Characterisation of Particles Emitted during Laser Cutting of Various Metal Sheets and an Exposure Assessment for the Laser Operators
Abstract
:1. Introduction
2. Materials and Methods
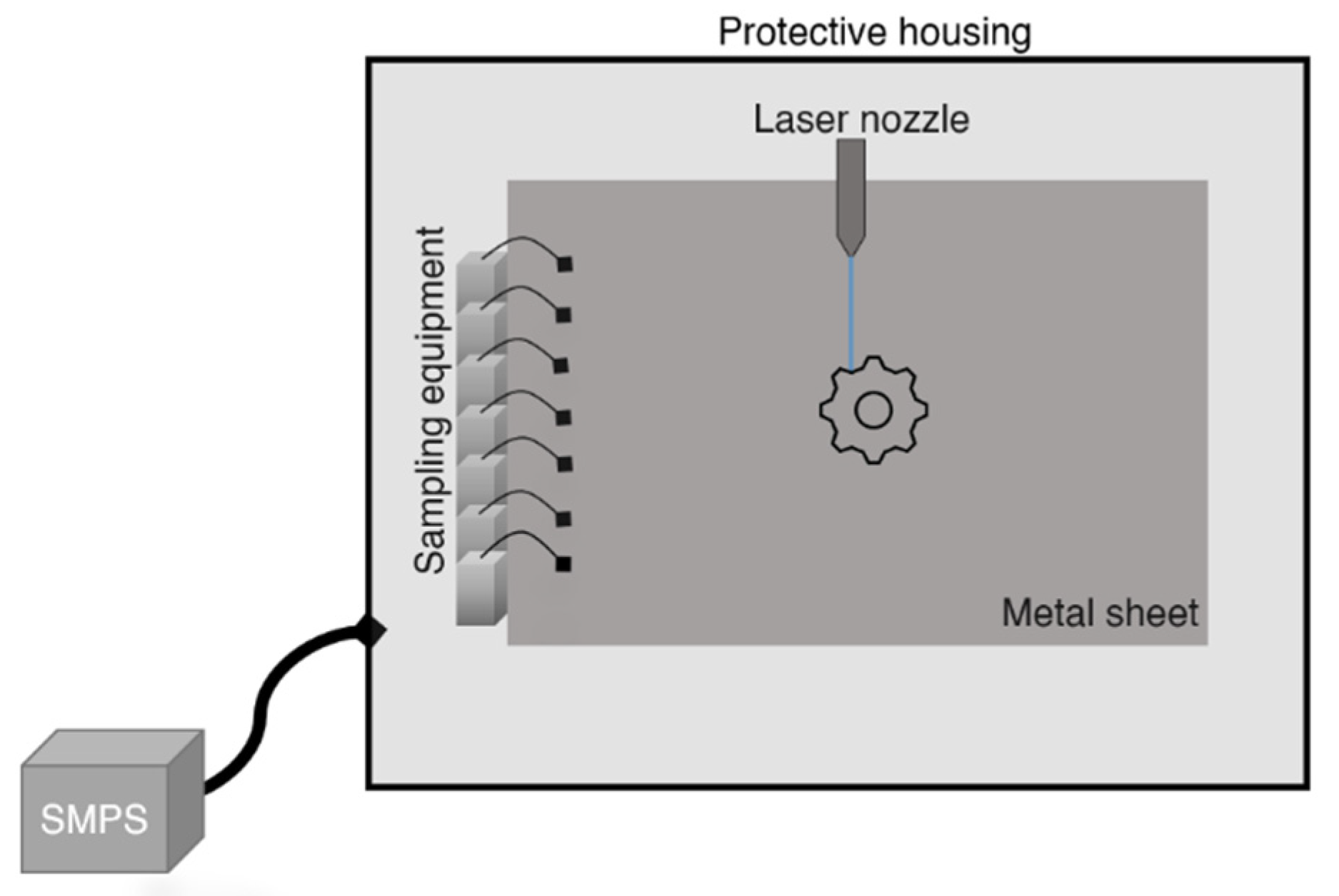
2.1. Stationary Sampling
2.2. Personal Sampling
2.3. Scanning Electron Microscopy
2.4. Gravimetric Analysis
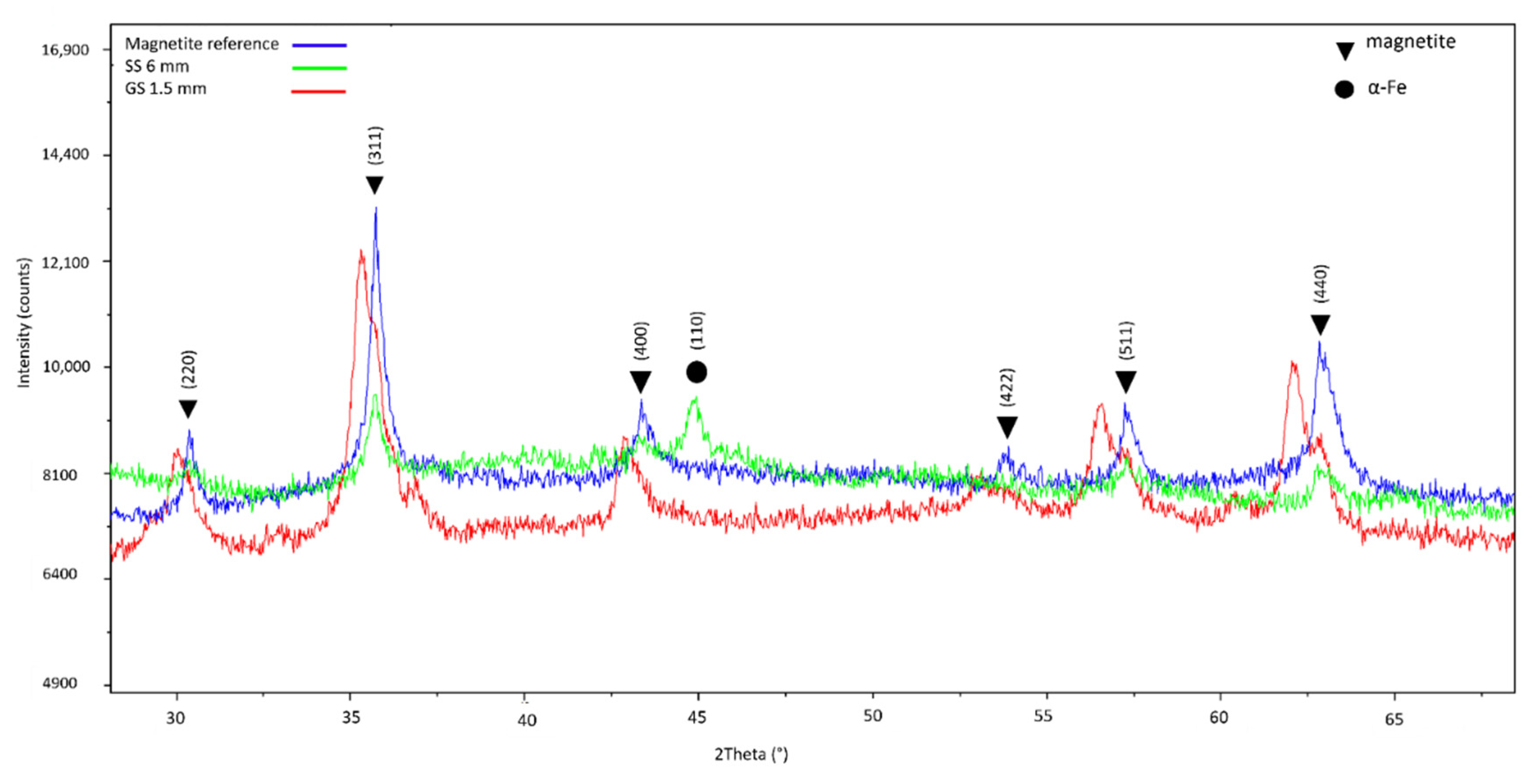
2.5. X-ray Diffraction
2.6. Inductively Coupled Plasma Mass Spectrometry
2.7. Statistics
3. Results
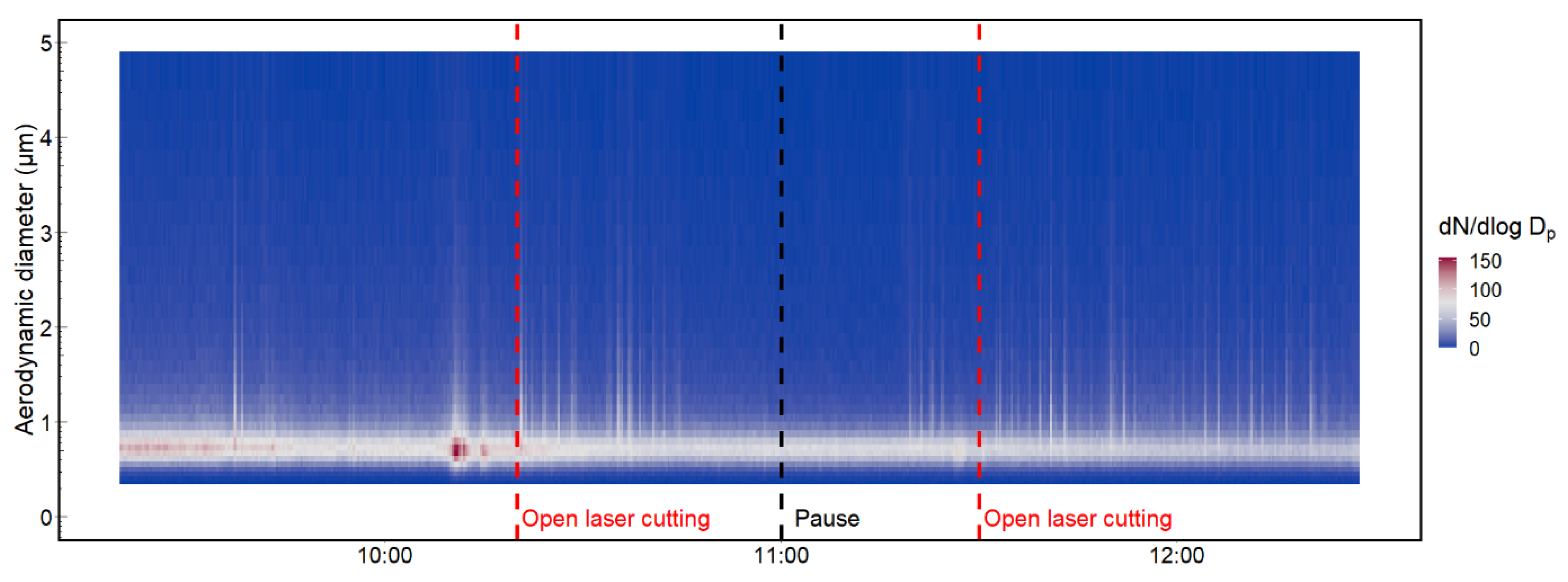
3.1. Particle Number Concentration and Size Distribution

3.2. Particle Morphology
3.3. Chemical Composition of Condensation Particles
3.4. Exposure Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, Y.; Xie, H.; Ge, Y.; Lin, Y.; Yao, Z.; Wang, B.; Jin, M.; Liu, J.; Chen, X.; Sun, Y. Laser Cutting Technologies and Corresponding Pollution Control Strategy. Processes 2022, 10, 732. [Google Scholar] [CrossRef]
- Sharma, A.; Yadava, V. Experimental analysis of Nd-YAG laser cutting of sheet materials—A review. Opt. Laser Technol. 2018, 98, 264–280. [Google Scholar] [CrossRef]
- Ivarson, A. On the Physics and Chemical Thermodynamics of Laser Cutting. Ph.D. Thesis, Luleå Tekniska Universitet, Luleå, Sweden, 1993. [Google Scholar]
- Riveiro, A.; Quintero, F.; Lusquiños, F.; Comesaña, R.; del Val, J.; Pou, J. The Role of the Assist Gas Nature in Laser Cutting of Aluminum Alloys. Phys. Procedia 2011, 12, 548–554. [Google Scholar] [CrossRef] [Green Version]
- Berlinger, B.; Benker, N.; Weinbruch, S.; L’Vov, B.; Ebert, M.; Koch, W.; Ellingsen, D.G.; Thomassen, Y. Physicochemical characterisation of different welding aerosols. Anal. Bioanal. Chem. 2011, 399, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Graff, P.; Ståhlbom, B.; Nordenberg, E.; Graichen, A.; Johansson, P.; Karlsson, H. Evaluating Measuring Techniques for Occupational Exposure during Additive Manufacturing of Metals: A Pilot Study. J. Ind. Ecol. 2017, 21, S120–S129. [Google Scholar] [CrossRef] [Green Version]
- Noskov, A.; Ervik, T.K.; Tsivilskiy, I.; Gilmutdinov, A.; Thomassen, Y. Characterization of ultrafine particles emitted during laser-based additive manufacturing of metal parts. Sci. Rep. 2020, 10, 20989. [Google Scholar] [CrossRef]
- Sutton, A.T.; Kriewall, C.S.; Karnati, S.; Leu, M.C.; Newkirk, J.W. Characterization of AISI 304L stainless steel powder recycled in the laser powder-bed fusion process. Addit. Manuf. 2020, 32, 100981. [Google Scholar] [CrossRef]
- Azzougagh, M.N.; Keller, F.-X.; Cabrol, E.; Cici, M.; Pourchez, J. Occupational exposure during metal additive manufacturing: A case study of laser powder bed fusion of aluminum alloy. J. Occup. Environ. Hyg. 2021, 18, 223–236. [Google Scholar] [CrossRef]
- Ervik, T.K.; Benker, N.; Weinbruch, S.; Thomassen, Y.; Ellingsen, D.G.; Berlinger, B. Size distribution and single particle characterization of airborne particulate matter collected in a silicon carbide plant. Environ. Sci. Processes Impacts 2019, 21, 564–574. [Google Scholar] [CrossRef]
- Hammer, S.E.; Ervik, T.K.; Ellingsen, D.G.; Thomassen, Y.; Weinbruch, S.; Benker, N.; Berlinger, B. Particle characterisation and bioaccessibility of manganese in particulate matter in silico-and ferromanganese smelters. Environ. Sci. Processes Impacts 2021, 23, 1488–1499. [Google Scholar] [CrossRef]
- Chan, Y.-J.; Yuan, T.-H.; Sun, H.-C.; Lin, T.-C. Characterization and Exposure Assessment of Odor Emissions from Laser Cutting of Plastics in the Optical Film Industry. Aerosol. Air Qual. Res. 2016, 16, 2216–2226. [Google Scholar] [CrossRef] [Green Version]
- Noskov, A.; Thomassen, Y.; Berlinger, B.; Olsen, R.; Ervik, T.K.; Weinbruch, S.; Gilmutdinov, A. Characterization of air contaminants emitted during laser cutting of carbon fiber-reinforced composite materials. Anal. Bioanal. Chem. 2019, 411, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Elihn, K.; Berg, P. Ultrafine Particle Characteristics in Seven Industrial Plants. Ann. Occup. Hyg. 2009, 53, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Pena, A.C.; Soler, J.G.; Caicedo, G.R. The Characterization of Aerosols Generated During the Cutting of Metallic Materials with Lasers. Environ. Technol. 1998, 19, 83–90. [Google Scholar] [CrossRef]
- Zubko, M.; Loskot, J.; Świec, P.; Prusik, K.; Janikowski, Z. Analysis of Stainless Steel Waste Products Generated during Laser Cutting in Nitrogen Atmosphere. Metals 2020, 10, 1572. [Google Scholar] [CrossRef]
- Bau, S.; Rousset, D.; Payet, R.; Keller, F.-X. Characterizing particle emissions from a direct energy deposition additive manufacturing process and associated occupational exposure to airborne particles. J. Occup. Environ. Hyg. 2020, 17, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Ljunggren, S.A.; Karlsson, H.; Ståhlbom, B.; Krapi, B.; Fornander, L.; Karlsson, L.E.; Bergström, B.; Nordenberg, E.; Ervik, T.K.; Graff, P. Biomonitoring of Metal Exposure During Additive Manufacturing (3D Printing). Saf. Health Work 2019, 10, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Ljunggren, S.A.; Ward, L.J.; Graff, P.; Persson, A.; Lind, M.L.; Karlsson, H. Metal additive manufacturing and possible clinical markers for the monitoring of exposure-related health effects. PLoS ONE 2021, 16, e0248601. [Google Scholar] [CrossRef]
- Ervik, T.K.; Benker, N.; Weinbruch, S.; Skogstad, A.; Thomassen, Y.; Ellingsen, D.G.; Berlinger, B. Phase identification of individual crystalline particles by combining EDX and EBSD: Application to workplace aerosols. Anal. Bioanal. Chem. 2018, 410, 2711–2721. [Google Scholar] [CrossRef] [PubMed]
- R Studio Team. R Studio: Integrated Development for R. Version 4.1.2; R Studio Team: Vienna, Austria, 2015. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, Version 3.3.2; CRAN; Springer: New York, NY, USA, 2016. [Google Scholar]
- Kassambara, A. ‘ggplot2’ Based Publication Ready Plots. Version 0.4.0.999. CRAN. 2020. Available online: https://rpkgs.datanovia.com/ggpubr/ (accessed on 7 August 2022).
- Xiao, N. ggsci: Scientific Journal and Sci-Fi Themed Color Palettes for ‘ggplot2’. Version 2.9, CRAN. 2018. Available online: https://nanx.me/ggsci/ (accessed on 7 August 2022).
- Halvorsen, J.Ø. Exposure Assessment and Particle Characterization of Workplace Aerosols in Norwegian Metal Laser-Cutting Industry. Master’s Thesis, Norwegian University of Life Sciences, Ås, Norway, 2021. [Google Scholar]
- Eggersdorfer, M.L.; Pratsinis, S.E. Agglomerates and aggregates of nanoparticles made in the gas phase. Adv. Powder Technol. 2014, 25, 71–90. [Google Scholar] [CrossRef]
- Stelzer, S.; Mahrle, A.; Wetzig, A.; Beyer, E. Experimental Investigations on Fusion Cutting Stainless Steel with Fiber and CO2 Laser Beams. Phys. Procedia 2013, 41, 399–404. [Google Scholar] [CrossRef]
- Lehtinen, K.E.J.; Windeler, R.S.; Friedlander, S.K. Prediction of nanoparticle size and the onset of dendrite formation using the method of characteristic times. J. Aerosol Sci. 1996, 27, 883–896. [Google Scholar] [CrossRef]
- Sanibondi, P. Numerical investigation of the effects of iron oxidation reactions on the fume formation mechanism in arc welding. J. Phys. D 2015, 48, 345202. [Google Scholar] [CrossRef]
- Wang, C.; Baer, D.R.; Amonette, J.E.; Engelhard, M.H.; Antony, J.; Qiang, Y. Morphology and Electronic Structure of the Oxide Shell on the Surface of Iron Nanoparticles. J. Am. Chem. Soc. 2009, 131, 8824–8832. [Google Scholar] [CrossRef]
- Hill, R.J. X-ray powder diffraction profile refinement of synthetic hercynite. Am. Mineral. 1984, 69, 937–942. [Google Scholar]
- Mistri, R.; Maiti, S.; Llorca, J.; Dominguez, M.; Mandal, T.K.; Mohanty, P.; Ray, B.C.; Gayen, A. Copper ion substituted hercynite (Cu0.03Fe0.97Al2O4): A highly active catalyst for liquid phase oxidation of cyclohexane. Appl. Catal. A Gen. 2014, 485, 40–50. [Google Scholar] [CrossRef]
- Simonelli, M.; Tuck, C.; Aboulkhair, N.T.; Maskery, I.; Ashcroft, I.; Wildman, R.D.; Hague, R. A Study on the Laser Spatter and the Oxidation Reactions During Selective Laser Melting of 316L Stainless Steel, Al-Si10-Mg, and Ti-6Al-4V. Metall. Mater. Trans. A 2015, 46, 3842–3851. [Google Scholar] [CrossRef]
- Braakhuis, H.M.; Park, M.V.D.Z.; Gosens, I.; De Jong, W.H.; Cassee, F.R. Physicochemical characteristics of nanomaterials that affect pulmonary inflammation. Part. Fibre Toxicol. 2014, 11, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartley, D.L.; Vincent, J.H. Sampling Conventions for Estimating Ultrafine and Fine Aerosol Particle Deposition in the Human Respiratory Tract. Ann. Occup. Hyg. 2011, 55, 696–709. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Gu, J.-M.; Huang, Y.; Duan, Y.-Y.; Huang, R.-X.; Hu, J.-A. Dose-Response Relationship between Cumulative Occupational Lead Exposure and the Associated Health Damages: A 20-Year Cohort Study of a Smelter in China. Int. J. Environ. Res. 2016, 13, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Material | Cutting Characteristics | Collection | ||||
|---|---|---|---|---|---|---|
| Type | Thickness (mm) | Sheet Type | Additional Information | Laser Type | Time (min) | Site |
| Aluminium (Al) | 0.5 | 5754 H34 | CO2 | 4 | B | |
| Aluminium a (Al) | 10 | EN AW 1050A | CO2 | 187 | C | |
| Black steel a (BKS) | 2.3 | GGB DU | PTFE and lead coating | CO2 | 100 | C |
| Black steel (BKS) | 3.2 | Hardox 450 | Hot rolled steel | Fibre | 54 | A |
| Galvanized steel (GS) | 1.5 | DX51D+Z275 | CO2 | 229 | B | |
| Mild steel (MS) | 2 | DC01AM | Cold-rolled steel covered with carbon-based oil | CO2 | 12 | B |
| Mild steel (MS) | 3 | S355MC | CO2 | 78 | B | |
| Stainless steel (SS) | 3 | AiSi 316 | CO2 | 9 | B | |
| Stainless steel (SS) | 5 | AiSi 304 | Fibre | 91 | A | |
| Stainless steel (SS) | 6 | AiSi 304 | Fibre | 200 | A | |
| Stainless steel (SS) | 15 | AiSi 316 | Fibre | 42 | A | |
| Titanium (Ti) | 4 | CO2 | 4 | B | ||
| Element | Recovery (%) |
|---|---|
| MSWF-1 Mild steel welding fume (n = 3) | |
| Fe | 99 ± 5.3 |
| Mn | 101 ± 5.7 |
| Zn | 101 ± 4.6 |
| GS 1.5 mm | MS 3 mm | BKS 3.2 mm | SS 5 mm | SS 6 mm | Al 10 mm | SS15 mm | |
|---|---|---|---|---|---|---|---|
| Particulate matter a | 1.5 [1.2, 1.9] | 0.1 [0.09, 0.13] | 1.0 [0.7, 1.9] | 0.8 [0.5, 1.2] | 0.4 * [0.4, 0.5] | 0.3 [0.2, 0.3] | 0.4 ** [0.3, 0.4] |
| Al | 2.4 | 2.1 | 5.5 | 5.7 | 5.8 | 40 | 4.2 |
| Cr | 0.3 | 0.04 | 1.9 | 1.2 | 0.5 | 0.3 | 0.5 |
| Cu | 1.2 | 0.05 | 4.3 | 0.6 | 0.3 | 20 | 0.4 |
| Fe a | 0.7 | 0.05 | 0.3 | 0.4 | 0.2 | 0.04 | 0.1 |
| Mn | 3.3 | 1.1 | 9.9 | 5.6 | 2.6 | 1.0 | 2.1 |
| Ni | 0.2 | 0.2 | 1.7 | 0.7 | 0.4 | 0.2 | 0.5 |
| Pb | 0.008 | 0.002 | 0.1 | 0.03 | 0.02 | 11 | 0.03 |
| Zn | <LOD | <LOD | <LOD | <LOD | <LOD | 4.2 | <LOD |
| Mineral phase | Magnetite b,c | Magnetite c | Magnetite b,c and α-Fe b | Magnetite b,c and α-Fe b | Magnetite b,c and α-Fe b | Hercynite c | Magnetite c |
| Median | Minimum | Maximum | Norwegian OEL (2022) | |
|---|---|---|---|---|
| Particulate matter | 52 | 29 | 115 | 5000 a |
| Fe | 5 | 2 | 14 | 3000 b |
| Al | 2 | 1 | 2 | 5000 c |
| Zn | 1 | 0.3 | 4 | 5000 d |
| Ti | 0.1 | 0.01 | 2 | 5000 e |
| Mn | 0.08 | 0.01 | 0.3 | 50 a |
| Cr | 0.07 | <LOD | 0.08 | 500 b |
| Cu | 0.03 | 0.02 | 24 | 100 c |
| Pb | 0.01 | 0.002 | 12 | 50 c |
| Sn | 0.002 | <LOD | 4 | 2000 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eriksen Hammer, S.; Halvorsen, J.Ø.; Graff, P.; Ervik, T.K. Characterisation of Particles Emitted during Laser Cutting of Various Metal Sheets and an Exposure Assessment for the Laser Operators. Int. J. Environ. Res. Public Health 2022, 19, 9888. https://doi.org/10.3390/ijerph19169888
Eriksen Hammer S, Halvorsen JØ, Graff P, Ervik TK. Characterisation of Particles Emitted during Laser Cutting of Various Metal Sheets and an Exposure Assessment for the Laser Operators. International Journal of Environmental Research and Public Health. 2022; 19(16):9888. https://doi.org/10.3390/ijerph19169888
Chicago/Turabian StyleEriksen Hammer, Stine, Johanne Østereng Halvorsen, Pål Graff, and Torunn Kringlen Ervik. 2022. "Characterisation of Particles Emitted during Laser Cutting of Various Metal Sheets and an Exposure Assessment for the Laser Operators" International Journal of Environmental Research and Public Health 19, no. 16: 9888. https://doi.org/10.3390/ijerph19169888





