The Effect of Exercise Intensity on Affective and Repetition Priming in Middle-Aged Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Size
2.2. Participants
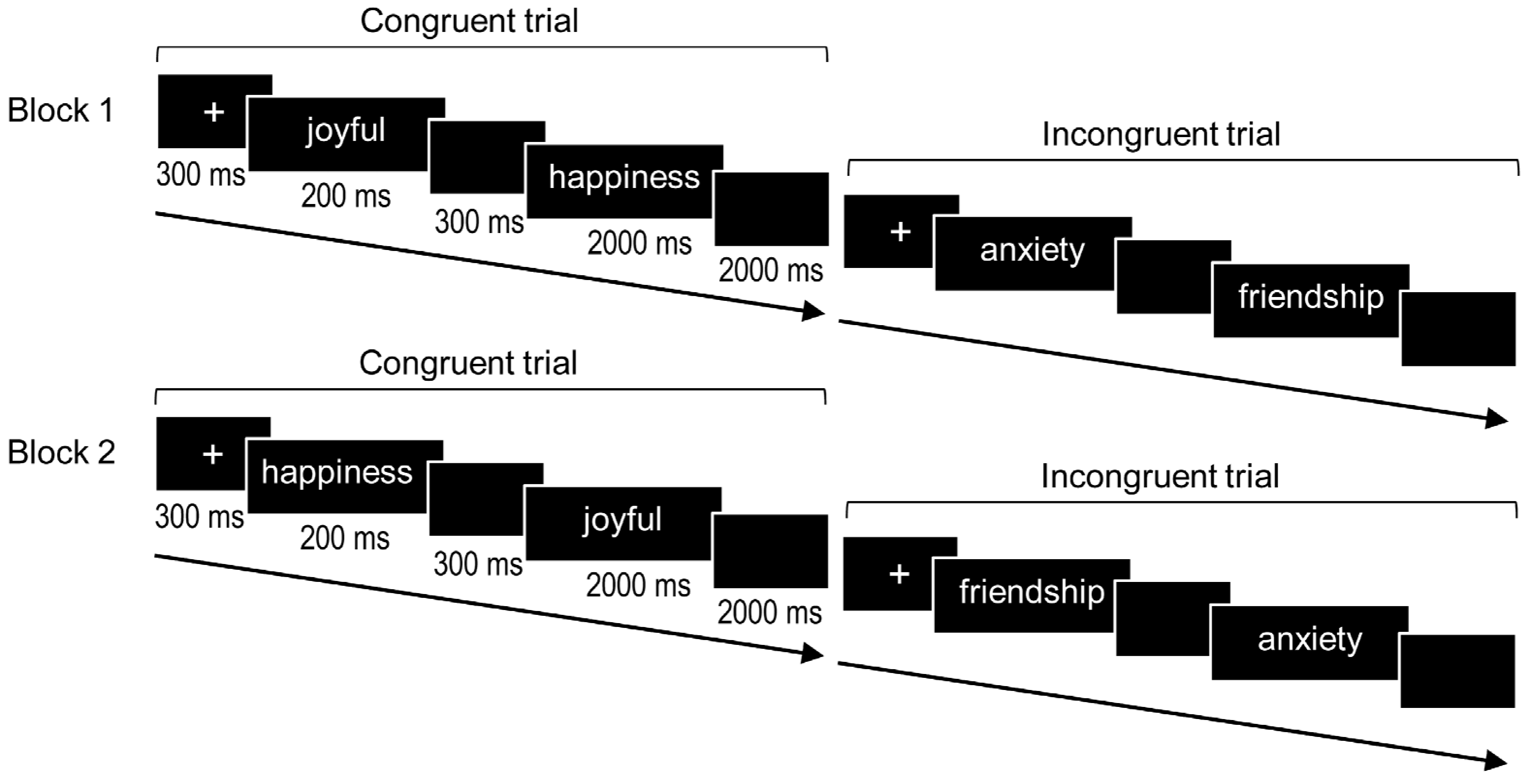
2.3. Affective and Repetition Priming Task
2.4. Procedure
2.5. Data Analysis
3. Results
Affective and Repetition Priming
4. Discussion
5. Conclusions
6. Limitations and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Action Plan on Physical Activity 2018–2030: More Active People for a Healthier World; World Health Organization: Geneva, Switzerland, 2018. Available online: https://apps.who.int/iris/bitstream/handle/10665/272722/9789241514187-eng.pdf (accessed on 15 May 2021).
- Pedersen, B.K.; Saltin, B. Exercise as medicine–evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. 2015, 25, 1–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warburton, D.E.; Bredin, S.S. Reflections on physical activity and health: What should we recommend? Can. J. Cardiol. 2016, 32, 495–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hötting, K.; Röder, B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci. Biobehav. Rev. 2013, 37, 2243–2257. [Google Scholar] [CrossRef] [PubMed]
- Scully, D.; Kremer, J.; Meade, M.M.; Graham, R.; Dudgeon, K. Physical exercise and psychological well-being: A critical review. Br. J. Sports Med. 1998, 32, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Wang, Y.; Li, Y.; Liu, D. Influence of1 exercises of different intensities on adolescent depression. Rev. Argent. Clín. Psicol. 2020, 29, 417–422. [Google Scholar] [CrossRef]
- Morgan, W.P.; OConnor, P.J. Exercise and mental health. In Exercise Adherence: Its Impact on Public Health; Dishman, R.K., Ed.; Human Kinetics: Champaign, IL, USA, 1988; pp. 91–121. [Google Scholar]
- Rocheleau, C.A.; Webster, G.D.; Bryan, A.; Frazier, J. Moderators of the relationship between exercise and mood changes: Gender, exertion level, and workout duration. Psychol. Health 2004, 19, 491–506. [Google Scholar] [CrossRef] [Green Version]
- Audiffren, M.; Tomporowski, P.D.; Zagrodnik, J. Acute aerobic exercise and information processing: Modulation of executive control in a random number generation task. Acta Psychol. 2009, 132, 85–95. [Google Scholar] [CrossRef]
- Murray, N.P.; Russoniello, C. Acute physical activity on cognitive function: A heart rate variability examination. Appl. Psychophysiol. Biofeedback 2012, 37, 219–227. [Google Scholar] [CrossRef]
- Aghjayan, S.L.; Bournias, T.; Kang, C.; Zhou, X.; Stillman, C.M.; Donofry, S.D.; Kamarck, T.W.; Marsland, A.L.; Voss, M.W.; Fraundorf, S.H.; et al. Aerobic exercise improves episodic memory in late adulthood: A systematic review and meta-analysis. Commun. Med. 2022, 2, 15. [Google Scholar] [CrossRef]
- Dietrich, A. Functional neuroanatomy of altered states of consciousness: The transient hypofrontality hypothesis. Conscious. Cogn. 2003, 12, 231–256. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, A. Transient hypofrontality as a mechanism for the psychological effects of exercise. Psychiatry Res. 2006, 145, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Kahneman, D. Attention and Effort; Prentice Hall Inc.: Englewood Cliffs, NJ, USA, 1973. [Google Scholar]
- Sanders, A.F. Towards a model of stress and human performance. Acta Psychol. 1983, 53, 61–97. [Google Scholar] [CrossRef]
- Zouhal, H.; Jacob, C.; Delamarche, P.; Gratas-Delamarche, A. Catecholamines and the effects of exercise, training and gender. Sports Med. 2008, 38, 401–423. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.R.R.; Tessaro, V.H.; Teixeira, L.A.C.; Murakava, K.; Roschel, H.; Gualano, B.; Takito, M.Y. Influence of acute high-intensity aerobic interval exercise bout on selective attention and short-term memory tasks. Percept. Mot. Ski. 2014, 118, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Kujach, S.; Byun, K.; Hyodo, K.; Suwabe, K.; Fukuie, T.; Laskowski, R.; Dan, I.; Soya, H. A transferable high-intensity intermittent exercise improves executive performance in association with dorsolateral prefrontal activation in young adults. NeuroImage 2018, 169, 117–125. [Google Scholar] [CrossRef]
- Ai, J.-Y.; Chen, F.-T.; Hsieh, S.-S.; Kao, S.-C.; Chen, A.-G.; Hung, T.-M.; Chang, Y.-K. The Effect of Acute High-Intensity Interval Training on Executive Function: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 3593. [Google Scholar] [CrossRef]
- Draper, S.; McMorris, T.; Parker, J.K. Effect of acute exercise of differing intensities on simple and choice reaction and movement times. Psychol. Sport Exerc. 2010, 11, 536–541. [Google Scholar] [CrossRef]
- Kashihara, K.; Nakahara, Y. Short-term effect of physical exercise at lactate threshold on choice reaction time. Percept. Mot. Ski. 2005, 100, 275–291. [Google Scholar] [CrossRef]
- Collardeau, M.; Brisswalter, J.; Audiffren, M. Effects of a prolonged run on simple reaction time of well-trained runners. Percept. Mot. Ski. 2001, 93, 679–689. [Google Scholar] [CrossRef]
- Chang, Y.K.; Labban, J.D.; Gapin, J.I.; Etnier, J.L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef] [Green Version]
- McMorris, T.; Hale, B.J. Differential effects of differing intensities of acute exercise on speed and accuracy of cognition: A meta-analytical investigation. Brain Cogn. 2012, 80, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Edwards, M.K. Exercise and implicit memory: A brief systematic review. Psychol. Rep. 2018, 121, 1072–1085. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, S.; Bischof, G.N.; Goh, J.O.; Park, D.C. Neural correlates of conceptual object priming in young and older adults: An event-related fMRI study. Neurobiol. Aging 2013, 34, 1254–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballesteros, S.; Reales, J.M.; Manga, D. Implicit and explicit memory for familiar and novel objects presented to touch. Psicothema. 1999, 11, pp. 785–800. Available online: http://www.psicothema.com/pdf/328.pdf (accessed on 16 February 2020).
- Mitchell, D.B.; Bruss, P.J. Age Differences in implicit memory: Conceptual, perceptual, or methodological? Psychol. Aging 2003, 18, 807–822. [Google Scholar] [CrossRef]
- Ballesteros, S.; Mayas, J.; Reales, J.M. Cognitive function in normal aging and in older adults with mild cognitive impairment. Psicothema 2013, 25, 18–24. [Google Scholar] [CrossRef]
- Ballesteros, S.; Reales, J.M.; Mayas, J. Picture priming in normal aging and Alzheimer’s disease. Psicothema. 2007, 19, pp. 239–244. Available online: http://www.psicothema.com/pdf/3354.pdf (accessed on 15 March 2020).
- Redondo, M.T.; Beltrán-Brotóns, J.L.; Reales, J.M.; Ballesteros, S. Word-stem priming and recognition in type 2 diabetes mellitus, Alzheimer’s disease patients and healthy older adults. Exp. Brain Res. 2015, 233, 3163–3174. [Google Scholar] [CrossRef]
- Schacter, D.L.; Cooper, L.A.; Valdiserri, M. Implicit and explicit memory for novel visual objects in older and younger adults. Psychol. Aging 1992, 7, 299–308. [Google Scholar] [CrossRef]
- Ballesteros, S.; Reales, J.M. Intact haptic priming in normal aging and Alzheimer’s disease: Evidence for dissociable memory systems. Neuropsychologia 2004, 42, 1063–1070. [Google Scholar] [CrossRef]
- Easton, R.D.; Greene, A.J.; Srinivas, K. Transfer between vision and touch: Memory for 2-D patterns and 3-D objects. Psych. Bull. Rev. 1997, 4, 403–410. [Google Scholar] [CrossRef] [Green Version]
- Caballero, A.; Reales, J.M.; Ballesteros, S. Taste priming and cross-modal taste-olfactory priming in normal aging and in older adults with mild cognitive impairment. Psicothema 2018, 30, 304–309. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Gilbert, M.; Robinson, G.; Dickerson, B. Experimental investigation examining the effects of acute exercise on implicit memory function. Eur. J. Psychol. 2019, 15, 700–716. [Google Scholar] [CrossRef] [Green Version]
- Klauer, K.C. Affective priming. Eur. Rev. Soc. Psychol. 1998, 8, 67–103. [Google Scholar] [CrossRef]
- Fazio, R.H. How do attitudes guide behavior? In Handbook of Motivation and Cognition: Foundations of Social Behavior; Sorrentino, R.M., Higgins, E.T., Eds.; Guilford Press: New York, NY, USA, 1986; pp. 204–243. [Google Scholar]
- Yao, Z.; Wang, Z. The effects of the concreteness of differently valenced words on affective priming. Acta Psychol. 2013, 143, 269–276. [Google Scholar] [CrossRef]
- Sass, K.; Habel, U.; Sachs, O.; Huber, W.; Gauggel, S.; Kircher, T. The influence of emotional associations on the neural correlates of semantic priming. Hum. Brain Mapp. 2012, 33, 676–694. [Google Scholar] [CrossRef]
- Kissler, J.; Koessler, S. Emotionally positive stimuli facilitate lexical decisions—An ERP study. Biol. Psychol. 2011, 86, 254–264. [Google Scholar] [CrossRef]
- Rossell, S.L.; Nobre, A.C. Semantic priming of different affective categories. Emotion 2004, 4, 354–363. [Google Scholar] [CrossRef] [Green Version]
- Rossell, S.L.; Shapleske, J.; David, A.S. Direct and indirect semantic priming with neutral and emotional words in schizophrenia: Relationship to delusions. Cogn. Neuropsychiatry 2000, 5, 271–292. [Google Scholar] [CrossRef]
- Bower, G.H. Mood and memory. Am. Psychol. 1981, 25, 443–455. [Google Scholar] [CrossRef]
- Clore, G.L.; Storbeck, J. Affect as information about liking, efficacy, and importance. In Affect in Social Thinking and Behavior; Forgas, J.P., Ed.; Psychology Press: Hove, UK, 2006; pp. 123–141. [Google Scholar]
- LeDoux, J.E. Emotional memory systems in the brain. Behav. Brain Res. 1993, 58, 69–79. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Frith, E.; Edwards, M.K. Exercise and emotional memory: A systematic review. J. Cogn. Enhanc. 2019, 3, 94–103. [Google Scholar] [CrossRef]
- Canli, T.; Zhao, Z.; Brewer, J.; Gabrieli, J.D.; Cahill, L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. J. Neurosc. 2000, 20, RC99. [Google Scholar] [CrossRef]
- Abbassi, E.; Blanchette, I.; Sirmon-Taylor, B.; Ansaldo, A.I.; Ska, B.; Joanette, Y. Lateralized affective word priming and gender effect. Front. Psychol. 2019, 9, 2591. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Eich, T.S.; Metcalfe, J. Effects of the stress of marathon running on implicit and explicit memory. Psych. Bull. Rev. 2009, 16, 475–479. [Google Scholar] [CrossRef] [Green Version]
- Kline, G.M.; Porcari, J.P.; Hintermeister, R.; Freedson, P.S.; Ward, A.; McCarron, R.F.; Ross, J.; Rippe, J.M. Estimation of VO2max from a one-mile track walk, gender, age, and body weight. Med. Sci. Sports Exerc. 1987, 19, 253–259. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [Green Version]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Beck Depression Inventory Manual; The Psychological Corporation: San Antonio, TX, USA, 1996; pp. 785–791. [Google Scholar]
- Powell, K.E.; Paluch, A.E.; Blair, S.N. Physical activity for health: What kind? How much? How intense? On top of what? Annu. Rev. Public Health 2011, 32, 349–365. [Google Scholar] [CrossRef] [Green Version]
- Borer, K.T. Exercise Endocrinology; Human Kinetics: Champaign, IL, USA, 2003. [Google Scholar]
- Karvonen, M.J.; Kentala, E.; Mustala, O. The effects of training on heart rate: A longitudinal study. Ann. Med. Exp. Biol. Fenn 1957, 35, 307–315. [Google Scholar]
- Gellish, R.L.; Goslin, B.R.; Olson, R.E.; McDonald, A.; Russi, G.D.; Moudgil, V.K. Longitudinal modeling of the relationship between age and maximal heart rate. Med. Sci. Sports Exerc. 2007, 39, 822–829. [Google Scholar] [CrossRef]
- Hinojosa, J.A.; Martínez-García, N.; Villalba-García, C.; Fernández-Folgueiras, U.; Sánchez-Carmona, A.; Pozo, M.A.; Montoro, P.R. Affective norms of 875 Spanish words for five discrete emotional categories and two emotional dimensions. Behav. Res. Methods 2016, 48, 272–284. [Google Scholar] [CrossRef]
- Light, L.L.; Singh, A. Implicit and explicit memory in young and older adults. J. Exp. Psychol. Learn. Mem. Cogn. 1987, 13, 531–541. [Google Scholar] [CrossRef]
- Ballesteros, S.; Mayas, J.; Reales, J.M. Does a physically active lifestyle attenuate decline in all cognitive functions in old age? Curr. Aging Sci. 2013, 6, 189–198. [Google Scholar] [CrossRef]
- Biederman, I.; Cooper, E.E. Size invariance in visual object priming. J. Exp. Psychol. Hum. Percept. Perform. 1992, 18, 121–133. [Google Scholar] [CrossRef]
- Cooper, L.A.; Schacter, S.L.; Ballesteros, S.; Moore, C. Priming and recognition of transformed three-dimensional objects: Effects of size and reflection. J. Exp. Psychol. Learn. Mem. Cogn. 1992, 18, 43–57. [Google Scholar] [CrossRef]
- Schacter, D.L.; Cooper, L.A.; Delaney, S.M. Implicit memory for unfamiliar objects depends on access to structural descriptions. J. Exp. Psychol. Gen 1990, 119, 5–24. [Google Scholar] [CrossRef]
- Yao, Z.; Wang, Y.; Lu, B.; Zhu, X. Effects of valence and arousal on affective priming vary with the degree of affective experience denoted by words. Intern. J. Psychophysiol. 2019, 140, 15–25. [Google Scholar] [CrossRef]
- Isen, A.M. Asymmetry of happiness and sadness in effects on memory in normal college students: Comment on Hasher, Rose, Zacks, Sanft, and Doren. J. Exp. Psychol. 1985, 114, 388–391. [Google Scholar] [CrossRef]
- Isen, A.M. Positive affect, cognitive processes, and social behavior. Adv. Exp. Soc. Psychol. 1987, 20, 203–253. [Google Scholar] [CrossRef]
- Niven, A.; Thow, J.; Holroyd, J.; Turner, A.P.; Phillips, S.M. Comparison of affective responses during and after low volume high-intensity interval exercise, continuous moderate- and continuous high-intensity exercise in active, untrained, healthy males. J. Sports Sci. 2018, 36, 1993–2001. [Google Scholar] [CrossRef] [Green Version]
- Alexopoulos, T.; Lemonnier, A.; Fiedler, K. Higher order influences on evaluative priming: Processing styles moderate congruity effects. Cogn. Emot. 2017, 31, 57–68. [Google Scholar] [CrossRef]
- Storbeck, J.; Clore, G.L. The affective regulation of cognitive priming. Emotion 2008, 8, 208–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Moderate-Intensity (n = 39) | High-Intensity (n = 40) | t (df) | p | |
|---|---|---|---|---|
| Men/women | 16/23 | 20/20 | t (77) = −0.794 | 0.430 |
| Age | 35.54 (7.78) | 34.65 (7.74) | t (77) = 0.509 | 0.612 |
| Education 1 | 1.38 (1.14) | 1.58 (1.11) | t (77) = −0.754 | 0.453 |
| Depression 2 | 4.77 (3.97) | 4.03 (3.29) | t (77) = 0.908 | 0.367 |
| VO2max 3 | 42.22 (6.53) | 43.80 (7.90) | t (77) = −0.965 | 0.338 |
| Positive Valence (n = 160) | Negative Valence (n = 160) | |
|---|---|---|
| Valence | 7.48 (0.59) | 2.14 (0.61) |
| Arousal | 6.01 (1.10) | 6.32 (1.01) |
| Length (letters) | 7.50 (1.10) | 7.75 (1.01) |
| Length (syllables) | 3.10 (0.69) | 3.17 (0.57) |
| Grammar class (a/n/v) 2 | 38/55/67 | 27/59/74 |
| Trial Run | Positive Valence | Negative Valence | |||
|---|---|---|---|---|---|
| Congruent | Incongruent | Congruent | Incongruent | ||
| Response Latencies | |||||
| Moderate-intensity exercise | 1 | 673.44 (129.70) | 682.59 (130.10) | 753.94 (139.62) | 730.80 (131.57) |
| 2 | 648.25 (118.74) | 665.35 (116.61) | 728.21 (138.76) | 712.85 (128.52) | |
| High-intensity exercise | 1 | 686.25 (122.25) | 677.48 (130.97) | 744.92 (131.38) | 725.53 (122.46) |
| 2 | 668.64 (120.79) | 667.59 (120.67) | 726.95 (120.14) | 709.13 (120.22) | |
| Error rates | |||||
| Moderate-intensity exercise | 1 | 3.0 (0.0–7.0) | 5.0 (3.0–7.0) | 10.0 (5.0–12.0) | 7.0 (5.0–15.0) |
| 2 | 3.0 (3.0–5.0) | 5.0 (3.0–5.0) | 5.0 (3.0–10.0) | 7.0 (5.0–10.0) | |
| High-intensity exercise | 1 | 4.0 (0.0–10.0) | 5.0 (0.7–10.0) | 7.0 (3.5–10.0) | 7.0 (3.0–11.5) |
| 2 | 3.0 (0.0–6.5) | 4.0 (3.0–7.0) | 5.0 (3.0–10.0) | 7.0 (5.0–12.0) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Rojo, C.; Rieker, J.A.; Ballesteros, S. The Effect of Exercise Intensity on Affective and Repetition Priming in Middle-Aged Adults. Int. J. Environ. Res. Public Health 2022, 19, 9873. https://doi.org/10.3390/ijerph19169873
Perez-Rojo C, Rieker JA, Ballesteros S. The Effect of Exercise Intensity on Affective and Repetition Priming in Middle-Aged Adults. International Journal of Environmental Research and Public Health. 2022; 19(16):9873. https://doi.org/10.3390/ijerph19169873
Chicago/Turabian StylePerez-Rojo, Cristina, Jennifer A. Rieker, and Soledad Ballesteros. 2022. "The Effect of Exercise Intensity on Affective and Repetition Priming in Middle-Aged Adults" International Journal of Environmental Research and Public Health 19, no. 16: 9873. https://doi.org/10.3390/ijerph19169873
APA StylePerez-Rojo, C., Rieker, J. A., & Ballesteros, S. (2022). The Effect of Exercise Intensity on Affective and Repetition Priming in Middle-Aged Adults. International Journal of Environmental Research and Public Health, 19(16), 9873. https://doi.org/10.3390/ijerph19169873






