Plague and Trace Metals in Natural Systems
Abstract
:1. Introduction
2. Survival of Plague between Epidemics and Epizootics
3. Plague Pathogens in Soil and Sapronoses
4. Host–Microbe Interactions and Chemical Elements
5. Investigation of Association between Microelements in Natural Environment and Plague Activity in Rodent Reservoirs
5.1. Research Methodology Applied by Evgeny Rotshild and Colleagues
5.2. Plague in Ground Squirrels and Trace Metals in Their Putative Plant Diets
5.3. Plague in Jirds and Trace Metals in Their Putative Plant Diets
5.4. Plague in Great Gerbils and Trace Metals in Their Putative Plant Diets
5.5. Plague in Pikas and Trace Metals in Their Putative Plant Diets
5.6. Plague in Marmots and Trace Metals in Their Putative Plant Diets
5.7. Overview of Russian Studies on Plague and Trace Metals
6. Other Infections
7. Discussion: Trace Metals and Risk of Zoonotic Diseases
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McLean, D.M. Diseases in Nature Communicable to Man. Can. Med. Assoc. J. 1961, 84, 995–999. [Google Scholar]
- Weiss, G. Impact of Metals on Immune Response and Tolerance, and Modulation of Metal Metabolism during Infection. In Trace Metals and Infectious Diseases; The MIT Press: Cambridge, MA, USA, 2015; pp. 147–159. [Google Scholar]
- Yersin, A. La peste bubonique a Hong Kong. Ann. L’ Inst. Pasteur 1894, 8, 662–667. [Google Scholar]
- Zabolotny, D.K. La Peste en Mongolie Orientale. Ann. L’ Inst. Pasteur 1899, 13, 833–840. [Google Scholar]
- Bacot, A.W.; Martin, C.J. Observations on the mechanism of the transmission of plague by fleas. J. Hyg. 1914, 13 (Suppl. III), 423–439. [Google Scholar] [PubMed]
- Gage, K.L.; Kosoy, M.Y. Natural history of plague: Perspectives from more than a century of research. Annu. Rev. Entomol. 2005, 50, 505–528. [Google Scholar] [CrossRef] [PubMed]
- Bertherat, E.; Bekhoucha, S.; Chougrani, S.; Razik, F.; Duchemin, J.B.; Houti, L.; Deharib, L.; Fayolle, C.; Makrerougrass, B.; Dali-Yahia, R.; et al. Plague Reappearance in Algeria after 50 years, 2003. Emerg. Infect. Dis. 2007, 13, 1459–1462. [Google Scholar] [CrossRef] [PubMed]
- Eisen, R.J.; Dennis, D.T.; Gage, K.L. The Role of Early-Phase Transmission in the Spread of Yersinia pestis. J. Med. Entomol. 2015, 52, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Baltazard, M. The Conservation of Plague in Invertebrate Foci. J. Hyg. Epidemiol. Microbiol. Immunol. 1964, 8, 9–21. [Google Scholar]
- Soldatkin, I.S.; Rudenchik, Y.V. Epizootic Process in Natural Foci of Plague (Revision of the Conception). In Ecology of Sapronoses; Litvin, V.Y., Ed.; Medicine Press: Moscow, Russia, 1988; pp. 117–131. [Google Scholar]
- Drancourt, M.; Houhamdi, L.; Raoult, D. Yersinia pestis as a Telluric, Human Ectoparasite-Borne Organism. Lancet Infect. Dis. 2006, 6, 234–241. [Google Scholar] [CrossRef]
- Bazanova, L.P.; Maevskiy, M.P.; Khabarov, A.V. Experimental Study of Possibility for Preservation of Causative Agent of Plague in Nest Substrate of Long-Tailed Suslik. Meditsinskaya Parazitol. [Med. Parasitol.] 1997, 4, 37–39. [Google Scholar]
- Ayyadurai, S.; Houhamdi, L.; Lepidi, H.; Nappez, C.; Raoult, D.; Drancourt, M. Long-Term Persistence of Virulent Yersinia pestis in soil. Microbiology 2008, 154, 2865–2871. [Google Scholar] [CrossRef] [PubMed]
- Reports on Plague Investigations in India by the Advisory Committee Appointed by the Secretary of State for India, the Royal Society, and the Lister Institute. J. Hyg. 1906, 6, 421–536.
- Eisen, R.J.; Petersen, J.M.; Higgins, C.L.; Wong, D.; Levy, C.E.; Mead, P.S.; Schriefer, M.E.; Griffith, K.S.; Gage, K.L.; Beard, C.B. Persistence of Yersinia pestis in Soil under Natural Conditions. Emerg. Infect. Dis. 2008, 14, 941–943. [Google Scholar] [CrossRef]
- Boegler, K.A.; Graham, C.B.; Montenieri, J.A.; MacMillan, K.; Holmes, J.L.; Petersen, J.M.; Gage, K.L.; Eisen, R.J. Evaluation of the infectiousness to mice of soil contaminated with Yersinia pestis-infected blood. Vector-Borne Zoonotic Dis. 2012, 12, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Domaradskiĭ, I.V. Is not Plague a Sapronosis? (The Role of Protozoa in the Epizootiology of Plague). Meditsinskaya Parazitol. [Med. Parasitol.] 1999, 2, 10–13. [Google Scholar]
- Nikul’shin, S.V.; Onatskaia, T.G.; Lukanina, L.M.; Bondarenko, A.L. Associations of Soil amoeba Hartmannella rhysodes with Bacterial Causative Agent of Plague and Y. pseudotuberculosis in Experiment. Zhurnal Mikrobiologii, Epidemiologii i Immunobiologii [J. Microbiol. Epidemiol. Immunobiol.] 1992, 9, 2–5. [Google Scholar]
- Benavides-Montaño, J.A.; Vadyvaloo, V. Yersinia pestis Resists Predation by Acanthamoeba castellanii and Exhibits Prolonged Intracellular Survival. Appl. Environ. Microbiol. 2017, 83, e00593-17. [Google Scholar] [CrossRef]
- Markman, D.W.; Antolin, M.F.; Bowen, R.A.; Wheat, W.H.; Woods; Gonzalez-Juarrero, M.; Jackson, M. Yersinia pestis Survival and Replication in Potential Ameba Reservoir. Emerg. Infect. Dis. 2018, 24, 294–302. [Google Scholar] [CrossRef]
- Terskikh, V.I. Diseases of Humans and Animals Caused by Microbes Able to Reproduce in Abiotic Environment That Represents Their Living Habitat. Zhurnal of microbiologii, Epidemiologii I Immunobiologii [J. Microbiol. Epidemiol. Immunobiol.] 1958, 8, 118–122. [Google Scholar]
- Hubalek, Z. Emerging Human Infectious Diseases: Anthroponoses, Zoonoses, and Sapronoses. Emerg. Infect. Dis. 2003, 9, 403–404. [Google Scholar] [CrossRef]
- Kuris, A.M.; Lafferty, K.D.; Sokolow, S.H. Sapronosis: A Distinctive Type of Infectious Agent. Trends Parasitol. 2014, 30, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Pushkareva, V.I. Bacterial Pathogens: Migration from Environmental Reservoirs to Human Host. Biol. Bull. Rev. 2020, 10, 150–157. [Google Scholar] [CrossRef]
- Nieder, R.; Benbi, D.K.; Reichl, F.X. Soil Components and Human Health; Springer: Dordrecht, The Netherlands, 2018; 919p. [Google Scholar]
- Weinberg, E.D. The Influence of Soil on Infectious Disease. Experientia 1987, 43, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Rail, C.D. Roles of Metallic Ions within an Area Endemic for the Plague Bacillus (Yersinia pestis). Med. Hypotheses 1980, 6, 105–112. [Google Scholar] [CrossRef]
- Nriagu, J.O.; Skaar, E.P. Trace Metals and Infectious Diseases; The MIT Press: Cambridge, MA, USA, 2015; p. 488. [Google Scholar]
- Ackland, M.L.; Bornhorst, J.; Dedoussis, G.V.; Dietert, R.; Nriagu, J.O.; Pacuna, J.M.; Pettifor, J.M. Metals in the Environment as Risk Factors for Infectious Diseases: Gaps and Opportunities. In Trace Metals and Infectious Disease; Nriagu, J.O., Skaar, E.P., Eds.; MIT Press: Cambridge, MA, USA, 2015; pp. 271–307. [Google Scholar]
- Rotshild, E.V. Spatial Structure of Plague Natural Focus and Methods of Its Investigation; Moscow University Press: Moscow, Russia, 1978; 192p. [Google Scholar]
- Rotshild, E.V.; Zhulidov, A.V. Changes in Microelement Composition of Plants as a Factor of Plague Epizootics among Gerbils. Bull. Mosc. Soc. Nat. Biol. Ser. 2000, 105, 10–20. [Google Scholar]
- Bates, R.L.; Jackson, J.A. Dictionary of Geological Terms, 3rd ed.; Anchor Academic Publishing: Hamburg, Germany, 1984; p. 576. [Google Scholar]
- Mason, B. Principles of Geochemistry, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1958; p. 310. [Google Scholar]
- Danilenko, I.D.; Kurolap, S.A.; Rotshild, E.V. Significance of Some Natural Factors for Indication of Plague Foci in the North-Eastern Caspian Region. News Mosc. State Univ. Geogr. Ser. 1983, 5, 47–52. [Google Scholar]
- Zhulidov, A.V.; Rotshild, E.V.; Dzyadevich, G.S. The Features of Microelement Composition of Plants within Local Plague Epizootics. Bull. Mosc. Univ. Ser. 1981, 5, 157–161. [Google Scholar]
- Rotshild, E.V. Infections in Nature: Dangerous Ailment through Eyes of a Naturalist; ABF Publisher: Moscow, Russia, 2012; p. 288. [Google Scholar]
- Rotshild, E.V.; Korobova, E.M.; Galatsevich, P.N.; Pil’nikov, A.E.; Fedorov, Y.N. Association Between Plague Epizootics and Anomalies of Microelements in Tuva Mountains. In Proceedings of the 3rd All-Union Conference on Epizootiology, Novosibirsk, Russia; 1991; pp. 397–399. [Google Scholar]
- Sorokin, V.V.; Rotshild, E.V.; Ampleeva, E.A. A Multiyear Dynamic of Plague Focus Structure in the Northeastern Precaspian Basin. Med. Parasitol. Parasit. Dis. 1981, 5, 70–76. [Google Scholar]
- Rotshild, E.V.; Derevschikov, A.G. Ecological and Geochemical Conditions of Manifestation of Plague in Gorno-Altai Republic. Bull. Mosc. Soc. Nat. Biol. Ser. 1994, 99, 15–22. [Google Scholar]
- Rotshild, E.V.; Evdokimov, A.K.; Amgalan, L. Anomalous Content of Microelements in Plants as a Factor of Gazelle Die-off in Mongolia. Bull. Mosc. Soc. Nat. Biol. Ser. 1988, 93, 35–42. [Google Scholar]
- Rotshild, E.V.; Kosoy, M.; Kushnarev, E. Microelement Composition of Plants and Structure of Foci of Viral Zoonoses. Probl. Espec. Danger. Infect. 1993, 73, 72–81. [Google Scholar]
- Rotshild, E.V. Dependence of Infectious Diseases from the Composition of Chemical Elements in Environment and the Periodic Law. Adv. Mod. Biol. 2001, 121, 252–265. [Google Scholar]
- Vernadsky, V. The Chemical Structure of Biosphere and Its Surroundings; Yaroshevsky, A.A., Ed.; Nauka Press: Moscow, Russia, 1987; p. 339. [Google Scholar]
- Vernadsky, V. Geochemistry and the Biosphere; Salisbury, F.B., Ed.; Synergetic Press: Santa Fe, NM, USA, 2007; p. 480. [Google Scholar]
- Nikolaenko, D.; Fiedler, B.A. Infectious Ecology: A New Dimension in Understanding the Phenomenon of Infection. In Three Facets of Public Health and Paths to Improvements: Behavior, Culture, and Environment; Fiedler, B.A., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 417–422. [Google Scholar]
- Nikolaenko, D. New Level of Understanding of Pathogenic Properties of Microorganisms and Infectious Ecology. Environ. Epidemiol. 2018, 12, 62–104. [Google Scholar]
| Plant Family | Samples | Mn | Cu | Zn | Mo | Co | |
|---|---|---|---|---|---|---|---|
| Poaceae | plague | 12 | 50.8–115.4 (85.5) | 0.29–0.54 (0.42) | 2.38–3.89 (3.07) | 0.48–0.82 (0.63) | 2.01–4.12 (3.32) |
| Poaceae | control | 24 | 30.9–45.8 (38.1) | 3.45–7.85 (5.45) | 15.34–24.73 (18.9) | 2.13–3.72 (3.14) | 0.12–0.34 (0.21) |
| Asteraceae | plague | 28 | 60.4–108.7 (84.0) | 0.84–3.05 (1.84) | 3.71–6.85 (4.68) | 0.12–0.52 (0.33) | 1.74–3.21 (2.40) |
| Asteraceae | control | 43 | 35.1–59.8 (46.6) | 4.85–22.12 (14.91) | 20.13–39.75 (28.1) | 1.75–2.95 (2.17) | 0.12–0.71 (0.45) |
| Ranunculaceae | plague | 11 | 77.4–80.9 (79.5) | 1.02–1.28 (1.13) | 3.21–3.72 (3.39) | 0.12–0.24 (0.16) | 0.9–1.95 (1.28) |
| Ranunculaceae | control | 10 | 35.4–37.6 (36.7) | 6.21–15.31 (11.56) | 17.12–26.84 (22.85) | 1.34–1.95 (1.6) | 0.1–0.51 (0.24) |
| Amaranthaceae | plague | 8 | 170.8–197.3 (183.5) | 0.84–0.89 (0.87) | 2.71–3.1 (2.91) | 0.32–0.39 (0.36) | 0.88–0.95 (0.92) |
| Amaranthaceae | control | 11 | 70.2–79.9 (76.3) | 3.71–4.32 (3.96) | 19.0 0–22.34 (20.40) | 1.33–1.54 (1.45) | 0.1–0.15 (0.13) |
| plague vs. control | p-values | 0.037 | 0.042 | 0.001 | 0.011 | 0.046 |
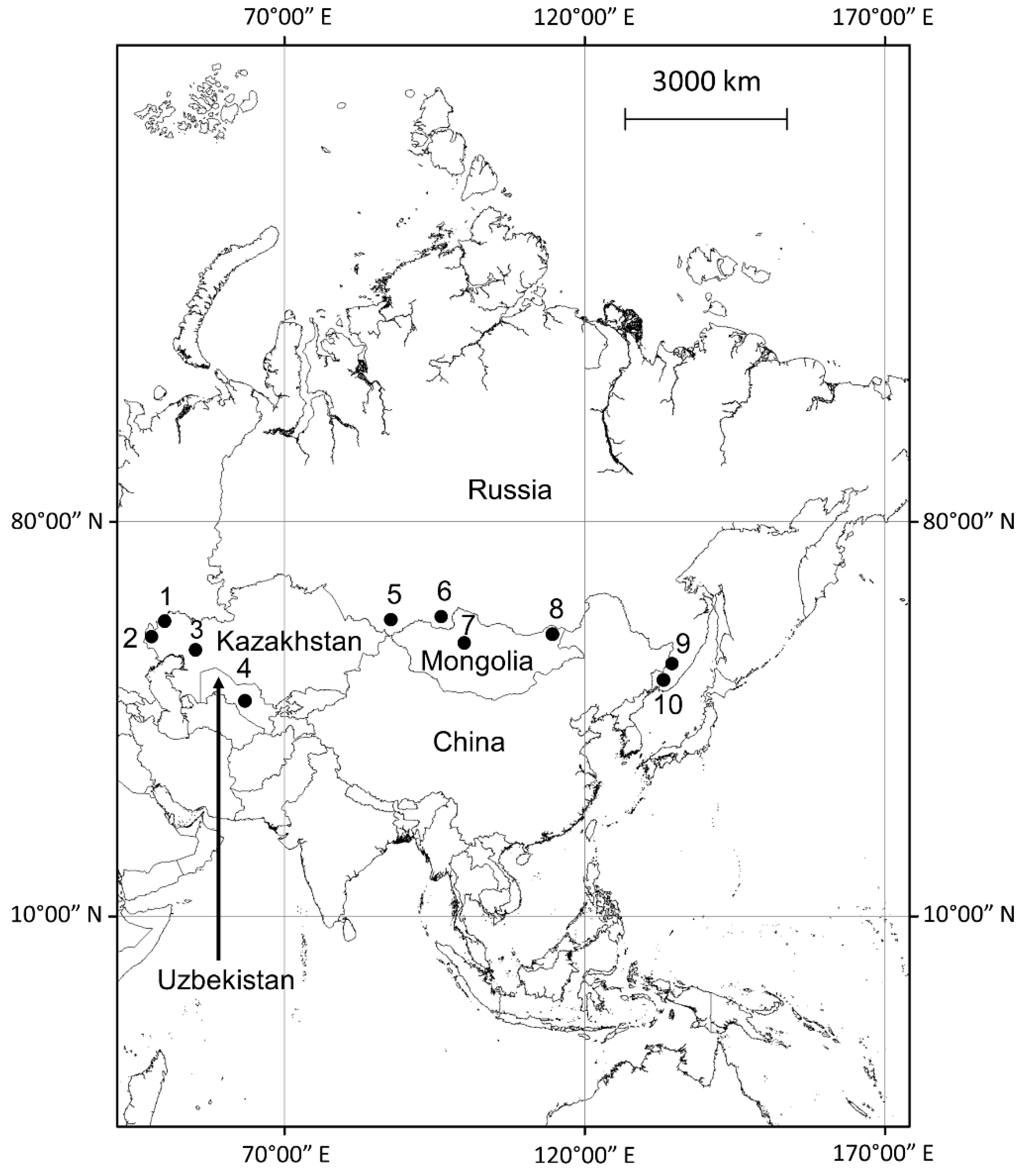
| Main Rodent Host/Region | Year | Plots (n) | Plants (n) | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Mo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spermophilus pygmaeus/Caspian Depression, Kazakhstan | 1979 | 11 | 71 | nd | nd | 2+ | nd | 2+ | nd | 2− | 2− | 2− |
| Spermophilus pygmaeus/Caspian Depression, Kazakhstan | 1980 | 7 | 13 | nd | nd | 2+ | nd | 2+ | nd | 2− | nd | nd |
| Urocitellus undulatus/Tannu-Ola Mtns., Tuva, Siberia | 1988 1989 | 15 | 70 | 1− | 1+ | 0 | 1+ | nd | 2+/2− | 2− | 0 | nd |
| Meriones tamariscinus and M. meridianus/Caspian Depression, Kazakhstan | 1981 | 61 | 61 | + | 1+ | 2− | 2- | 1− | ||||
| Rhombomys opimus/western Kazakhstan | 1980 | 6 | 11 | + | 2+ | 2− | 2- | 1− | ||||
| Rhombomys opimus/Kyzyl-Kum, Uzbekistan | 1980 | 3 | 6 | + | 2+ | 2− | 2- | 1− | ||||
| Ochotona pallasi/Altay Mtns., southern Siberia | 1981 1982 1985 | 13 | 79 | 1− | 0 | 1+ | 1+ | 1+ | 2− | 2− | 0 | nd |
| Marmota sibirica/Khangai Mtns., Mongolia | 1987 | 5 | 14 | 1− | 1− | 0 | 0 | nd | 2− | 2− | 0 | nd |
| p-value | 0.13 | 0.50 | 0.02 | 0.25 | 0.02 | 0.02 | 0.06 | 0.06 |
| Type of Plot | Beetles (n) | Plants (n) | Cobalt in Beetles | Cobalt in Plants | Zinc in Beetles | Zinc in Plants |
|---|---|---|---|---|---|---|
| 1 | 9 | 10 | 1.48 | 1.24 | 3.84 | 0.69 |
| 2 | 7 | 15 | 1.64 | 1.40 | 3.23 | 0.57 |
| 3 | 10 | 15 | 2.28 | 1.04 | 1.98 | 0.89 |
| 4 | 11 | 21 | 2.92 | 1.00 | 1.20 | 1.00 |
| Infection/Main Rodent Host/Region | Year | Plots (n) | Plants (n) | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Mo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pasteurellosis/Procapra gutturosa/western Mongolia | 1983–1984 | 34 | 76 | nd | 2+ | 1+ | 1+ | 2+ | 2+ | 0 | 0 | 2+ |
| TBE virus/Apodemus and Clethrionomys spp./Far-Eastern Russia | 1982 | 12 | 12 | 2+ | 0 | 0 | 1+ | nd | 2− | 1+ | 0 | nd |
| Hantaviruses/Apodemus agrarius/far eastern Russia | 1982 | 19 | 19 | 2+ | 1+ | 2+ | 1+ | nd | 2− | 1− | 0 | nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosoy, M.; Biggins, D. Plague and Trace Metals in Natural Systems. Int. J. Environ. Res. Public Health 2022, 19, 9979. https://doi.org/10.3390/ijerph19169979
Kosoy M, Biggins D. Plague and Trace Metals in Natural Systems. International Journal of Environmental Research and Public Health. 2022; 19(16):9979. https://doi.org/10.3390/ijerph19169979
Chicago/Turabian StyleKosoy, Michael, and Dean Biggins. 2022. "Plague and Trace Metals in Natural Systems" International Journal of Environmental Research and Public Health 19, no. 16: 9979. https://doi.org/10.3390/ijerph19169979
APA StyleKosoy, M., & Biggins, D. (2022). Plague and Trace Metals in Natural Systems. International Journal of Environmental Research and Public Health, 19(16), 9979. https://doi.org/10.3390/ijerph19169979







