Using Telehealth to Guarantee the Continuity of Rehabilitation during the COVID-19 Pandemic: A Systematic Review
Abstract
:1. Introduction
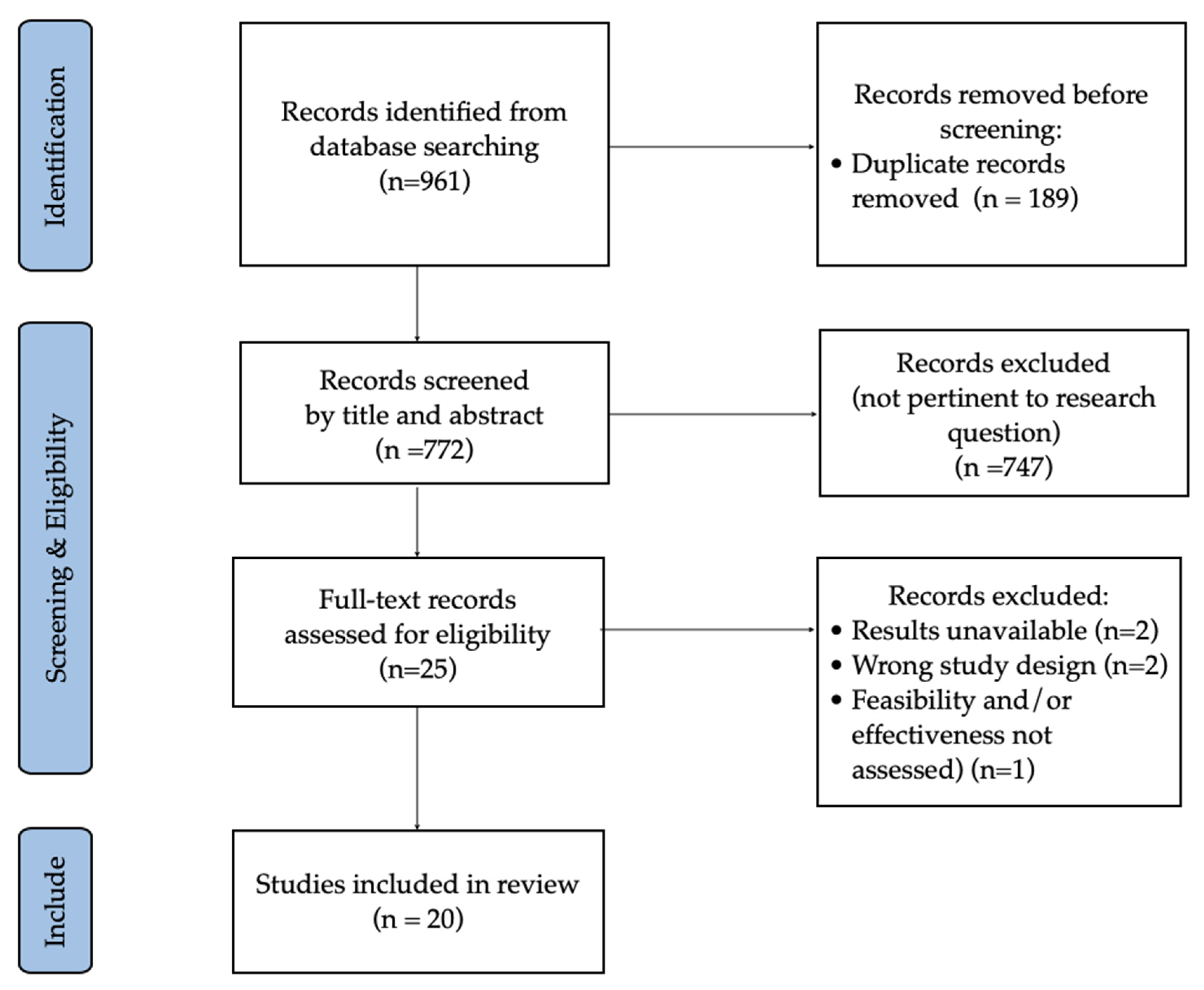
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Quality Assessment
2.5. Data Extraction
2.6. Ethical Approval and Reporting
3. Results
| Author, Year, Country | Method. Quality | Population (N, Pathology, Age—Years) | Intervention (Type, Sessions, Length, Frequency, Total Duration) | Control | Delivery Method | Main Outcomes | Conclusion |
|---|---|---|---|---|---|---|---|
| Batalik et al. (2021) Czech Republic [23] | PEDro Scale 6/10 | 44 patients with coronary heart disease (CAD) (56.6 ± 7.3) | n = 21 Home-based cardiac telerehabilitation w/wrist monitor and telemonitoring/coaching; exercise 3×/week for 60 min at 70–80% of heart rate reserve for 12 weeks | n = 23 Traditional center-based cardiac rehabilitation (same intervention but supervised in person) | Mixed | Cardio- respiratory fitness (CRF) and health-related quality of life (HRQL) | At 1 year follow-up, home-based cardiac telerehabilitation was more effective than center-based cardiac rehabilitation in maintaining long-term CRF levels (p = 0.047). No statistically significant difference between the two groups for HRQL. |
| Gonzalez- Gerez et al. (2021) Spain [24] | PEDro Scale 8/10 | 38 COVID-19 patients with mild to moderate symptoms in the acute stage (28–53) | n = 19 7-day pulmonary rehabilitation (10 breathing exercises, performed daily at home); additional two videoconferences with a physiotherapist and daily text messages | n = 19 No intervention | Mixed | 6 min walk test (6MWT), dyspnea (MD12), 30 s sit-to-stand test (30STST), Borg Scale | Significant differences were found for all outcomes in favor of the intervention group, with 90% adherence. A 1-week telerehabilitation program based on respiratory exercises is effective, safe and feasible in COVID-19 patients with acute mild–moderate symptoms. |
| Hernando- Garijo et al. (2021) Spain [25] | PEDro Scale 7/10 | 34 women with fibromyalgia (53.44 ± 8.8) | n = 17 50 min sessions over 15 weeks (two sessions/week) of telerehabilitation (low-impact rhythmic movements, guided by video) | n = 17 No additional intervention (asked to maintain the same medical prescription during the study) | Mixed | Pain intensity, mechanical pain sensitivity, psychological distress | A telerehabilitation aerobic exercise program yielded statistically significant improvements in pain intensity (p = 0.022), mechanical pain sensitivity (p < 0.05) and psychological distress (p = 0.005) compared to the control group, which showed no statistically significant changes in any variable (p > 0.05). |
| Li et al. (2021) China [26] | PEDro Scale 8/10 | 120 formerly hospitalized COVID-19 survivors with remaining dyspnea complaint (18–75) | n = 59 TERECO: unsupervised home-based 6-week exercise program; three–four sessions/week of breathing, aerobic exercise and strengthening exercises; delivered via smartphone app and monitored via heart rate telemetry | n = 61 No intervention (only received short educational instructions at baseline) | Mixed | 6MWT, lower limb strength, pulmonary function (spirometry), HRQL, dyspnea. Outcomes assessed at week 6 (post-treatment) and 28 (follow-up) | Results demonstrated the superiority of TERECO over no rehabilitation for 6MWD (p < 0.001), lower limb strength (p < 0.001) and HRQOL (p = 0.004). |
| Ozturk et al. (2022) Turkey [27] | PEDro Scale 5/10 | 41 patients with BMI > 25 (18–65) | n = 21 Exercise training with remote live connection (warm-up, trunk stabilization and breathing exercises) supervised by a physiotherapist (3×/week for 6 weeks) | n = 20 Only informed about the importance of exercise for one session | Synchronous | Physical fitness (Senior Fitness Test protocol), HRQL (SF-36), evaluated at baseline and after 6 weeks (post-treatment) | All parameters were statistically significantly different in favor of the telerehabilitation group (p < 0.05). Exercise training via telerehabilitation during the COVID-19 pandemic was effective, safe and feasible in overweight and obese individuals (BMI > 25). |
| Rodríguez- Blanco et al. (2021) Spain [28] | PEDro Scale 8/10 | 77 subjects with COVID-19 in the acute stage (39.40 ± 11.71) | n = 29 Breathing exercises n = 26 Strengthening exercises Both groups performed exercises 1×/day for 14 days; they were taught on day 1 via videoconference; reinforced 1×/week; additional daily text message | n = 22 No intervention | Mixed | Visual analog scale for fatigue, 6MWT, 30STST, dyspnea (MD-12), Borg scale Assessed at baseline and 14 days later | The strength and breathing groups achieved significant improvements in fatigue, dyspnea, perceived effort and physical state compared to control group (p < 0.05). The greatest benefits were found for dyspnea and aerobic capacity in the breathing group (p < 0.001). |
| Author, Year, Country | Method. Quality | Population (Pathology, Age—Years) | Intervention (Type, Sessions, Length, Frequency, Total Duration) | Comparison | Delivery Method | Main Outcomes | Conclusion |
|---|---|---|---|---|---|---|---|
| Cancino- López et al. (2021) Chile [29] | STROBE Checklist 18/22 | 50 COVID-19 patients (54.1 ± 15.4) | 24 exercise sessions of 50–60 min each (10 min warm up, 25 min resistance training, 10 min aerobic training, 5 min cool down), 2–3×/week, via video calls | (No comparison) | Synchronous | Functionality (Barthel’s index) and physical fitness (2 min step test), elbow flexion—one repetition maximum (1RM), short physical performance battery, hand grip strength, 30 s chair stand, skeletal muscle index, body fat percentage, resting pulse, arterial blood pressure and pulse oximetry | 24 sessions of in-home telerehabilitation exercise program promoted the recovery of physical independence, with significant improvements in functionality and physical fitness (p < 0.0001). |
| De Marchi et al. (2020) Italy [30] | STROBE Checklist 19/22 | 19 patients with ALS (51.48) | Televisit of 80–120 min, 3×/month for 3 months (multidisciplinary approach: neurologist, dietician, psychologist, physiotherapist) | (No comparison) | Synchronous | Anxiety and depression (HADS and ALSAQ-40), functional status (ALSFRS-R, Barthel scale), exertion (Borg scale) and pain intensity (VAS) | ALS patients managed by telemedicine received a comparable quality of care to those seen via traditional face-to-face methods; this needs to become an integrated platform for delivering high-quality tertiary ALS care. |
| Lamberti et al. (2021) Italy [31] | STROBE Checklist 21/22 | 66 patients with peripheral artery disease (PAD) (72) | 2 × 8 min daily sessions of slow intermittent in-home walking. Additional regular phone calls to check in on patients | (No comparison) | Synchronous | 6MWD, pain-free walking distance, body weight blood pressure, ankle–brachial index | Pain-free walking distance improved significantly (p < 0.001), body weight decreased, while 6MWD, blood pressure and ankle–brachial index remained stable. A structured in-home walking program guided by phone was adhered to by patients with PAD and improved their mobility. |
| Milani et al. (2021) Italy [32] | STROBE Checklist 19/22 | 23 patients with physical disabilities (44–70.6) | Physiotherapist-led telerehabilitation program with customized exercises; 1 h sessions 2–3 times/week from March to May 2020, delivered in real time via Skype | No tele-rehabilitation | Synchronous | Feasibility and acceptability | Telerehabilitation was a feasible solution, with high adherence and well accepted by patients. |
| Negrini et al. (2020) Italy [33] | STROBE 16/22 | 1207 patients with spinal disorders, (3–18) | Teleconsultations and telephysiotherapy delivered over 3 weeks (15 working days) | Traditional in-person physiotherapy (13 working days) | Mixed | Number of services provided and patient satisfaction | Telephysiotherapy was feasible and allowed health professionals to continue providing outpatient services with a high patient satisfaction, reducing face-to-face contact and the need for travel to a minimum. |
| Oprandi et al. (2021) Italy [34] | STROBE Checklist 19/22 | 13 children and young adults with acquired brain injury (ABI) (10.7) | Neuropsychological and speech telerehabilitation sessions (2×/week for 10 weeks) | (No comparison) | Synchronous | Feasibility and acceptability | Feasibility and acceptability of synchronous telerehabilitation for young patients with ABI was demonstrated. Telerehabilitation can be a successful intervention for this population. |
| Patel et al. (2021) India [35] | STROBE Checklist 16/22 | 47 patients (23 cardio-vascular, 15 pulmonary, 9 oncology) (61.2 ± 12.5) | Exercise telerehabilitation program (5–10 min warm-up, 20–25 min aerobic and strengthening exercises; plus +30 min brisk walk); 3×/week for 1 month | (No comparison) | Synchronous | 6MWT, HRQL (FACIT), daily step count | A short-term, supervised telerehabilitation program yielded significantly positive effects on 6MWT (p = 0.0418) and HRQL (p = 0.0313) in cardiac, pulmonary and oncology patients during COVID-19. |
| Romano et al. (2021) Italy [36] | STROBE Checklist 20/22 | 13 patients with Rett syndrome (RTT) (17 y 11 m) | 3-month home-based, individualized rehabilitation program of motor activities, remotely supervised via Skype calls | (No comparison) | Synchronous | Gross motor function | A total of 76.9% of participants significantly increased their gross motor function. A high level of usefulness, adherence and general satisfaction was observed. Findings strongly support the implementation of telerehabilitation programs for this population. |
| Sakai et al. (2020) Japan [37] | STROBE Checklist 18/22 | 43 COVID-19 patients undergoing rehabilitation (21–95) | n = 18 Remote rehabilitation via videocalls on iPad, with exercises to develop strength, endurance, range of motion and flexibility. Daily 20 min sessions for 1 month | n = 25 In-person rehabilitation with exercises to develop strength, endurance, range of motion and flexibility | Synchronous | ADLs (Barthel Index), mobility scores | The remote rehabilitation group had significantly higher scores in the Barthel Index than the in-person group. Remote rehabilitation is an effective and safe modality and can facilitate rehabilitation in various situations, including patients that can be treated at a distance. |
| Werneke et al. (2021) USA [22] | STROBE Checklist 20/22 | 222,680 patients with a variety of conditions (55 ± 18) | Telerehabilitation (6% of all episodes of care) | Traditional in-person visits | Synchronous (60%), asynchronous (21%), mixed (19%) | Physical function, number of visits, patient satisfaction, telerehabilitation frequency and modes | Telerehabilitation rate was 6%, decreasing from 10% to 5% between the second and third quarters of 2020. The rate of patients very satisfied with their treatment was 3% higher for no telerehabilitation. More studies are needed to understand what facilitates and inhibits the use of telerehabilitation by rehabilitation therapists in order to promote it when appropriate. |
| Author, Year, Country | Method. Quality | Population (Mean Age, Pathology) | Intervention (Type, Sessions, Length, Frequency, Total Duration) | Comparison | Delivery Methods | Main Outcomes | Conclusion |
|---|---|---|---|---|---|---|---|
| Lowe et al. (2021) UK [38] | CONSORT Checklist 17/25 | 21 patients with MS (18+) | LEAP-MS Online Intervention (3 months) delivered via Zoom calls and a web-based online physical activity tool | (No comparison) | Mixed | Fatigue (MFIS) impact of MS(MSIS-29), HRQOL (EQ-5D-5 L), impact of ill health on participation and activities (OxPAQ), self-efficacy (UW-SES-SF), impression of change (PGIC) | This feasibility study allowed meeting the needs of people with MS during the COVID-19 pandemic. |
| Martin et al. (2021) Belgium [39] | CONSORT Checklist 17/25 | 27 patients with COVID-19 (61.5 ± 10.5) | n = 14 telerehabilitation via videoconference; 50 min sessions 3×/week for 6 weeks (30 min endurance exercises, 20 min strengthening exercises); Borg Scale: 6 | n = 13 No intervention (patients who refused the telerehabilitation intervention) | Synchronous | Functional exercise capacity (1 min STST), SpO2 at rest | At 3 months follow-up, improvements were significantly and clinically greater in the telerehabilitation group (p = 0.005). The feasibility and effectiveness of a simple telerehabilitation program were verified. |
| Nakayama et al. (2020) Japan [40] | CONSORT Checklist 16/25 | 236 patients hospitalized for heart failure (HF) (59) | n = 30 remote cardiac rehabilitation (CR) | n = 69 outpatient CR n = 137 non-CR | Mixed | HRQL (EQ5D) 30 days after discharge; Number of emergency readmissions (%) | Emergency readmission rate within 30 days of discharge was lower in the remote CR group than in the non-CR group (n = 137) (p = 0.02). HRQL score was higher in the remote CR group than in the outpatient CR group (p = 0.03) 30 days after discharge. The remote CR program can be a good alternative to outpatient CR. |
| Tanguay et al. (2021) Canada [41] | CONSORT Checklist 15/25 | Seven COVID-19 patients (49–80) | Physiotherapist- led telerehabilitation intervention delivering a pulmonary telerehabilitation program (2×/week for 8 weeks) | (No comparison) | Mixed | Severity of pulmonary symptoms (CAT), HRQL (EQ-5D-5L, EQ-VAS) | All participants increased their quality-of-life scores by at least 10 points. Eight weeks of telerehabilitation seem to improve symptoms, quality of life and return to physical activities in COVID-19 patients. |
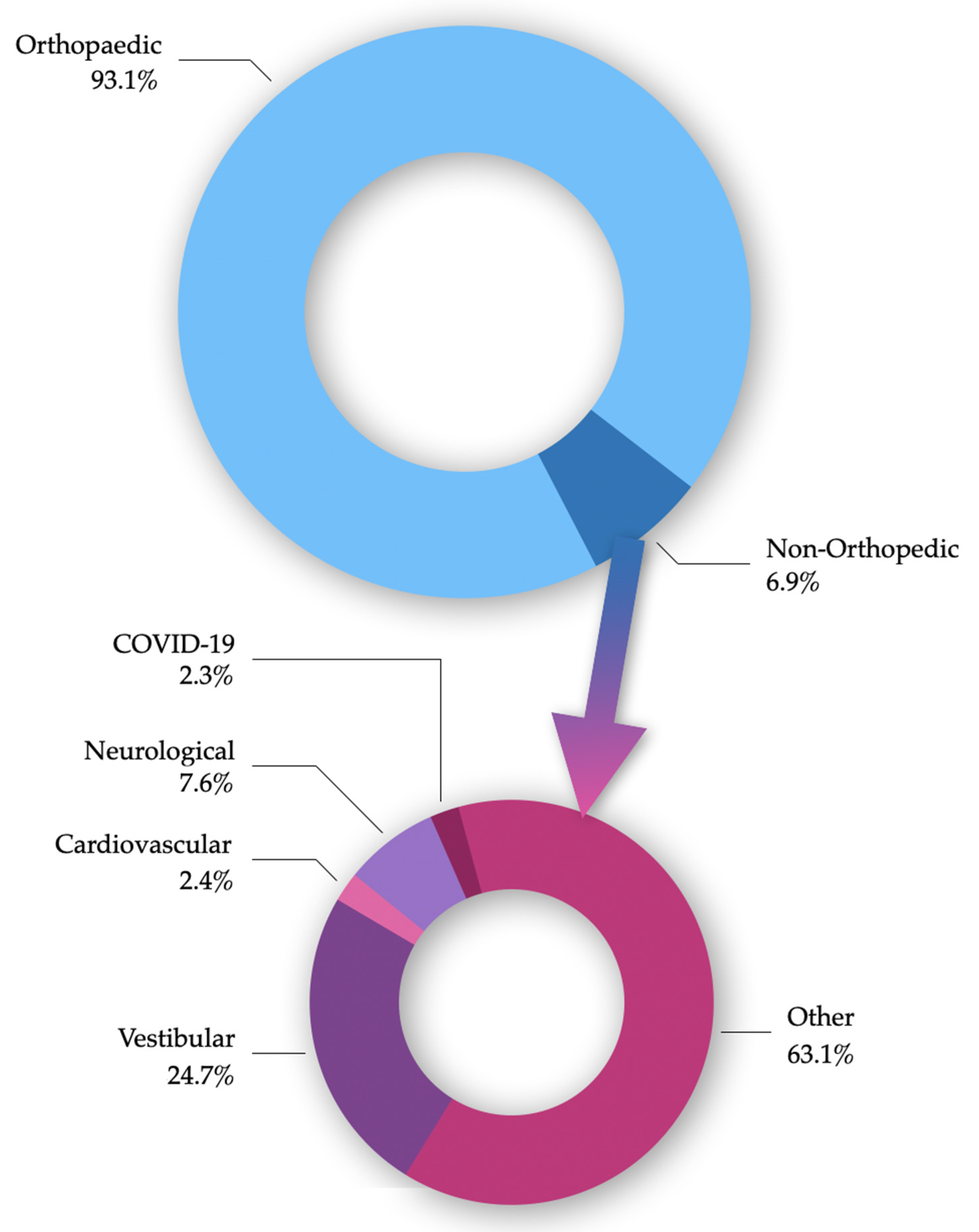
3.1. COVID-19
3.2. Neurological Diseases
3.3. Cardiovascular Diseases
3.4. Other Conditions
4. Discussion
4.1. Limitations and Strengths
4.2. Implications for Clinical Practice and Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Monaghesh, E.; Hajizadeh, A. The role of telehealth during COVID-19 outbreak: A systematic review based on current evidence. BMC Public Health 2020, 20, 1193. [Google Scholar] [CrossRef] [PubMed]
- WHO. Rehabilitation. Available online: https://www.who.int/news-room/fact-sheets/detail/rehabilitation (accessed on 13 March 2022).
- Darkins, A.; Darkins, A.W.; Cary, M.A.; Cary, M. Telemedicine and Telehealth: Principles, Policies, Performances and Pitfalls; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Shaw, T.; McGregor, D.; Brunner, M.; Keep, M.; Janssen, A.; Barnet, S. What is eHealth (6)? Development of a conceptual model for eHealth: Qualitative study with key informants. J. Med. Internet Res. 2017, 19, e324. [Google Scholar] [CrossRef] [PubMed]
- Brennan, D.; Tindall, L.; Theodoros, D.; Brown, J.; Campbell, M.; Christiana, D.; Smith, D.; Cason, J.; Lee, A. A blueprint for telerehabilitation guidelines. Int. J. Telerehabil. 2010, 2, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, M.A.; Galea, O.A.; O’Leary, S.P.; Hill, A.J.; Russell, T.G. Real-time telerehabilitation for the treatment of musculoskeletal conditions is effective and comparable to standard practice: A systematic review and meta-analysis. Clin. Rehabil. 2017, 31, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Howard, I.M.; Kaufman, M.S. Telehealth applications for outpatients with neuromuscular or musculoskeletal disorders. Muscle Nerve 2018, 58, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Ericsson Mobility Report. 2021. Available online: https://www.ericsson.com/4ad7e9/assets/local/reports-papers/mobility-report/documents/2021/ericsson-mobility-report-november-2021.pdf (accessed on 15 March 2022).
- Bennell, K.L.; Marshall, C.J.; Dobson, F.; Kasza, J.; Lonsdale, C.; Hinman, R.S. Does a web-based exercise programming system improve home exercise adherence for people with musculoskeletal conditions? A randomised controlled trial. Am. J. Phys. Med. Rehabil. 2019, 98, 850–858. [Google Scholar] [CrossRef]
- Lambert, T.E.; Harvey, L.A.; Avdalis, C.; Chen, L.W.; Jeyalingam, S.; Pratt, C.A.; Tatum, H.J.; Bowden, J.L.; Lucas, B.R. An app with remote support achieves better adherence to home exercise programs than paper handouts in people with musculoskeletal conditions: A randomized trial. J. Physiother. 2017, 63, 161–167. [Google Scholar] [CrossRef]
- Lawford, B.J.; Delany, C.; Bennell, K.L.; Hinman, R.S. “I was really sceptical...But it worked really well”: A qualitative study of patient perceptions of telephone-delivered exercise therapy by physiotherapists for people with knee osteoarthritis. Osteoarthr. Cartil. 2018, 26, 741–750. [Google Scholar] [CrossRef]
- Moffet, H.; Tousignant, M.; Nadeau, S.; Mérette, C.; Boissy, P.; Corriveau, H.; Marquis, F.; Cabana, F.; Belzile, E.L.; Ranger, P.; et al. Patient satisfaction with in-home telerehabilitation after total knee arthroplasty: Results from a randomized controlled trial. Telemed. J. E Health 2017, 23, 80–87. [Google Scholar] [CrossRef]
- Tousignant, M.; Boissy, P.; Moffet, H.; Corriveau, H.; Cabana, F.; Marquis, F.; Simard, J. Patients’ satisfaction of healthcare services and perception with in-home telerehabilitation and physiotherapists’ satisfaction toward technology for post-knee arthroplasty: An embedded study in a randomized trial. Telemed. J. E Health 2011, 17, 376–382. [Google Scholar] [CrossRef]
- Anil, K.; Freeman, J.A.; Buckingham, S.; Demain, S.; Gunn, H.; Jones, R.B.; Logan, A.; Marsden, J.; Playford, D.; Sein, K.; et al. Scope, context and quality of telerehabilitation guidelines for physical disabilities: A scoping review. BMJ Open 2021, 11, e049603. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Cashin, A.G.; McAuley, J.H. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J. Physiother. 2020, 66, 59. [Google Scholar] [CrossRef]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13 (Suppl. S1), S31–S34. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A. CONSORT 2010 statement: Extension to randomized pilot and feasibility trials. BMJ 2016, 355, i5239. [Google Scholar] [CrossRef]
- Thabane, L.; Hopewell, S.; Lancaster, G.A.; Bond, C.M.; Coleman, C.L.; Campbell, M.J.; Eldridge, S.M. Methods and processes for development of a CONSORT extension for reporting pilot randomized controlled trials. Pilot Feasibility Stud. 2016, 2, 25. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereauz, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Werneke, M.W.; Deutscher, D.; Grigsby, D.; Tucker, C.A.; Mioduski, J.E.; Hayes, D. Telerehabilitation during the COVID-19 pandemic in outpatient rehabilitation settings: A descriptive study. Phys. Ther. 2021, 101, pzab110. [Google Scholar] [CrossRef]
- Batalik, L.; Dosbaba, F.; Hartman, M.; Konecny, V.; Batalikova, K.; Spinar, J. Long-term exercise effects after cardiac telerehabilitation in patients with coronary artery disease: 1-year follow-up results of the randomized study. Eur. J. Phys. Rehabil. Med. 2021, 57, 807–814. [Google Scholar] [CrossRef]
- Gonzalez-Gerez, J.J.; Saavedra-Hernandez, M.; Anarte-Lazo, E.; Bernal-Utrera, C.; Perez-Ale, M.; Rodriguez-Blanco, C. Short-term effects of a respiratory telerehabilitation program in confined COVID-19 patients in the acute phase: A pilot study. Int. J. Environ. Res. Public Health 2021, 18, 7511. [Google Scholar] [CrossRef] [PubMed]
- Hernando-Garijo, I.; Ceballos-Laita, L.; Mingo-Gómez, M.T.; Medrano-de-la-Fuente, R.; Estébanez-de-Miguel, E.; Martínez-Pérez, M.N.; Jiménez-Del-Barrio, S. Immediate effects of a telerehabilitation program based on aerobic exercise in women with fibromyalgia. Int. J. Environ. Res. Public Health 2021, 18, 2075. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xia, W.; Zhan, C.; Liu, S.; Yin, Z.; Wang, J.; Chong, Y.; Reinhardt, J.D. A telerehabilitation programme in post-discharge COVID-19 patients (TERECO): A randomized controlled trial. Thorax 2021, 77, 697–706. [Google Scholar] [CrossRef]
- Ozturk, B.; Duruturk, N. Effect of telerehabilitation applied during COVID-19 isolation period on physical fitness and quality of life in overweight and obese individuals. Int. J. Obes. 2022, 46, 95–99. [Google Scholar] [CrossRef]
- Rodríguez-Blanco, C.; Bernal-Utrera, C.; Anarte-Lazo, E.; Saavedra-Fernandez, M.; De-La-Barrera-Aranda, E.; Serrera-Figallo, M.A.; Gonzalez-Martin, M.; Gonzalez-Gerez, J.J. Breathing exercises versus strength exercises through telerehabilitation in coronavirus disease 2019 patients in the acute phase: A randomized controlled trial. Clin. Rehabil. 2022, 36, 486–497. [Google Scholar] [CrossRef]
- Cancino-López, J.; Vergara, P.Z.; Dinamarca, B.L.; Contreras, P.F.; Cárcamo, L.M.; Ibarra, N.C.; Soto-Sánchez, J. Telerehabilitation is Effective to Recover Functionality and Increase Skeletal Muscle Mass Index in COVID-19 Survivors. IJT 2021, 13, e6415. [Google Scholar] [CrossRef]
- De Marchi, F.; Sarnelli, M.F.; Serioli, M.; De Marchi, I.; Zani, E.; Bottone, N.; Ambrosi, S.; GArone, R.; CAntello, R.; Mazzini, L.; et al. Telehealth approach for amyotrophic lateral sclerosis patients: The experience during COVID-19 pandemic. Acta Neurol. Scand. 2021, 143, 489–496. [Google Scholar] [CrossRef]
- Lamberti, N.; Straudi, S.; Manfredini, R.; De Giorgi, A.; Gasbarro, V.; Zamboni, P.; Manfredini, F. Don’t stop walking: The in-home rehabilitation program for peripheral artery disease patients during the COVID-19 pandemic. Intern. Emerg. Med. 2021, 16, 1307–1315. [Google Scholar] [CrossRef]
- Milani, G.; Demattè, G.; Ferioli, M.; Dallagà, G.; Lavezzi, S.; Basaglia, N.; Straudi, S. Telerehabilitation in Italy during the COVID-19 lockdown: A feasibility and acceptability study. Int. J. Telerehabil. 2021, 13, e6334. [Google Scholar] [CrossRef]
- Negrini, S.; Donzelli, S.; Negrini, A.; Negrini, A.; Romano, M.; Zaina, F. Feasibility and acceptability of telemedicine to substitute outpatient rehabilitation services in the COVID-19 emergency in Italy: An observational everyday clinical-life study. Arch. Phys. Med. Rehabil. 2020, 101, 2027–2032. [Google Scholar] [CrossRef]
- Oprandi, M.C.; Bardoni, A.; Corno, L.; Guerrini, A.M.; Molatore, L.; Negri, L.; Beretta, E.; Locatelli, F.; Strazzer, S.; Poggi, G. Feasibility and acceptability of a real-time telerehabilitation intervention for children and young adults with acquired brain injury during the COVID-19 pandemic: An experience report. IJT 2021, 13, e6423. [Google Scholar] [CrossRef]
- Patel, J.; Franklin, B.A.; Pujary, D.; Kaur, G.; Deodhar, A.; Kharbanda, S.; Contractor, A. Effects of supervised exercise-based telerehabilitation on walk test performance and quality of life in patients in India with chronic disease: Combatting COVID-19. Int. J. Telerehabil. 2021, 13, e6349. [Google Scholar] [CrossRef]
- Romano, A.; Di Rosa, G.; Tisano, A.; Fabio, R.A.; Lotan, M. Effects of a remotely supervised motor rehabilitation program for individuals with Rett syndrome at home. Disabil. Rehabil. 2021, 1–11. [Google Scholar] [CrossRef]
- Sakai, T.; Hoshino, C.; Yamaguchi, R.; Hirao, M.; Nakahara, R.; Okawa, A. Remote rehabilitation for patients with COVID-19. J. Rehabil. Med. 2020, 52, 1–8. [Google Scholar] [CrossRef]
- Lowe, R.; Barlow, C.; Lloyd, B.; Latchem-Hastings, J.; Poile, V.; Scoble, C.; Dean-Young, A.; Button, K.; Playle, R.; Busse, M. Lifestyle, exercise and activity package for people living with Progressive Multiple Sclerosis (LEAP-MS): Adaptions during the COVID-19 pandemic and remote delivery for improved efficiency. Trials 2021, 22, 286. [Google Scholar] [CrossRef]
- Martin, I.; Braem, F.; Baudet, L.; Poncin, W.; Fizaine, S.; Aboubakar, F.; Friodure, A.; Pilette, C.; Liistro, G.; De Greef, J.; et al. Follow-up of functional exercise capacity in patients with COVID-19: It is improved by telerehabilitation. Respir Med. 2021, 183, 106438. [Google Scholar] [CrossRef]
- Nakayama, A.; Takayama, N.; Kobayashi, M.; Hyodo, K.; Maeshima, N.; Takayuki, F.; Komuro, I. Remote cardiac rehabilitation is a good alternative of outpatient cardiac rehabilitation in the COVID-19 era. Environ. Health Prev. Med. 2020, 25, 48. [Google Scholar] [CrossRef]
- Tanguay, P.; Marquis, N.; Gaboury, I.; Kairy, D.; Touchette, M.; Tousignant, M.; Décary, S. Telerehabilitation for post-hospitalized COVID-19 patients: A proof-of-concept study during a pandemic. Int. J. Telerehabil. 2021, 13, e6383. [Google Scholar] [CrossRef]
- Agostini, M.; Moja, L.; Banzi, R.; Pistotti, V.; Tonin, P.; Venneri, A.; Turolla, A. Telerehabilitation and recovery of motor function: A systematic review and meta-analysis. J. Telemed. Telecare 2015, 21, 202–213. [Google Scholar] [CrossRef]
- Barker-Davies, R.M.; O’Sullivan, O.; Senaratne, K.P.P.; Baker, P.; Cranley, M.; Dharm-Datta, S.; Ellis, H.; Goodall, D.; Gough, M.; Lewis, S.; et al. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. BJSM 2020, 54, 949–959. [Google Scholar] [CrossRef]
- Agostini, F.; Mangone, M.; Ruiu, P.; Paolucci, T.; Santilli, V.; Bernetti, A. Rehabilitation setting during and after COVID-19: An overview on recommendations. J. Rehabil. Med. 2021, 53, jrm00141. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.L.; Lotke, P.A.; Lau, E.; Manley, M.T.; Kurtz, S.M. Prevalence and costs of rehabilitation and physical therapy after primary TJA. J. Arthroplast. 2015, 30, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Marra, C.; Gordon, W.J.; Stern, A.D. Use of connected digital products in clinical research following the COVID-19 pandemic: A comprehensive analysis of clinical trials. BMJ Open 2021, 11, e047341. [Google Scholar] [CrossRef] [PubMed]
- Carl, J.R.; Jones, D.J.; Lindhiem, O.J.; Doss, B.D.; Weingardt, K.R.; Timmons, A.C.; Comer, J.S. Regulating digital therapeutics for mental health: Opportunities, challenges, and the essential role of psychologists. Br. J. Clin. Psychol. 2022, 61 (Suppl. S1), 130–135. [Google Scholar] [CrossRef]
- Negrini, S.; Kiekens, C.; Bernetti, A.; Capecci, M.; Ceravolo, M.G.; Lavezzi, S.; Zampolini, M.; Boldrini, P. Telemedicine from research to practice during the pandemic. “Instant paper from the field” on rehabilitation answers to the COVID-19 emergency. Eur. J. Phys. Rehabil. Med. 2020, 56, 327–330. [Google Scholar] [CrossRef]
- Rangachari, P.; Mushiana, S.S.; Herbert, K.A. Narrative review of factors historically influencing telehealth use across six medical specialties in the United States. Int. J. Environ. Res. Public Health 2021, 18, 4995. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brigo, E.; Rintala, A.; Kossi, O.; Verwaest, F.; Vanhoof, O.; Feys, P.; Bonnechère, B. Using Telehealth to Guarantee the Continuity of Rehabilitation during the COVID-19 Pandemic: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 10325. https://doi.org/10.3390/ijerph191610325
Brigo E, Rintala A, Kossi O, Verwaest F, Vanhoof O, Feys P, Bonnechère B. Using Telehealth to Guarantee the Continuity of Rehabilitation during the COVID-19 Pandemic: A Systematic Review. International Journal of Environmental Research and Public Health. 2022; 19(16):10325. https://doi.org/10.3390/ijerph191610325
Chicago/Turabian StyleBrigo, Elisabetta, Aki Rintala, Oyéné Kossi, Fabian Verwaest, Olivier Vanhoof, Peter Feys, and Bruno Bonnechère. 2022. "Using Telehealth to Guarantee the Continuity of Rehabilitation during the COVID-19 Pandemic: A Systematic Review" International Journal of Environmental Research and Public Health 19, no. 16: 10325. https://doi.org/10.3390/ijerph191610325
APA StyleBrigo, E., Rintala, A., Kossi, O., Verwaest, F., Vanhoof, O., Feys, P., & Bonnechère, B. (2022). Using Telehealth to Guarantee the Continuity of Rehabilitation during the COVID-19 Pandemic: A Systematic Review. International Journal of Environmental Research and Public Health, 19(16), 10325. https://doi.org/10.3390/ijerph191610325









