the Risk of Sudden Infant Death Syndrome: Can We Resolve the Debate? J. Pediatrics 2012, 160, 44–48.e2. [Google Scholar] [CrossRef]
- Onland, W.; Debray, T.P.; Laughon, M.M.; Miedema, M.; Cools, F.; Askie, L.M.; Asselin, J.M.; Calvert, S.A.; Courtney, S.E.; Dani, C.; et al. Clinical Prediction Models for Bronchopulmonary Dysplasia: A Systematic Review and External Validation Study. BMC Pediatrics 2013, 13, 207. [Google Scholar] [CrossRef]
- Stata Statistical Software; StataCorp LLC: College Station, TX, USA, 2017.
- Mehta, H.B.; Mehta, V.; Girman, C.J.; Adhikari, D.; Johnson, M.L. Regression Coefficient-Based Scoring System Should Be Used to Assign Weights to the Risk Index. J. Clin. Epidemiol. 2016, 79, 22–28. [Google Scholar] [CrossRef]
- Hoffman, H.J.; Damus, K.; Hillman, L.; Krongrad, E. Risk Factors for SIDS. Results of the National Institute of Child Health and Human Development SIDS Cooperative Epidemiological Study. Ann. N. Y. Acad. Sci. 1988, 533, 13–30. [Google Scholar] [CrossRef]
- American Academy of Pediatrics (AAP) Task Force on Infant Positioning and SIDS. Positioning and SIDS. Pediatrics 1992, 89, 1120–1126. [Google Scholar] [CrossRef]
- Peters, T.J.; Golding, J. Prediction of Sudden Infant Death Syndrome: An Independent Evaluation of Four Scoring Methods. Stat. Med. 1986, 5, 113–126. [Google Scholar] [CrossRef]
- O’Brien, S.J.; Matthews, T.G. Sheffield Cot Death Risk Score Applied to an Irish Population. Lancet 1985, 1, 706. [Google Scholar] [CrossRef]
- Brooks, J.G.; Fleming, P.J.; Berry, P.J.; Golding, J. Evaluation of the Oxford and Sheffield SIDS Risk Prediction Scores. Pediatr. Pulmonol. 1992, 14, 171–179. [Google Scholar] [CrossRef]
- Paine, S.M.L.; Jacques, T.S.; Sebire, N.J. Review: Neuropathological Features of Unexplained Sudden Unexpected Death in Infancy: Current Evidence and Controversies. Neuropathol. Appl. Neurobiol. 2014, 40, 364–384. [Google Scholar] [CrossRef]
- Franco, P.; Scaillet, S.; Wermenbol, V.; Valente, F.; Groswasser, J.; Kahn, A. The Influence of a Pacifier on Infants’ Arousals from Sleep. J. Pediatrics 2000, 136, 775–779. [Google Scholar]
- Horne, R.S.C.; Parslow, P.M.; Ferens, D.; Watts, A.-M.; Adamson, T.M. Comparison of Evoked Arousability in Breast and Formula Fed Infants. Arch. Dis. Child. 2004, 89, 22–25. [Google Scholar]
- Thompson, J.M.D. The Relationship Between Breastfeeding and SIDS. In Investigation of Sudden Infant Death Syndrome; Hauck, F.R., Scheimberg, I.B., Beckwith, J.B., Cohen, M.C., Eds.; Diagnostic Pediatric Pathology; Cambridge University Press: Cambridge, UK, 2019; pp. 142–145. ISBN 9781108185981. [Google Scholar]
- Klonoff-Cohen, H.S.; Srinivasan, I.P.; Edelstein, S.L. Prenatal and Intrapartum Events and Sudden Infant Death Syndrome. Paediatr. Perinat. Epidemiol. 2002, 16, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, X. Maternal Smoking and Increased Risk of Sudden Infant Death Syndrome: A Meta-Analysis. Leg. Med. 2013, 15, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.E.; Johnson, D.C.; Batal, H.A. Sudden Infant Death Syndrome and Prenatal Maternal Smoking: Rising Attributed Risk in the Back to Sleep Era. BMC Med. 2005, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Elliott, A.J.; Kinney, H.C.; Haynes, R.L.; Dempers, J.D.; Wright, C.; Fifer, W.P.; Angal, J.; Boyd, T.K.; Burd, L.; Burger, E.; et al. Concurrent Prenatal Drinking and Smoking Increases Risk for SIDS: Safe Passage Study Report. eClinicalMedicine 2020, 19, 100247. [Google Scholar] [CrossRef]
- Engelberts, A. Prenatal and Postpartum Nicotine Exposure. In Investigation of Sudden Infant Death Syndrome; Hauck, F.R., Scheimberg, I.B., Beckwith, J.B., Cohen, M.C., Eds.; Diagnostic Pediatric Pathology; Cambridge University Press: Cambridge, UK, 2019; pp. 131–133. ISBN 9781108185981. [Google Scholar]
- Cirik, V.; Efe, E. PO-0897 Sudden Infant Death Syndrome in Low Birth Weight Infants. Arch. Dis. Child. 2014, 99, A544. [Google Scholar] [CrossRef]
- Caraballo, M.; Shimasaki, S.; Johnston, K.; Tung, G.; Albright, K.; Halbower, A.C. Knowledge, Attitudes, and Risk for Sudden Unexpected Infant Death in Children of Adolescent Mothers: A Qualitative Study. J. Pediatrics 2016, 174, 78–83.e2. [Google Scholar] [CrossRef]
- CDC. Breastfeeding Rates: National Immunization Survey (NIS). Available online: https://www.cdc.gov/breastfeeding/data/nis_data/index.htm (accessed on 28 July 2022).
- Smith, P.H.; Coley, S.L.; Labbok, M.H.; Cupito, S.; Nwokah, E. Early Breastfeeding Experiences of Adolescent Mothers: A Qualitative Prospective Study. Int. Breastfeed. J. 2012, 7, 13. [Google Scholar] [CrossRef]
- Elhaik, E. Neonatal Circumcision and Prematurity Are Associated with Sudden Infant Death Syndrome (SIDS). J. Clin. Transl. Res. 2019, 4, 136–151. [Google Scholar] [CrossRef]
- Blair, P.S.; Sidebotham, P.; Pease, A.; Fleming, P.J. Bed-Sharing in the Absence of Hazardous Circumstances: Is There a Risk of Sudden Infant Death Syndrome? An Analysis from Two Case-Control Studies Conducted in the UK. PLoS ONE 2014, 9, e107799. [Google Scholar] [CrossRef]
- Trachtenberg, F.L.; Haas, E.A.; Kinney, H.C.; Stanley, C.; Krous, H.F. Risk Factor Changes for Sudden Infant Death Syndrome after Initiation of Back-to-Sleep Campaign. Pediatrics 2012, 129, 630–638. [Google Scholar] [CrossRef]
- Hamayasu, H.; Miyao, M.; Kawai, C.; Osamura, T.; Yamamoto, A.; Minami, H.; Abiru, H.; Tamaki, K.; Kotani, H. A Proof-of-Concept Study to Construct Bayesian Network Decision Models for Supporting the Categorization of Sudden Unexpected Infant Death. Sci. Rep. 2022, 12, 9773. [Google Scholar] [CrossRef]
- Shaw, P.A.; Pepe, M.S.; Alonzo, T.A.; Etzioni, R. Methods for Assessing Improvement in Specificity When a Biomarker Is Combined with a Standard Screening Test. Stat. Biopharm. Res. 2009, 1, 18–25. [Google Scholar] [CrossRef]
- Power, M.; Fell, G.; Wright, M. Principles for High-Quality, High-Value Testing. Evid. Based Med. 2013, 18, 5–10. [Google Scholar] [CrossRef]
| Risk Score | Variables |
|---|---|
| Sheffield birth score [22] | Mother’s age |
| Previous pregnancies | |
| Twin pregnancy | |
| Mother’s blood group | |
| Urinary infection during pregnancy | |
| Duration of 2nd stage labor | |
| Birthweight | |
| Feeding intention at the time of delivery | |
| California score [23] | Maternal age < 25 years |
| Maternal smoking | |
| Pregnancy < 12 months | |
| <11 perinatal visits | |
| Birthweight < 3000 g | |
| Gestational age < 4 0 wk | |
| Male sex | |
| Blue collar family | |
| Cardiff score [24] | Age of mother at birth |
| Maternal smoking | |
| Antenatal clinic visits | |
| Gestation at booking | |
| Previous deliveries | |
| Pregnancy complications | |
| 2nd stage of labor | |
| Admission to SCBU | |
| Mode of delivery | |
| Birthweight | |
| Maternal birth injury | |
| Season of birth | |
| Sex of infant | |
| Infant feeding | |
| Area of residence | |
| Social class | |
| Mother’s employment | |
| Employment of partner | |
| Sheffield multistage score [26] | Mother’s age |
| Birth order | |
| Twin | |
| Blood group A | |
| Urinary infection during pregnancy | |
| Duration 2nd stage labor | |
| Birthweight | |
| Breastfeeding intention at delivery | |
| Oxford score [25] | Mother’s age at delivery and birth order |
| Maternal drug addiction | |
| Mother took barbiturates | |
| Previous SIDS | |
| Interpregnancy interval | |
| Infection during pregnancy | |
| Multiple births/twins | |
| Gestation | |
| Marital status | |
| Husband’s social class | |
| Abbreviated Oxford score [27] | Maternal age and parity |
| Infants’ birthweight | |
| Marital status | |
| New Zealand CID birth score [27] | Maternal age |
| Parity | |
| Infants’ birthweight | |
| Marital status | |
| New Zealand CID multistage score [27] | Birth score |
| Mother’s age | |
| Parity | |
| Birth weight | |
| Marital status | |
| 1-month score | |
| Birth score | |
| Feeding change | |
| Comments on home |
| Characteristics | SIDS No. (%) | Controls No. (%) |
|---|---|---|
| Gender | ||
| Females | 109 (37.46) | 94 (38.84) |
| Males | 182 (62.54) | 148 (61.16) |
| Race/Ethnicity | ||
| White | 117 (40.21) | 107 (44.21) |
| Black | 38 (13.06) | 27 (11.16) |
| Hispanic | 92 (31.62) | 80 (33.06) |
| Asian | 27 (9.28) | 22 (9.09) |
| Others | 17 (5.84) | 6 (2.48) |
| Risk Factor | SIDS No. (%) | Controls No. (%) | p |
|---|---|---|---|
| Birth weight z-score | |||
| More than −1 | 55 (19.3) | 16 (6.93) | Reference |
| Less than or equal to −1 | 230 (80.7) | 215 (93.07) | <0.001 * |
| Maternal age | |||
| More than or equal to 25 years | 156 (54.17) | 173 (73.31) | Reference |
| Less than 25 years | 132 (45.83) | 63 (26.69) | <0.001 * |
| Maternal smoking | |||
| Never smoked | 140 (48.11) | 156 (64.46) | Reference |
| Smoker but not during pregnancy | 56 (19.24) | 39 (16.12) | 0.049 |
| Smoker and smoked during pregnancy | 95 (32.65) | 47 (19.42) | <0.001 * |
| Paternal smoking during pregnancy | |||
| No | 180 (61.86) | 196 (80.99) | Reference |
| Yes | 110 (37.8) | 46 (19.01) | <0.001 * |
| Infants’ exposure to passive smoking | |||
| No | 166 (57.24) | 196 (81.67) | Reference |
| Yes | 124 (42.76) | 44 (18.33) | <0.001 * |
| Maternal use of recreational drugs during pregnancy | |||
| No | 268 (92.17) | 233 (96.28) | Reference |
| Yes | 22 (7.59) | 9 (3.72) | 0.06 |
| Maternal use of alcohol during pregnancy | |||
| No | 197 (67.93) | 153 (63.93) | Reference |
| Yes | 93 (32.07) | 89 (36.78) | 0.26 |
| Anemia during pregnancy | |||
| No | 239 (82.13) | 216 (89.26) | Reference |
| Yes | 52 (17.87) | 26 (10.74) | 0.02 * |
| Breastfeeding duration | |||
| More than 4 months | 5 (1.77) | 58 (23.97) | Reference |
| 2–4 months | 19 (6.74) | 39 (16.12) | <0.001 * |
| Less than 2 months | 258 (91.49) | 145 (59.92) | 0.001 * |
| Family history of SIDS | |||
| No | 210 (74.47) | 216 (92.7) | Reference |
| Yes | 72 (25.53) | 17 (7.3) | <0.001 * |
| Co-sleeping | |||
| No | 188 (65.51) | 173 (71.49) | Reference |
| Yes | 65 (22.65) | 49 (20.25) | 0.36 |
| Sometimes | 34 (11.85) | 20 (8.26) | 0.14 |
| Circumcision in male infants (n = 330) | |||
| No | 70 (38.46) | 65 (43.92) | Reference |
| Yes | 36 (19.78) | 29 (19.59) | 0.64 |
| Missing data | 76 (41.76) | 54 (36.49) | |
| Routine sleep position | |||
| Stomach | 182 (62.98) | 147 (61) | Reference |
| Side | 24 (8.3) | 19 (7.88) | 0.95 |
| Back | 37 (12.8) | 30 (12.45) | 0.99 |
| No usual position | 44 (15.22) | 45 (18.67) | 0.32 |
| Variable | Coefficient | Odds Ratio (95% Confidence Interval) | p Value | Risk Score |
|---|---|---|---|---|
| Birth weight z score | ||||
| More than or equal to −1 | Reference | 0 | ||
| Less than −1 | 1.0 | 2.74 (1.41 to 5.33) | 0.003 * | 1 |
| Passive smoke | ||||
| No exposure | Reference | 0 | ||
| Exposed | 1.0 | 2.64 (1.65 to 4.20) | <0.001 * | 1 |
| Maternal age at the time of childbirth | ||||
| More than or equal to 25 years | Reference | 0 | ||
| Below 25 years | 1.0 | 1.77 (1.13 to 2.79) | 0.01 * | 1 |
| Family History of SIDS | ||||
| No | 0 | |||
| Yes | 1.5 | 4.31 (2.24 to 8.31) | <0.001 * | 2 |
| Breastfeeding duration | ||||
| More than 4 months | Reference | 0 | ||
| 2–4 months | 1.8 | 6.11 (1.99 to 18.73) | 0.002 * | 2 |
| Less than 2 months | 2.6 | 13.85 (5.25 to 36.55) | <0.001 * | 3 |
| Anemia during pregnancy | ||||
| No | Reference | 0 | ||
| Yes | 1.0 | 2.07 (1.09 to 3.94) | 0.03 * | 1 |
| Risk Score | Author | Population | Cutoff Score | Sensitivity | Specificity |
|---|---|---|---|---|---|
| Our model | 291 SIDS infants and 242 healthy controls from Southern California, 1989–1992 | 6 | 44% | 90% | |
| Sheffield birth score [22] | Peters [48] | 34 SIDS and 318 controls from British Birth Cohort, 1970 | 492 | 62% | 80% |
| Cameron [26] | 61 SIDS and 131 controls from Melbourne, 1980 | 500 | 43% | 89% | |
| O’Brien [49] | 48 SIDS and 192 controls from Dublin, 1979–1981 | 500 | 29% | 85% | |
| Brooks [50] | 123 SIDS and 637 controls from England, 1983–1987 | 500 | 35% | 89% | |
| California score [23] | Peters [48] | 34 SIDS and 318 controls from British Birth Cohort, 1970 | 5 | 53% | 80% |
| Cardiff score [24] | Murphy [24] | 99 SIDS cases from the 47,413 live births from the Cardiff Births Survey | 102 | NA * | NA * |
| Sheffield multistage score [26] | Cameron [26] | 61 SIDS and 131 controls from Melbourne, 1980 | 754 | 44% | 85% |
| O’Brien [49] | 48 SIDS and 192 controls from Dublin, 1979–1981 | 745 | 38% | 85% | |
| Oxford score [25] | Peters [48] | 34 SIDS and 318 controls from British Birth Cohort, 1970 | 1.745 | 70% | 80% |
| Brooks [50] | 123 SIDS and 637 controls from England, 1983–1987 | 1.745 | 57% | 77% | |
| Abbreviated Oxford score [27] | Nelson [27] | 377 possible preventable post neonatal deaths and 936 controls from New Zealand, 1979–1984 | 1.44 | 50% | 77% |
| New Zealand CID birth score [27] | Nelson [27] | 377 possible preventable post neonatal deaths and 936 controls from New Zealand, 1979–1984 | 342 | 50% | 79% |
| New Zealand CID multistage score [27] | Nelson [27] | 514 consecutive births in 1986 and 49 SIDS from New Zealand, 1986–1987 | 355 | 50% | 80% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polavarapu, M.; Klonoff-Cohen, H.; Joshi, D.; Kumar, P.; An, R.; Rosenblatt, K. Development of a Risk Score to Predict Sudden Infant Death Syndrome. Int. J. Environ. Res. Public Health 2022, 19, 10270. https://doi.org/10.3390/ijerph191610270
Polavarapu M, Klonoff-Cohen H, Joshi D, Kumar P, An R, Rosenblatt K. Development of a Risk Score to Predict Sudden Infant Death Syndrome. International Journal of Environmental Research and Public Health. 2022; 19(16):10270. https://doi.org/10.3390/ijerph191610270
Chicago/Turabian StylePolavarapu, Mounika, Hillary Klonoff-Cohen, Divya Joshi, Praveen Kumar, Ruopeng An, and Karin Rosenblatt. 2022. "Development of a Risk Score to Predict Sudden Infant Death Syndrome" International Journal of Environmental Research and Public Health 19, no. 16: 10270. https://doi.org/10.3390/ijerph191610270





