Association between Benign Paroxysmal Positional Vertigo and Previous Proton Pump Inhibitor Use: A Nested Case–Control Study Using a National Health Screening Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Ethical Consideration
2.2. Participant Selection
2.2.1. Proton Pump Inhibitor (Exposure)
2.2.2. Benign Paroxysmal Positional Vertigo (Outcome)
2.2.3. Covariates
2.3. Statistical Analyses
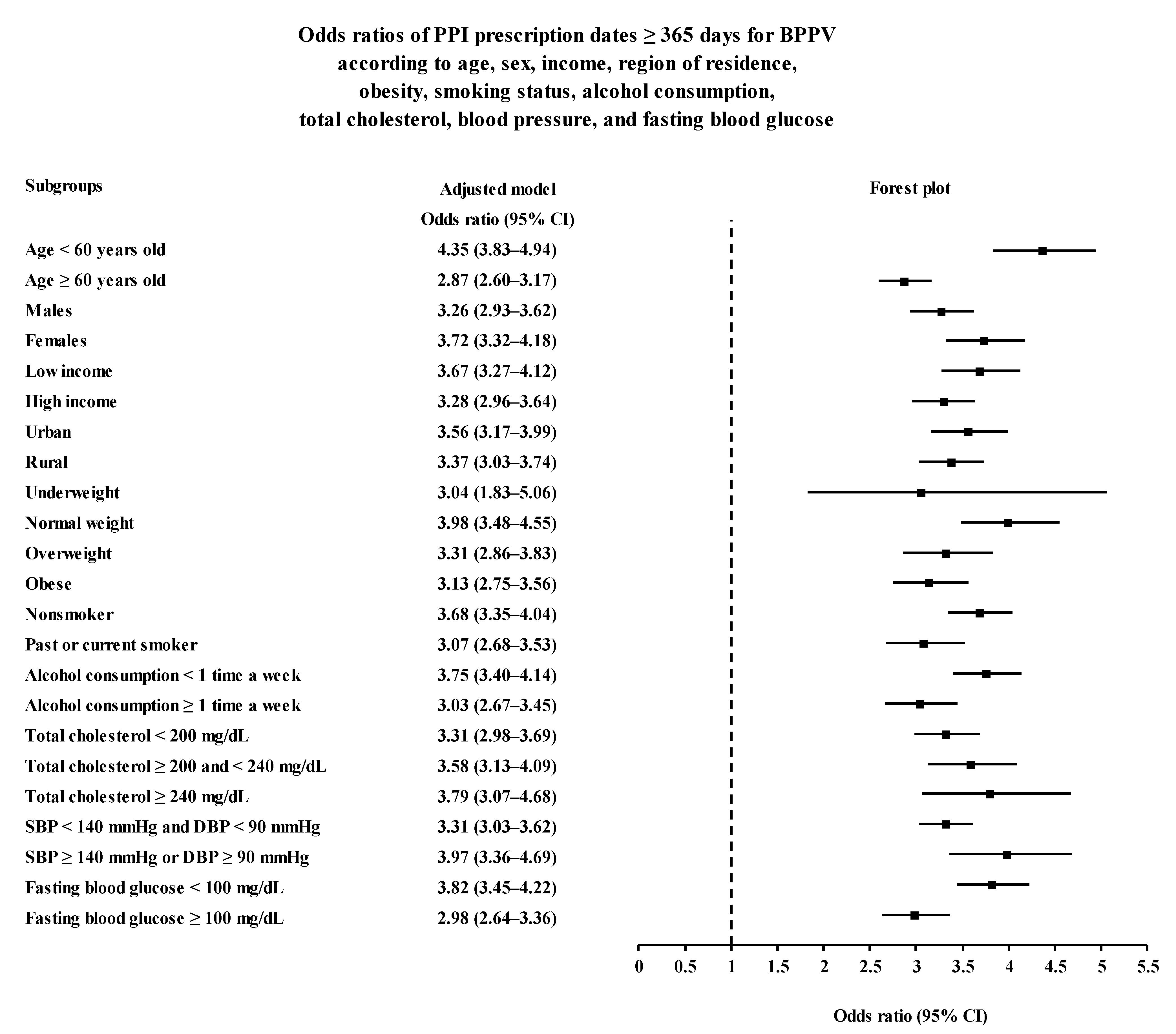
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chubineh, S.; Birk, J. Proton Pump Inhibitors. South. Med. J. 2012, 105, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Klotz, U. Proton pump inhibitors: An update of their clinical use and pharmacokinetics. Eur. J. Clin. Pharmacol. 2008, 64, 935–951. [Google Scholar] [CrossRef] [PubMed]
- Hassall, E. Over-Prescription of Acid-Suppressing Medications in Infants: How It Came About, Why It’s Wrong, and What to Do About It. J. Pediatr. 2012, 160, 193–198. [Google Scholar] [CrossRef]
- Ummarino, D.; Miele, E.; Masi, P.; Tramontano, A.; Staiano, A.; Vandenplas, Y. Impact of antisecretory treatment on respiratory symptoms of gastroesophageal reflux disease in children. Dis. Esophagus 2012, 25, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Tofil, N.M.; Benner, K.W.; Fuller, M.P.; Winkler, M.K. Histamine 2 receptor antagonists vs intravenous proton pump inhibitors in a pediatric intensive care unit: A comparison of gastric pH. J. Crit. Care 2008, 23, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Haastrup, P.F.; Thompson, W.; Søndergaard, J.; Jarbøl, D. Side Effects of Long-Term Proton Pump Inhibitor Use: A Review. Basic Clin. Pharmacol. Toxicol. 2018, 123, 114–121. [Google Scholar] [CrossRef]
- Ghosh, G.; Schnoll-Sussman, F.; Mathews, S.; Katz, P.O. Reported proton pump inhibitor side effects: What are physician and patient perspectives and behaviour patterns? Aliment. Pharmacol. Ther. 2020, 51, 121–128. [Google Scholar] [CrossRef]
- Makunts, T.; Alpatty, S.; Lee, K.C.; Atayee, R.S.; Abagyan, R. Proton-pump inhibitor use is associated with a broad spectrum of neurological adverse events including impaired hearing, vision, and memory. Sci. Rep. 2019, 9, 17280. [Google Scholar] [CrossRef]
- Kim, J.-S.; Zee, D.S. Benign Paroxysmal Positional Vertigo. N. Engl. J. Med. 2014, 370, 1138–1147. [Google Scholar] [CrossRef]
- Bhattacharyya, N.; Gubbels, S.P.; Schwartz, S.R.; Edlow, J.A.; El-Kashlan, H.; Fife, T.; Holmberg, J.M.; Mahoney, K.; Hollingsworth, D.B.; Roberts, R.; et al. Clinical Practice Guideline: Benign Paroxysmal Positional Vertigo (Update). Otolaryngol. Neck Surg. 2017, 156, S1–S47. [Google Scholar] [CrossRef]
- Shim, D.B. Treatment of Benign Paroxysmal Positional Vertigo: An Approach Considering Patients’ Convenience. Clin. Exp. Otorhinolaryngol. 2020, 13, 320–321. [Google Scholar] [CrossRef] [PubMed]
- Von Brevern, M.; Radtke, A.; Lezius, F.; Feldmann, M.; Ziese, T.; Lempert, T.; Neuhauser, H. Epidemiology of benign paroxysmal positional vertigo: A population based study. J. Neurol. Neurosurg. Psychiatry 2007, 78, 710–715. [Google Scholar] [CrossRef]
- Parnes, L.S.; Agrawal, S.K.; Atlas, J. Diagnosis and management of benign paroxysmal positional vertigo (BPPV). Can. Med Assoc. J. 2003, 169, 681–693. [Google Scholar]
- Lee, H.J.; Jeon, E.-J.; Lee, D.-H.; Seo, J.-H. Therapeutic Efficacy of the Modified Epley Maneuver with a Pillow Under the Shoulders. Clin. Exp. Otorhinolaryngol. 2020, 13, 376–380. [Google Scholar] [CrossRef]
- Kim, H.-J.; Park, J.; Kim, J.-S. Update on benign paroxysmal positional vertigo. J. Neurol. 2021, 268, 1995–2000. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, S.; Cui, K.; Liu, C. Risk factors for benign paroxysmal positional vertigo recurrence: A systematic review and meta-analysis. J. Neurol. 2021, 268, 4117–4127. [Google Scholar] [CrossRef]
- Lin, B.M.; Curhan, S.G.; Wang, M.; Jacobson, B.C.; Eavey, R.; Stankovic, K.M.; Curhan, G.C. Prospective Study of Gastroesophageal Reflux, Use of Proton Pump Inhibitors and H2-Receptor Antagonists, and Risk of Hearing Loss. Ear Hear. 2017, 38, 21–27. [Google Scholar] [CrossRef]
- Pirodda, A.; Brandolini, C.; Raimondi, M.C.; Modugno, G.C. The possible role of proton pump inhibitors of the homeostasis of the inner ear. Med. Hypotheses 2009, 72, 325–326. [Google Scholar] [CrossRef]
- Seong, S.C.; Kim, Y.-Y.; Park, S.K.; Khang, Y.-H.; Kim, H.C.; Park, J.H.; Kang, H.-J.; Do, C.-H.; Song, J.-S.; Lee, E.-J.; et al. Cohort profile: The National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open 2017, 7, e016640. [Google Scholar] [CrossRef]
- Kim, S.K.; Hong, S.M.; Park, I.-S.; Choi, H.G. Association Between Migraine and Benign Paroxysmal Positional Vertigo Among Adults in South Korea. JAMA Otolaryngol. Neck Surg. 2019, 145, 307–312. [Google Scholar] [CrossRef]
- Kim, S.Y.; Min, C.; Yoo, D.M.; Chang, J.; Lee, H.-J.; Park, B.; Choi, H.G. Hearing Impairment Increases Economic Inequality. Clin. Exp. Otorhinolaryngol. 2021, 14, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Min, C.; Oh, D.J.; Choi, H.G. Bidirectional Association Between GERD and Asthma: Two Longitudinal Follow-Up Studies Using a National Sample Cohort. J. Allergy Clin. Immunol. Pr. 2020, 8, 1005–1013.e9. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Oh, D.J.; Park, B.; Choi, H.G. Bell’s palsy and obesity, alcohol consumption and smoking: A nested case-control study using a national health screening cohort. Sci. Rep. 2020, 10, 4248. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.-M.; Sundararajan, V. Updating and Validating the Charlson Comorbidity Index and Score for Risk Adjustment in Hospital Discharge Abstracts Using Data From 6 Countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kong, I.G.; Lim, H.; Choi, H.G. Increased Risk of Sudden Sensory Neural Hearing Loss in Osteoporosis: A Longitudinal Follow-Up Study. J. Clin. Endocrinol. Metab. 2018, 103, 3103–3109. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; E Thomas, L. Addressing Extreme Propensity Scores via the Overlap Weights. Am. J. Epidemiology 2019, 188, 250–257. [Google Scholar] [CrossRef]
- Thomas, L.E.; Li, F.; Pencina, M.J. Overlap Weighting. JAMA 2020, 323, 2417. [Google Scholar] [CrossRef]
- Zhu, Y.; Schonbach, M.; Coffman, D.L.; Williams, J.S. Variable Selection for Propensity Score Estimation via Balancing Covariates. Epidemiology 2015, 26, e14–e15. [Google Scholar] [CrossRef]
- Wiciński, M.; Malinowski, B.; Puk, O.; Górski, K.; Adamkiewicz, D.; Chojnacki, G.; Walczak, M.; Wódkiewicz, E.; Szambelan, M.; Adamska, P.; et al. Possible Effects of Proton Pump Inhibitors on Hearing Loss Development. BioMed Res. Int. 2019, 2019, 4853695. [Google Scholar] [CrossRef]
- Yee, J.; Han, H.W.; Gwak, H.S. Proton pump inhibitor use and hearing loss in patients with type 2 diabetes: Evidence from a hospital-based case-control study and a population-based cohort study. Br. J. Clin. Pharmacol. 2022, 88, 2738–2746. [Google Scholar] [CrossRef]
- Kim, S.; Lee, C.; Min, C.; Yoo, D.; Choi, H. Association between Proton Pump Inhibitors and Hearing Impairment: A Nested Case-Control Study. Curr. Issues Mol. Biol. 2021, 43, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Pirodda, A.; Modugno, G.C.; Manzari, L.; Raimondi, M.C.; Brandolini, C.; Ferri, G.G.; Borghi, C. Meniere’s disease and the use of proton pump inhibitors. Swiss Med. Wkly. 2010, 140, w13104. [Google Scholar] [CrossRef] [PubMed]
- Takumida, M.; Takumida, H.; Anniko, M. Gastric-type H+,K+-ATPase in mouse vestibular end organs. Acta Oto-Laryngol. 2016, 137, 455–459. [Google Scholar] [CrossRef]
- Lecain, E.; Robert, J.-C.; Thomas, A.; Huy, P.T.B. Gastric proton pump is expressed in the inner ear and choroid plexus of the rat. Hear. Res. 2000, 149, 147–154. [Google Scholar] [CrossRef]
- Maléth, J.; Hegyi, P. Long-term proton pump inhibitor therapy and osteoporosis. Is there a real danger? Orvosi Hetil. 2013, 154, 1005–1009. [Google Scholar] [CrossRef]
- Lin, S.-M.; Yang, S.-H.; Liang, C.-C.; Huang, H.-K. Proton pump inhibitor use and the risk of osteoporosis and fracture in stroke patients: A population-based cohort study. Osteoporos. Int. 2018, 29, 153–162. [Google Scholar] [CrossRef]
- Guo, T.; Xing, Y.; Zhu, H.; Yang, L.; Xiao, Y.; Xu, J. Relationship between osteoporosis and benign paroxysmal positional vertigo based on evidence-based medicine and bioinformatics. Arch. Osteoporos. 2021, 16, 173. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, H.-J.; Min, C.; Choi, H.G. Association between benign paroxysmal positional vertigo and osteoporosis: Two nested case-control studies. Osteoporos. Int. 2020, 31, 2017–2024. [Google Scholar] [CrossRef]
- Vibert, D.; Sans, A.; Kompis, M.; Travo, C.; Mühlbauer, R.C.; Tschudi, I.; Boukhaddaoui, H.; Häusler, R. Ultrastructural Changes in Otoconia of Osteoporotic Rats. Audiol. Neurotol. 2008, 13, 293–301. [Google Scholar] [CrossRef]
- Bell, E.J.; Bielinski, S.J.; Sauver, J.L.S.; Chen, L.Y.; Rooney, M.R.; Larson, N.B.; Takahashi, P.Y.; Folsom, A.R. Association of Proton Pump Inhibitors with Higher Risk of Cardiovascular Disease and Heart Failure. Mayo Clin. Proc. 2021, 96, 2540–2549. [Google Scholar] [CrossRef]
- Sfakianaki, I.; Binos, P.; Karkos, P.; Dimas, G.G.; Psillas, G. Risk Factors for Recurrence of Benign Paroxysmal Positional Vertigo. A Clinical Review. J. Clin. Med. 2021, 10, 4372. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.M.; Corser, W.D.; Monsell, E.M. Cardiovascular Risk Factors and Benign Paroxysmal Positional Vertigo in Community Otolaryngology–Head and Neck Surgery. Otolaryngol. Neck Surg. 2020, 162, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Feng, H.; Liu, H.; Xu, X.; Wang, J.; Jin, Z. Carotid imaging changes and serum IL-1β, sICAM-1, and sVAP-1 levels in benign paroxysmal positional vertigo. Sci. Rep. 2020, 10, 21494. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Before Overlap Weighting Adjustment | Standardized Difference | After Overlap Weighting Adjustment | Standardized Difference | ||
|---|---|---|---|---|---|---|
| BPPV | Comparison | BPPV | Comparison | |||
| Number | 34,441 | 137,764 | 26,737 | 26,737 | ||
| Age (years, mean, SD) | 61.9 (9.1) | 61.6 (9.0) | 0.03 | 61.9 (8.0) | 61.6 (4.0) | 0.04 |
| Age (years, n, %) | 0.00 | 0.03 | ||||
| 40–44 | 460 (1.3) | 1840 (1.3) | 359 (1.3) | 366 (1.4) | ||
| 45–49 | 2299 (6.7) | 9196 (6.7) | 1799 (6.7) | 1804 (6.8) | ||
| 50–54 | 4871 (14.1) | 19,484 (14.1) | 3812 (14.3) | 3780 (14.1) | ||
| 55–59 | 6928 (20.1) | 27,712 (20.1) | 5417 (20.3) | 5301 (19.8) | ||
| 60–64 | 7280 (21.1) | 29,120 (21.1) | 5649 (21.1) | 5664 (21.2) | ||
| 65–69 | 5704 (16.6) | 22,816 (16.6) | 4368 (16.3) | 4535 (17.0) | ||
| 70–74 | 3551 (10.3) | 14,204 (10.3) | 2721 (10.2) | 2787 (10.4) | ||
| 75–79 | 2152 (6.3) | 8608 (6.3) | 1670 (6.2) | 1635 (6.1) | ||
| 80–84 | 964 (2.8) | 3856 (2.8) | 758 (2.8) | 698 (2.6) | ||
| 85+ | 232 (0.7) | 928 (0.7) | 186 (0.7) | 167 (0.6) | ||
| Sex (n, %) | 0.00 | 0.00 | ||||
| Males | 14,694 (42.7) | 58,776 (42.7) | 11,399 (42.6) | 11,399 (42.6) | ||
| Females | 19,747 (57.3) | 78,988 (57.3) | 15,338 (57.4) | 15,338 (57.4) | ||
| Income (n, %) | 0.00 | 0.01 | ||||
| 1 (lowest) | 5658 (16.4) | 22,632 (16.4) | 4356 (16.3) | 4386 (16.4) | ||
| 2 | 4492 (13.0) | 17,968 (13.0) | 3490 (13.1) | 3454 (12.9) | ||
| 3 | 5408 (15.7) | 21,632 (15.7) | 4201 (15.7) | 4172 (15.6) | ||
| 4 | 7454 (21.6) | 29,816 (21.6) | 5785 (21.6) | 5829 (21.8) | ||
| 5 (highest) | 11,429 (33.2) | 45,716 (33.2) | 8905 (33.3) | 8896 (33.3) | ||
| Region of residence (n, %) | 0.00 | 0.00 | ||||
| Urban | 15,318 (44.5) | 61,272 (44.5) | 11,914 (44.6) | 11,914 (44.6) | ||
| Rural | 19,123 (55.5) | 76,492 (55.5) | 14,823 (55.4) | 14,823 (55.4) | ||
| Total cholesterol level (mg/dL, mean, SD) | 199.6 (38.2) | 200.0 (38.9) | 0.01 | 199.7 (33.6) | 199.7 (17.1) | 0.00 |
| SBP (mmHg, mean, SD) | 126.3 (16.4) | 126.7 (17.2) | 0.02 | 126.3 (14.5) | 126.3 (7.5) | 0.00 |
| DBP (mmHg, mean, SD) | 77.8 (10.4) | 78.0 (10.9) | 0.01 | 77.9 (9.2) | 77.9 (4.8) | 0.00 |
| Fasting blood glucose level (mg/dL, mean, SD) | 99.9 (26.7) | 101.4 (30.1) | 0.05 | 100.2 (24.2) | 100.2 (11.8) | 0.00 |
| Obesity (n, %) ‡ | 0.08 | 0.00 | ||||
| Underweight | 711 (2.1) | 3689 (2.7) | 577 (2.2) | 577 (2.2) | ||
| Normal | 11,613 (33.7) | 50,402 (36.6) | 9172 (34.3) | 9172 (34.3) | ||
| Overweight | 9760 (28.3) | 36,894 (26.8) | 7500 (28.1) | 7500 (28.1) | ||
| Obese I | 11,288 (32.8) | 42,333 (30.7) | 8653 (32.4) | 8653 (32.4) | ||
| Obese II | 1069 (3.1) | 4446 (3.2) | 836 (3.1) | 836 (3.1) | ||
| Smoking status (n, %) | 0.13 | 0.00 | ||||
| Nonsmoker | 26,432 (76.8) | 102,093 (74.1) | 20,395 (76.3) | 20,395 (76.3) | ||
| Past smoker | 4620 (13.4) | 16,483 (12.0) | 3523 (13.2) | 3523 (13.2) | ||
| Current smoker | 3389 (9.8) | 19,188 (13.9) | 2819 (10.5) | 2819 (10.5) | ||
| Alcohol consumption (n, %) | 0.09 | 0.00 | ||||
| <1 time a week | 24,574 (71.4) | 92,741 (67.3) | 18,851 (70.5) | 18,851 (70.5) | ||
| ≥1 time a week | 9867 (28.7) | 45,023 (32.7) | 7886 (29.5) | 7886 (29.5) | ||
| CCI score (score, mean, SD) | 1.1 (1.7) | 0.9 (1.7) | 0.09 | 1.0 (1.4) | 1.0 (0.8) | 0.00 |
| CCI score (n, %) | 0.20 | 0.15 | ||||
| 0 score | 18,851 (54.7) | 88,690 (64.4) | 14,878 (55.6) | 16,525 (61.8) | ||
| 1 score | 6798 (19.7) | 19,354 (14.1) | 5257 (19.7) | 3826 (14.3) | ||
| ≥2 scores | 8792 (25.5) | 29,720 (21.6) | 6603 (24.7) | 6386 (23.9) | ||
| Osteoporosis (n, %) | 11,131 (32.3) | 32,456 (23.6) | 0.20 | 8064 (30.2) | 8064 (30.2) | 0.00 |
| H2 blocker prescription dates (days, mean, SD) | 29.1 (62.4) | 16.5 (49.9) | 0.22 | 25.2 (48.4) | 25.2 (29.8) | 0.00 |
| No. of GERD treatments (no., mean, SD) | 0.7 (2.3) | 0.3 (1.4) | 0.22 | 0.6 (1.5) | 0.6 (1.0) | 0.00 |
| No. of GERD treatments (n, %) | 0.32 | 0.17 | ||||
| 0 time | 26,696 (77.5) | 122,936 (89.2) | 21,256 (79.5) | 22,857 (85.5) | ||
| 1 time | 2865 (8.3) | 6013 (4.4) | 2198 (8.2) | 1275 (4.8) | ||
| ≥2 times | 4880 (14.2) | 8815 (6.4) | 3283 (12.3) | 2605 (9.7) | ||
| PPI prescription history (n, %) | 0.47 | 0.39 | ||||
| PPI nonuser | 1574 (4.6) | 17,662 (12.8) | 1283 (4.8) | 3010 (11.3) | ||
| Past PPI user | 9698 (28.2) | 57,071 (41.4) | 7700 (28.8) | 10,713 (40.1) | ||
| Current PPI user | 23,169 (67.3) | 63,031 (45.8) | 17,754 (66.4) | 13,015 (48.7) | ||
| PPI prescription dates (days, mean, SD) | 234.8 (243.6) | 167.7 (206.1) | 0.30 | 226.4 (209.1) | 185.5 (96.1) | 0.25 |
| PPI prescription dates (n, %) | 0.39 | 0.29 | ||||
| PPI nonuser | 1574 (4.6) | 17,662 (12.8) | 1283 (4.8) | 3010 (11.3) | ||
| ≥1 day and <30 days PPI user | 7416 (21.5) | 40,139 (29.1) | 5959 (22.3) | 7264 (27.2) | ||
| ≥30 days and < 365 days PPI user | 15,327 (44.5) | 52,642 (38.2) | 11,932 (44.6) | 10,483 (39.2) | ||
| ≥365 days PPI user | 10,124 (29.4) | 27,321 (19.8) | 7563 (28.3) | 5980 (22.4) | ||
| Characteristics | BPPV | Comparison | Odds Ratios (95% Confidence Intervals) | |||
|---|---|---|---|---|---|---|
| (Exposure/Total, %) | (Exposure/Total, %) | Crude | p-Value | Adjusted Model with OW † | p-Value | |
| User of PPI | ||||||
| Past PPI user | 9698/66,769 (14.5) | 57,071/66,769 (85.5) | 1.91 (1.80–2.02) | <0.001 * | 1.76 (1.64–1.89) | <0.001 * |
| Current PPI user | 23,169/86,200 (26.9) | 63,031/86,200 (73.1) | 4.12 (3.91–4.35) | <0.001 * | 3.57 (3.33–3.83) | <0.001 * |
| PPI dates | ||||||
| ≥1 day and <30 days | 7416/47,555 (15.6) | 40,139/47,555 (84.4) | 2.07 (1.96–2.19) | <0.001 * | 1.95 (1.81–2.10) | <0.001 * |
| ≥30 days and < 365 days | 15,327/67,969 (22.6) | 52,642/67,969 (77.5) | 3.27 (3.09–3.45) | <0.001 * | 2.88 (2.68–3.10) | <0.001 * |
| ≥365 days | 10,124/37,445 (27.0) | 27,321/37,445 (73.0) | 4.16 (3.93–4.40) | <0.001 * | 3.45 (3.19–3.73) | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.Y.; Yoo, D.M.; Kwon, M.J.; Kim, J.H.; Kim, J.-H.; Lee, J.S.; Choi, H.G. Association between Benign Paroxysmal Positional Vertigo and Previous Proton Pump Inhibitor Use: A Nested Case–Control Study Using a National Health Screening Cohort. Int. J. Environ. Res. Public Health 2022, 19, 10280. https://doi.org/10.3390/ijerph191610280
Kim SY, Yoo DM, Kwon MJ, Kim JH, Kim J-H, Lee JS, Choi HG. Association between Benign Paroxysmal Positional Vertigo and Previous Proton Pump Inhibitor Use: A Nested Case–Control Study Using a National Health Screening Cohort. International Journal of Environmental Research and Public Health. 2022; 19(16):10280. https://doi.org/10.3390/ijerph191610280
Chicago/Turabian StyleKim, So Young, Dae Myoung Yoo, Mi Jung Kwon, Ji Hee Kim, Joo-Hee Kim, Joong Seob Lee, and Hyo Geun Choi. 2022. "Association between Benign Paroxysmal Positional Vertigo and Previous Proton Pump Inhibitor Use: A Nested Case–Control Study Using a National Health Screening Cohort" International Journal of Environmental Research and Public Health 19, no. 16: 10280. https://doi.org/10.3390/ijerph191610280
APA StyleKim, S. Y., Yoo, D. M., Kwon, M. J., Kim, J. H., Kim, J.-H., Lee, J. S., & Choi, H. G. (2022). Association between Benign Paroxysmal Positional Vertigo and Previous Proton Pump Inhibitor Use: A Nested Case–Control Study Using a National Health Screening Cohort. International Journal of Environmental Research and Public Health, 19(16), 10280. https://doi.org/10.3390/ijerph191610280







