Advances in Technologies for Boron Removal from Water: A Comprehensive Review
Abstract
:1. Introduction

| Waters | Concentration (mg∙L−1) | Waters | Concentration (mg∙L−1) |
|---|---|---|---|
| Continental geothermal waters | 1080 | Rivers, Agricultural regions | 0.193–0.387 |
| Waters of active volcanic and geothermal activities | 0.2–72 | Rhine and Meuse rivers, The Netherlands | 0.04–0.20 |
| Rains, Germany, Switzerland | 0.0003–0.007 | Rivers, northern France | 0.10 (<0.01–0.93) |
| Rains, Paris, France | 0.002 | Natural rivers, Liaoning, China | 0.002–0.51 |
| Rains, southern Asia | 0.0003–0.009 | Polluted rivers, Liaoning, China | 0.039–25.1 |
| Snowpack | 0.002 | Groundwater, average | 0.04 |
| Surface fresh | 0.002 | Mediterranean basin | 3–13 |
| River water, average | 0.0003–0.002 | Seawater, average | 4.6 |
2. Boron Overview
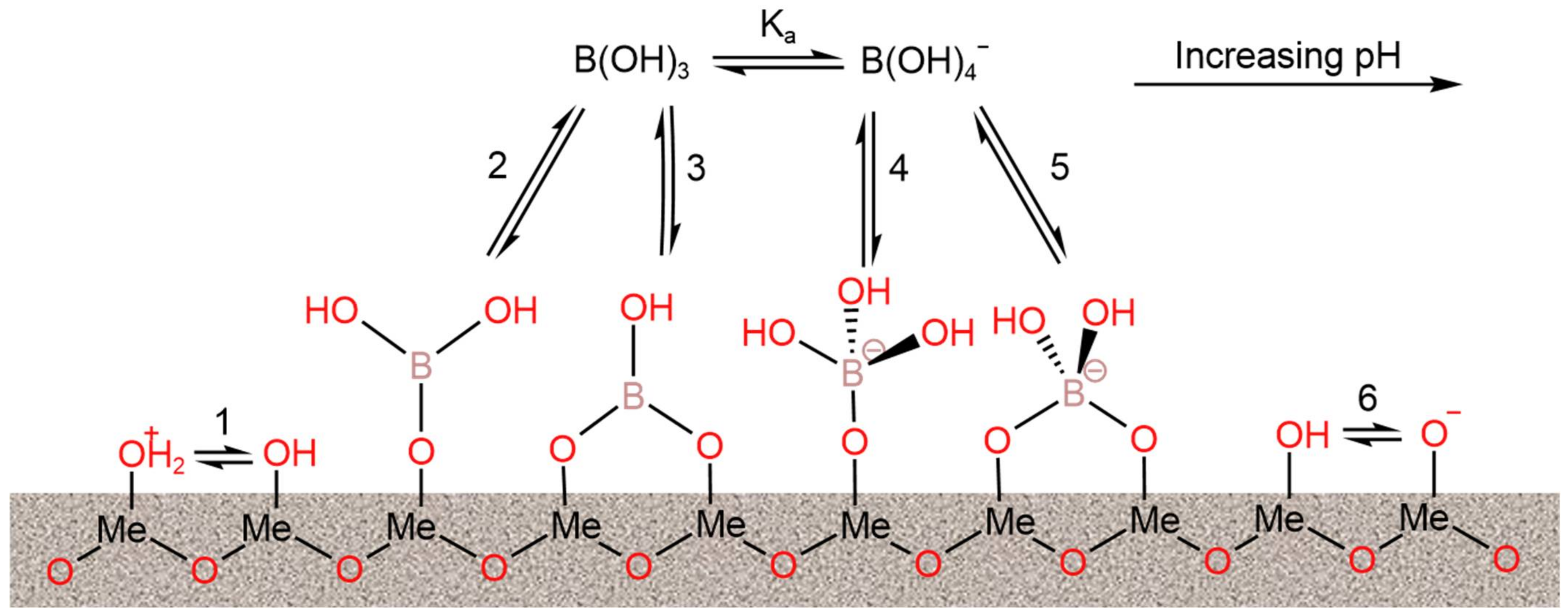
2.1. Reasons for the Difficulties of Boron Removal from Water
2.2. Toxic Effects of Boron
3. Processes for Boron Removal from Water
3.1. Membrane Processes
3.1.1. RO Process
3.1.2. FO Process
3.1.3. ED Process and DD Process
3.1.4. MD Process
3.2. Adsorption Technologies
3.2.1. Carbon-Based Materials
| Adsorbent | Conditions | Equilibrium a/Maximum b Adsorption Capacity | Refs. |
|---|---|---|---|
| F400 | [B]0 = 30 mg·L−1, 24 h, pH = 7, adsorbent dose = 0.04 g·L−1, 20 ± 1 °C | a 0.319 mg·g−1 | [59] |
| WD Extra | [B]0 = 30 mg·L−1, 24 h, pH = 7, adsorbent dose = 0.04 g·L−1, 20 ± 1 °C | a 0.152 mg·g−1 | [59] |
| WG-12 | [B]0 = 30 mg·L−1, 24 h, pH = 7, adsorbent dose = 0.04 g·L−1, 20 ± 1 °C | a 0.144 mg·g−1 | [59] |
| Norit SX2 | [B]0 = 30 mg·L−1, 24 h, pH = 7, adsorbent dose = 0.04 g·L−1, 20 ± 1 °C | a 0.238 mg·g−1 | [59] |
| Norit AZO | [B]0 = 30 mg·L−1, 24 h, pH = 7, adsorbent dose = 0.04 g·L−1, 20 ± 1 °C | a 0.191 mg·g−1 | [59] |
| AquaSorb BP2 | [B]0 = 30 mg·L−1, 24 h, pH = 7, adsorbent dose = 0.04 g·L−1, 20 ± 1 °C | a 0.191 mg·g−1 | [59] |
| CWZ-22 | [B]0 = 30 mg·L−1, 24 h, pH = 7, adsorbent dose = 0.04 g·L−1, 20 ± 1 °C | a 0.193 mg·g−1 | [59] |
| F400 + mannitol | [B]0 = 60 mg·L−1, 4 h, pH = 7, adsorbent dose = 20 g·L−1, 25 °C | a 1.50 mg·g−1 | [59] |
| F400 + xylitol | [B]0 = 60 mg·L−1, 4 h, pH = 7, adsorbent dose = 20 g·L−1, 25 °C | a 1.45 mg·g−1 | [59] |
| F400 + sodium gluconate | [B]0 = 60 mg·L−1, 4 h, pH = 7, adsorbent dose = 20 g·L−1, 25 °C | a 1.04 mg·g−1 | [59] |
| Cur-AC | [B]0 = 1000 mg·L−1,2 h, pH = 5.5, adsorbent dose = 40 g·L−1, 25 °C | b 5.0 mg·g−1 | [60] |
| CWZ-30 | [B]0 = 30 mg·L−1, 2 h, pH = 6, adsorbent dose = 20 g·L−1, 20 °C | a 0.294 mg·g−1 | [61] |
| CWZ-30 + glucose | [B]0 = 30 mg·L−1, 2 h, pH = 6, adsorbent dose = 20 g·L−1, 20 °C | a 0.335 mg·g−1 | [61] |
| CWZ-30 + CaCl2 | [B]0 = 30 mg·L−1, 2 h, pH = 6, adsorbent dose = 20 g·L−1, 20 °C | a 0.568 mg·g−1 | [61] |
| CWZ-30 + citric acid | [B]0 = 30 mg·L−1, 2 h, pH = 6, adsorbent dose = 20 g·L−1, 20 °C | a 0.671 mg·g−1 | [61] |
| CWZ-30 + H3PO4 | [B]0 = 30 mg·L−1, 2 h, pH = 6, adsorbent dose = 20 g·L−1, 20 °C | a 0.384 mg·g−1 | [61] |
| CWZ-30 + tartaric acid | [B]0 = 30 mg·L−1, 2 h, pH = 6, adsorbent dose = 20 g·L−1, 20 °C | a 0.648 mg·g−1 | [61] |
| CWZ-30 + salicylic acid | [B]0 = 30 mg·L−1, 2 h, pH = 6, adsorbent dose = 20 g·L−1, 20 °C | a 0.325 mg·g−1 | [61] |
| N-GO | [B]0 = 5 mg·L−1, 48 h, pH = 8.5, adsorbent dose = 1.6 g·L−1, 25 °C | b 58.7 mg·g−1 | [62] |
| GO/ZIF-67 | pH = 11, 25 °C, adsorbent dose = 1 g·L−1 | b 66.65 mg·g−1 | [63] |
| CNTs | [B]0 = 20 mg·L−1, 24 h, pH = 8.7, adsorbent dose = 4 g·L−1, 25 °C | b 1.28 mg·g−1 | [64] |
| PVA–CNTs | [B]0 = 20 mg·L−1, 24 h, pH = 8.7, adsorbent dose = 4 g·L−1, 25 °C | b 1.19 mg·g−1 | [64] |
3.2.2. Commercial Boron-Specific Resins and Fibers
3.2.3. LDHs Adsorbents
3.2.4. Waste Industrial Materials
3.2.5. Natural Materials
3.2.6. Porous Organic Polymers (POPs)
3.2.7. Metal Oxide-Based Adsorbents
3.2.8. Other Materials
3.3. Chemical Precipitation and (Electric) Coagulation
3.4. Extraction Method
3.5. Capacitive Deionization (CDI) Process and Electrodeionization (EDI) Process
3.6. Integrated Methods
4. Conclusions
- (1)
- The RO process is a suitable technology for seawater desalination along with boron restriction. Nevertheless, the need for multiple RO stages to decrease the boron concentration below recommended standard poses a major restriction and increases the investment costs. Consequently, the combination of the RO process with other processes, such as adsorption or a membrane, such as ED, EDI, or MD, or even the EC process, is recommended.
- (2)
- Adsorption techniques are only efficient for solutions with low boron concentrations and mineral concentrations when the goal is to prevent repeated regeneration operations. To overcome this limitation and improve the applicability of these techniques, developing novel magnetic porous support materials, in which the boron-specific chelating functional groups are embedded, is encouraged. Furthermore, with respect to the novel adsorption materials, assessments of their risks to human health should also receive more attention.
- (3)
- Coagulation, electrocoagulation, and direct chemical precipitation, in essence, involve transforming the dissolved boron into undissolved boron-bearing solids, which immobilizes the boron inside their chemical structure. This class of methods are characterized by the excessive dosage of the chemicals; thus, it is suggested that, when the target is to remove multiple pollutants, including boron, researchers should weigh up the input costs and output benefits.
- (4)
- As regards the integration methods, RO separation combined with coagulation, MF/UF combined with adsorption, and complexing membrane filtration (CMF) are considered to be economically, ecologically profitable, and promising techniques.
- (5)
- Regarding the recycle of the treated boron, the boron immobilized by the adsorbents with a poor capacity for regeneration, or in the flocs from EC and co-precipitation, are expected to be difficult to recycle. More effort should be put into developing technologies with a high boron selectivity and high boron recyclability.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bilgin Simsek, E.; Beker, U.; Senkal, B.F. Predicting the dynamics and performance of selective polymeric resins in a fixed bed system for boron removal. Desalination 2014, 349, 39–50. [Google Scholar] [CrossRef]
- Najid, N.; Kouzbour, S.; Ruiz-García, A.; Fellaou, S.; Gourich, B.; Stiriba, Y. Comparison analysis of different technologies for the removal of boron from seawater: A review. J. Environ. Chem. Eng. 2021, 9, 105133. [Google Scholar] [CrossRef]
- Tagliabue, M.; Reverberi, A.P.; Bagatin, R. Boron removal from water: Needs, challenges and perspectives. J. Cleaner Prod. 2014, 77, 56–64. [Google Scholar] [CrossRef]
- Dydo, P.; Turek, M. Boron transport and removal using ion-exchange membranes: A critical review. Desalination 2013, 310, 2–8. [Google Scholar] [CrossRef]
- Guan, Z.M.; Lv, J.F.; Bai, P.; Guo, X.H. Boron removal from aqueous solutions by adsorption—A review. Desalination 2016, 383, 29–37. [Google Scholar] [CrossRef]
- Güler, E.; Kaya, C.; Kabay, N.; Arda, M. Boron removal from seawater: State-of-the-art review. Desalination 2015, 356, 85–93. [Google Scholar] [CrossRef]
- Hilal, N.; Kim, G.J.; Somerfield, C. Boron removal from saline water: A comprehensive review. Desalination 2011, 273, 23–35. [Google Scholar] [CrossRef]
- Nasef, M.M.; Nallappan, M.; Ujang, Z. Polymer-based chelating adsorbents for the selective removal of boron from water and wastewater: A review. React. Funct. Polym. 2014, 85, 54–68. [Google Scholar] [CrossRef]
- Tang, Y.P.; Luo, L.; Thong, Z.; Chung, T.S. Recent advances in membrane materials and technologies for boron removal. J. Membr. Sci. 2017, 541, 434–446. [Google Scholar] [CrossRef]
- Bhagyaraj, S.; Al-Ghouti, M.A.; Kasak, P.; Krupa, I. An updated review on boron removal from water through adsorption processes. Emergent Mater. 2021, 4, 1167–1186. [Google Scholar] [CrossRef]
- Kot, F.S. Boron sources, speciation and its potential impact on health. Rev. Environ. Sci. Biotechnol. 2009, 8, 3–28. [Google Scholar] [CrossRef]
- González, P.; Sixto, A.; Knochen, M. Multi-pumping flow system for the determination of boron in eye drops, drinking water and ocean water. Talanta 2017, 166, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Wang, S.H.; Song, X.X.; Zhou, Y.; Shen, H.M.; Cao, X.Z.; Zhang, P.; Gao, C.J. High boron removal polyamide reverse osmosis membranes by swelling induced embedding of a sulfonyl molecular plug. J. Membr. Sci. 2020, 597, 117716. [Google Scholar] [CrossRef]
- Park, J.K.; Lee, K.J. Diffusion coefficients for aqueous boric acid. J. Chem. Eng. Data 1994, 39, 891–894. [Google Scholar] [CrossRef]
- Shultz, S.; Bass, M.; Semiat, R.; Freger, V. Modification of polyamide membranes by hydrophobic molecular plugs for improved boron rejection. J. Membr. Sci. 2018, 546, 165–172. [Google Scholar] [CrossRef]
- Wolska, J.; Bryjak, M. Methods for boron removal from aqueous solutions—A review. Desalination 2013, 310, 18–24. [Google Scholar] [CrossRef]
- Power, P.P.; Woods, W.G. The chemistry of boron and its speciation in plants. Plant Soil 1997, 193, 1–13. [Google Scholar] [CrossRef]
- Nielsen, F.H. Update on human health effects of boron. J. Trace Elem. Med. Biol. 2014, 28, 383–387. [Google Scholar] [CrossRef]
- Shireen, F.; Nawaz, M.A.; Chen, C.; Zhang, Q.K.; Zheng, Z.H.; Sohail, H.; Sun, J.Y.; Cao, H.S.; Huang, Y.; Bie, Z.L. Boron: Functions and approaches to enhance its availability in plants for sustainable agriculture. Int. J. Mol. Sci. 2018, 19, 1856. [Google Scholar] [CrossRef]
- Shorrocks, V.M. The occurrence and correction of boron deficiency. Plant Soil 1997, 193, 121–148. [Google Scholar] [CrossRef]
- Sun, L.Y.; Lu, C.H.; Ni, Y.R.; Xu, Z.Z. Research and development of leadless low-melting glasses. Mater. Rep. 2007, 21, 331–334. [Google Scholar]
- Christogerou, A.; Kavas, T.; Pontikes, Y.; Rathossi, C.; Angelopoulos, G.N. Evolution of microstructure, mineralogy and properties during firing of clay-based ceramics with borates. Ceram. Int. 2010, 36, 567–575. [Google Scholar] [CrossRef]
- Kurama, S.; Kara, A.; Kurama, H. Investigation of borax waste behaviour in tile production. J. Eur. Ceram. Soc. 2007, 27, 1715–1720. [Google Scholar] [CrossRef]
- Bermingham, M.J.; Kent, D.; Zhan, H.; StJohn, D.H.; Dargusch, M.S. Controlling the microstructure and properties of wire arc additive manufactured Ti-6Al-4V with trace boron additions. Acta Mater. 2015, 91, 289–303. [Google Scholar] [CrossRef]
- An, J.; Xue, X.X. Study on ecological development strategies of boron industry in China. Ecol. Econ. 2014, 30, 88–90. [Google Scholar] [CrossRef]
- Fang, C.S.; Qu, H.; Geng, Z.; Li, X.C.; Shi, H.C.; Wang, J. Removal efficiency of boron in waste water from simulation nuclear power plant with reverse osmosis membrane. J. Jilin Univ. Sci. Ed. 2018, 56, 743–746. [Google Scholar] [CrossRef]
- Boubakri, A.; Bouguecha, S.A.-T.; Dhaouadi, I.; Hafiane, A. Effect of operating parameters on boron removal from seawater using membrane distillation process. Desalination 2015, 373, 86–93. [Google Scholar] [CrossRef]
- Khaliq, H.; Zhong, J.M.; Peng, K.-M. The physiological role of boron on health. Biol. Trace Elem. Res. 2018, 186, 31–51. [Google Scholar] [CrossRef]
- Korkmaz, M. Encyclopedia of Environmental Health, 2nd ed.; Elsevier: Oxford, UK, 2011; pp. 456–459. [Google Scholar]
- Hu, J.J. Studies on Development of the Trace Boron Adsorbents and the Mechanism for the Removal of Nature Water. Master’s Thesis, Donghua University, Shanghai, China, 2014. [Google Scholar]
- Lin, J.Y.; Mahasti, N.N.N.; Huang, Y.H. Recent advances in adsorption and coagulation for boron removal from wastewater: A comprehensive review. J. Hazard. Mater. 2021, 407, 124401. [Google Scholar] [CrossRef]
- Fujioka, T.; Oshima, N.; Suzuki, R.; Price, W.E.; Nghiem, L.D. Probing the internal structure of reverse osmosis membranes by positron annihilation spectroscopy: Gaining more insight into the transport of water and small solutes. J. Membr. Sci. 2015, 486, 106–118. [Google Scholar] [CrossRef]
- Rodríguez Pastor, M.; Ferrándiz Ruiz, A.; Chillón, M.F.; Prats Rico, D. Influence of pH in the elimination of boron by means of reverse osmosis. Desalination 2001, 140, 145–152. [Google Scholar] [CrossRef]
- Bernstein, R.; Belfer, S.; Freger, V. Toward improved boron removal in RO by membrane modification: Feasibility and challenges. Environ. Sci. Technol. 2011, 45, 3613–3620. [Google Scholar] [CrossRef] [PubMed]
- Sagiv, A.; Semiat, R. Analysis of parameters affecting boron permeation through reverse osmosis membranes. J. Membr. Sci. 2004, 243, 79–87. [Google Scholar] [CrossRef]
- Tu, K.L.; Nghiem, L.D.; Chivas, A.R. Boron removal by reverse osmosis membranes in seawater desalination applications. Sep. Purif. Technol. 2010, 75, 87–101. [Google Scholar] [CrossRef]
- Liu, L.F.; Xie, X.; Qi, S.R.; Li, R.H.; Zhang, X.; Song, X.X.; Gao, C.J. Thin film nanocomposite reverse osmosis membrane incorporated with UiO-66 nanoparticles for enhanced boron removal. J. Membr. Sci. 2019, 580, 101–109. [Google Scholar] [CrossRef]
- Shultz, S.; Freger, V. In situ modification of membrane elements for improved boron rejection in RO desalination. Desalination 2018, 431, 66–72. [Google Scholar] [CrossRef]
- Wang, S.H.; Zhou, Y.; Gao, C.J. Novel high boron removal polyamide reverse osmosis membranes. J. Membr. Sci. 2018, 554, 244–252. [Google Scholar] [CrossRef]
- Hu, J.H.; Pu, Y.L.; Ueda, M.; Zhang, X.; Wang, L.J. Charge-aggregate induced (CAI) reverse osmosis membrane for seawater desalination and boron removal. J. Membr. Sci. 2016, 520, 1–7. [Google Scholar] [CrossRef]
- Tu, K.L.; Chivas, A.R.; Nghiem, L.D. Enhanced boron rejection by NF/RO membranes by complexation with polyols: Measurement and mechanisms. Desalination 2013, 310, 115–121. [Google Scholar] [CrossRef]
- Dydo, P.; Turek, M.; Milewski, A. Removal of boric acid, monoborate and boron complexes with polyols by reverse osmosis membranes. Desalination 2014, 334, 39–45. [Google Scholar] [CrossRef]
- Darwish, N.B.; Alkhudhiri, A.; AlAlawi, A.; AlRomaih, H.; Hilal, N. Experimental investigation of forward osmosis process for boron removal from water. J. Water Process. Eng. 2020, 38, 101570. [Google Scholar] [CrossRef]
- Wang, Y.N.; Li, W.Y.; Wang, R.; Tang, C.Y. Enhancing boron rejection in FO using alkaline draw solutions. Water Res. 2017, 118, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Fam, W.; Phuntsho, S.; Lee, J.H.; Cho, J.; Shon, H.K. Boron transport through polyamide-based thin film composite forward osmosis membranes. Desalination 2014, 340, 11–17. [Google Scholar] [CrossRef]
- Sun, M.J.; Li, M.; Zhang, X.; Wu, C.M.; Wu, Y.H. Graphene oxide modified porous P84 co-polyimide membranes for boron recovery by bipolar membrane electrodialysis process. Sep. Purif. Technol. 2020, 232, 115963. [Google Scholar] [CrossRef]
- Dydo, P. The effect of process parameters on boric acid transport during the electrodialytic desalination of aqueous solutions containing selected salts. Desalination 2013, 310, 43–49. [Google Scholar] [CrossRef]
- Noguchi, M.; Nakamura, Y.; Shoji, T.; Iizuka, A.; Yamasaki, A. Simultaneous removal and recovery of boron from waste water by multi-step bipolar membrane electrodialysis. J. Water Process. Eng. 2018, 23, 299–305. [Google Scholar] [CrossRef]
- Ayyildiz, H.F.; Kara, H. Boron removal by ion exchange membranes. Desalination 2005, 180, 99–108. [Google Scholar] [CrossRef]
- Trifi, I.M.; Chaabane, L.; Dammak, L.; Baklouti, L.; Hamrouni, B. Response surface methodology for boron removal by Donnan dialysis: Doehlert experimental design. Membranes 2021, 11, 731. [Google Scholar] [CrossRef]
- Ping, Q.; Abu-Reesh, I.M.; He, Z. Boron removal from saline water by a microbial desalination cell integrated with donnan dialysis. Desalination 2015, 376, 55–61. [Google Scholar] [CrossRef]
- Hou, D.Y.; Wang, J.; Sun, X.C.; Luan, Z.K.; Zhao, C.W.; Ren, X.J. Boron removal from aqueous solution by direct contact membrane distillation. J. Hazard. Mater. 2010, 177, 613–619. [Google Scholar] [CrossRef]
- Hou, D.Y.; Dai, G.H.; Wang, J.; Fan, H.; Luan, Z.K.; Fu, C.C. Boron removal and desalination from seawater by PVDF flat-sheet membrane through direct contact membrane distillation. Desalination 2013, 326, 115–124. [Google Scholar] [CrossRef]
- Wen, X.; Li, F.Z.; Zhao, X. Removal of nuclides and boron from highly saline radioactive wastewater by direct contact membrane distillation. Desalination 2016, 394, 101–107. [Google Scholar] [CrossRef]
- Luo, Q.L.; He, L.; Wang, X.Y.; Huang, H.; Wang, X.F.; Sang, S.H.; Huang, X.L. Cyclodextrin derivatives used for the separation of boron and the removal of organic pollutants. Sci. Total Environ. 2020, 749, 141487. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, A.; Takahashi, M.; Nakamura, T.; Yamasaki, A. Boron removal performance of a solid sorbent derived from waste concrete. Ind. Eng. Chem. Res. 2014, 53, 4046–4051. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Salih, N.R. Application of eggshell wastes for boron remediation from water. J. Mol. Liq. 2018, 256, 599–610. [Google Scholar] [CrossRef]
- Kluczka, J.; Gnus, M.; Kazek-Kęsik, A.; Dudek, G. Zirconium-chitosan hydrogel beads for removal of boron from aqueous solutions. Polymer 2018, 150, 109–118. [Google Scholar] [CrossRef]
- Kluczka, J.; Pudło, W.; Krukiewicz, K. Boron adsorption removal by commercial and modified activated carbons. Chem. Eng. Res. Des. 2019, 147, 30–42. [Google Scholar] [CrossRef]
- Halim, A.A.; Roslan, N.A.; Yaacub, N.S.; Latif, M.T. Boron removal from aqueous solution using curcumin-impregnated activated carbon. Sains Malays. 2013, 42, 1293–1300. [Google Scholar] [CrossRef]
- Kluczka, J.; Ciba, J.; Trojanowska, J.; Zolotajkin, M.; Turek, M.; Dydo, P. Removal of boron dissolved in water. Environ. Prog. 2007, 26, 71–77. [Google Scholar] [CrossRef]
- Chen, F.; Guo, L.; Zhang, X.; Leong, Z.Y.; Yang, S.; Yang, H.Y. Nitrogen-doped graphene oxide for effectively removing boron ions from seawater. Nanoscale 2017, 9, 326–333. [Google Scholar] [CrossRef]
- Hu, G.Z.; Zhang, W.; Chen, Y.T.; Xu, C.; Liu, R.; Han, Z. Removal of boron from water by GO/ZIF-67 hybrid material adsorption. Environ. Sci. Pollut. Res. Int. 2020, 27, 28396–28407. [Google Scholar] [CrossRef] [PubMed]
- Ismanto, A.E.; Liu, J.C. Enhanced boron adsorption using PVA-modified carbonaceous materials. Compos. Interfaces 2014, 21, 639–650. [Google Scholar] [CrossRef]
- Jung, S.; Kim, M.-J. Optimal conditions for recovering boron from seawater using boron selective resins. Korean J. Chem. Eng. 2016, 33, 2411–2417. [Google Scholar] [CrossRef]
- Hou, R.X.; Gu, P.; Wei, X.Z.; Zhang, G.H. Study on the removal of boron from water. Ind. Water Treat. 2012, 32, 14–18. [Google Scholar] [CrossRef]
- Recepoğlu, Y.K.; Kabay, N.; Ipek, I.Y.; Arda, M.; Yüksel, M.; Yoshizuka, K.; Nishihama, S. Packed bed column dynamic study for boron removal from geothermal brine by a chelating fiber and breakthrough curve analysis by using mathematical models. Desalination 2018, 437, 1–6. [Google Scholar] [CrossRef]
- Ide, T.; Hirayama, Y. How boron is adsorbed by D-glucamine: A density functional theory study. Comput. Theor. Chem. 2019, 1150, 85–90. [Google Scholar] [CrossRef]
- Ikeda, K.; Umeno, D.; Saito, K.; Koide, F.; Miyata, E.; Sugo, T. Removal of boron using nylon-based chelating fibers. Ind. Eng. Chem. Res. 2011, 50, 5727–5732. [Google Scholar] [CrossRef]
- Ting, T.M.; Nasef, M.M.; Aravindan, D.; Rosslan, I.F.N.; Ruslan, N. Selective removal of boron from industrial wastewater containing high concentration of ammonia by radiation grafted fibrous adsorbent in fixed bed column. J. Environ. Chem. Eng. 2021, 9, 104993. [Google Scholar] [CrossRef]
- Darwish, N.B.; Kochkodan, V.; Hilal, N. Boron removal from water with fractionized Amberlite IRA743 resin. Desalination 2015, 370, 1–6. [Google Scholar] [CrossRef]
- Kamcev, J.; Taylor, M.K.; Shin, D.M.; Jarenwattananon, N.N.; Colwell, K.A.; Long, J.R. Functionalized porous aromatic frameworks as high-performance adsorbents for the rapid removal of boric acid from water. Adv. Mater. 2019, 31, 1808027. [Google Scholar] [CrossRef]
- Şen, F.; Altıok, E.; Cyganowski, P.; Wolska, J.; Bryjak, M.; Kabay, N.; Arda, M.; Yüksel, M. Reclamation of RO permeate and concentrate of geothermal water by new chelating resins having N-methyl-D-glucamine ligands. Sep. Purif. Technol. 2021, 254, 117558. [Google Scholar] [CrossRef]
- Sun, L.; Huang, J.C.; Liu, H.N.; Zhang, Y.J.; Ye, X.S.; Zhang, H.F.; Wu, A.G.; Wu, Z.J. Adsorption of boron by CA@KH-550@EPH@NMDG (CKEN) with biomass carbonaceous aerogels as substrate. J. Hazard. Mater. 2018, 358, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Choi, H.; Hong, S.; Yoon, S.J.; Kim, T.-H.; Lee, J.Y.; Hong, Y.T.; So, S. Surface-initiated ATRP of glycidyl methacrylate in the presence of divinylbenzene on porous polystyrene-based resins for boron adsorption. Desalination 2020, 473, 114166. [Google Scholar] [CrossRef]
- Wang, B.Y.; Lin, H.; Guo, X.H.; Bai, P. Boron removal using chelating resins with pyrocatechol functional groups. Desalination 2014, 347, 138–143. [Google Scholar] [CrossRef]
- Hussain, A.; Sharma, R.; Minier-Matar, J.; Hirani, Z.; Adham, S. Application of emerging ion exchange resin for boron removal from saline groundwater. J. Water Process. Eng. 2019, 32, 100906. [Google Scholar] [CrossRef]
- Nan, X.R.; Liu, J.; Wang, X.L.; Pan, X.H.; Wang, X.M.; Zhang, X. Preparation of superhydrophilic adsorbents with 3DOM structure by water-soluble colloidal crystal templates for boron removal from natural seawater. ACS Appl. Mater. Interfaces 2018, 10, 36918–36925. [Google Scholar] [CrossRef]
- Meng, F.Q.; Ma, W.; Wu, L.; Hao, H.X.; Xin, L.; Chen, Z.; Wang, M.Y. Selective and efficient adsorption of boron (III) from water by 3D porous CQDs/LDHs with oxygen-rich functional groups. J. Taiwan Inst. Chem. Eng. 2018, 83, 192–203. [Google Scholar] [CrossRef]
- Demirçivi, P.; Saygılı, G.N. Comparative study of modified expanded perlite with hexadecyltrimethylammonium-bromide and gallic acid for boron adsorption. J. Mol. Liq. 2018, 254, 383–390. [Google Scholar] [CrossRef]
- Shu, Z.; Guo, Q.H.; Chen, Y.; Zhou, J.; Guo, W.; Cao, Y.W. Accelerated sorption of boron from aqueous solution by few-layer hydrotalcite nanosheets. Appl. Clay Sci. 2017, 149, 13–19. [Google Scholar] [CrossRef]
- Gao, Z.S.; Xie, S.L.; Zhang, B.; Qiu, X.H.; Chen, F.X. Ultrathin Mg-Al layered double hydroxide prepared by ionothermal synthesis in a deep eutectic solvent for highly effective boron removal. Chem. Eng. J. 2017, 319, 108–118. [Google Scholar] [CrossRef]
- Qiu, X.H.; Sasaki, K.; Osseo-Asare, K.; Hirajima, T.; Ideta, K.; Miyawaki, J. Sorption of H3BO3/B(OH)4- on calcined LDHs including different divalent metals. J. Colloid Interface Sci. 2015, 445, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Kluczka, J.; Trojanowska, J.; Zołotajkin, M. Utilization of fly ash zeolite for boron removal from aqueous solution. Desalin. Water Treat. 2014, 54, 1839–1849. [Google Scholar] [CrossRef]
- Babiker, E.; Al-Ghouti, M.A.; Zouari, N.; McKay, G. Removal of boron from water using adsorbents derived from waste tire rubber. J. Environ. Chem. Eng. 2019, 7, 102948. [Google Scholar] [CrossRef]
- Öztürk, N.; Kavak, D. Boron removal from aqueous solutions by adsorption on waste sepiolite and activated waste sepiolite using full factorial design. Adsorption 2004, 10, 245–257. [Google Scholar] [CrossRef]
- Balidakis, A.; Matsi, T. Boron adsorption-desorption by steelmaking slag for boron removal from irrigation waters. Water Air Soil Pollut. 2020, 231, 383. [Google Scholar] [CrossRef]
- Jalali, M.; Rajabi, F.; Ranjbar, F. The removal of boron from aqueous solutions using natural and chemically modified sorbents. Desalin. Water Treat. 2015, 57, 8278–8288. [Google Scholar] [CrossRef]
- Masindi, V.; Gitari, M.W.; Tutu, H.; Debeer, M. Removal of boron from aqueous solution using magnesite and bentonite clay composite. Desalin. Water Treat. 2016, 57, 8754–8764. [Google Scholar] [CrossRef]
- Demircivi, P.; Saygili, G.N. Response surface modeling of boron adsorption from aqueous solution by vermiculite using different adsorption agents: Box-behnken experimental design. Water Sci. Technol. 2017, 76, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Khan, M. Eggshell membrane as a novel bio sorbent for remediation of boron from desalinated water. J. Environ. Manag. 2018, 207, 405–416. [Google Scholar] [CrossRef]
- Lyu, J.F.; Zhang, N.; Liu, H.X.; Zeng, Z.L.Z.; Zhang, J.S.; Bai, P.; Guo, X.H. Adsorptive removal of boron by zeolitic imidazolate framework: Kinetics, isotherms, thermodynamics, mechanism and recycling. Sep. Purif. Technol. 2017, 187, 67–75. [Google Scholar] [CrossRef]
- Zhang, J.L.; Cai, Y.N.; Liu, K.X. Extremely effective boron removal from water by stable metal organic framework ZIF-67. Ind. Eng. Chem. Res. 2019, 58, 4199–4207. [Google Scholar] [CrossRef]
- Lyu, J.F.; Liu, H.X.; Zeng, Z.L.Z.; Zhang, J.S.; Xiao, Z.X.; Bai, P.; Guo, X.H. Metal-organic framework UiO-66 as an efficient adsorbent for boron removal from aqueous solution. Ind. Eng. Chem. Res. 2017, 56, 2565–2572. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, M.Y.; Wang, X. A novel chitosan based adsorbent for boron separation. Sep. Purif. Technol. 2019, 211, 162–169. [Google Scholar] [CrossRef]
- Kameda, T.; Yamamoto, Y.; Kumagai, S.; Yoshioka, T. Mechanism and kinetics of aqueous boron removal using MgO. J. Water Process. Eng. 2018, 26, 237–241. [Google Scholar] [CrossRef]
- Li, P.; Liu, C.; Zhang, L.; Zheng, S.L.; Zhang, Y. Enhanced boron adsorption onto synthesized MgO nanosheets by ultrasonic method. Ultrason. Sonochem. 2017, 34, 938–946. [Google Scholar] [CrossRef]
- Demey, H.; Barron-Zambrano, J.; Mhadhbi, T.; Miloudi, H.; Yang, Z.; Ruiz, M.; Sastre, A.M. Boron removal from aqueous solutions by using a novel alginate-based sorbent: Comparison with Al2O3 particles. Polymers 2019, 11, 1509. [Google Scholar] [CrossRef]
- Kluczka, J.; Gnus, M.; Dudek, G.; Turczyn, R. Removal of boron from aqueous solution by composite chitosan beads. Sep. Sci. Technol. 2017, 52, 1559–1571. [Google Scholar] [CrossRef]
- Tural, S.; Ece, M.S.; Tural, B. Synthesis of novel magnetic nano-sorbent functionalized with N-methyl-D-glucamine by click chemistry and removal of boron with magnetic separation method. Ecotoxicol. Environ. Saf. 2018, 162, 245–252. [Google Scholar] [CrossRef]
- Liao, X.P.; Zhang, Q. Mesoporous polymer nanosponges immobilized with functional polyols for rapid removal of boric acid and organic micropollutants. ACS Appl. Polym. Mater. 2019, 1, 2089–2098. [Google Scholar] [CrossRef]
- Luo, Q.L.; Zeng, M.T.; Wang, X.Y.; Huang, H.; Wang, X.F.; Liu, N.; Huang, X.L. Glycidol-functionalized macroporous polymer for boron removal from aqueous solution. React. Funct. Polym. 2020, 150, 104543. [Google Scholar] [CrossRef]
- Duran, H.; Yavuz, E.; Sismanoglu, T.; Senkal, B.F. Functionalization of gum arabic including glycoprotein and polysaccharides for the removal of boron. Carbohydr. Polym. 2019, 225, 115139. [Google Scholar] [CrossRef] [PubMed]
- Ben Amor, T.; Dhaouadi, I.; Lebeau, B.; Tlili, M.; Ben Amor, M. Synthesis, characterization and application of glucamine-modified mesoporous silica type SBA-15 for the removal of boron from natural water. Desalination 2014, 351, 82–87. [Google Scholar] [CrossRef]
- Padmaja, K.; Cherukuri, J.; Anji Reddy, M. A comparative study of the efficiency of chemical coagulation and electrocoagulation methods in the treatment of pharmaceutical effluent. J. Water Process. Eng. 2020, 34, 101153. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, C.; Du, W.C.; Chen, G. A review on research progress on mechanism and methods of boron-concentration control in water. Environ. Prot. Technol. 2018, 24, 56–64. [Google Scholar] [CrossRef]
- Tang, M.L.; Deng, T.L.; Liao, M.X. Study on the extraction of boric acid from post-salt mother liquor by precipitation. Sea-Lake Salt Chem. Ind. 1994, 23, 17–19. [Google Scholar] [CrossRef]
- Xu, Y.L.; Jiang, J.-Q. Technologies for boron removal. Ind. Eng. Chem. Res. 2008, 47, 16–24. [Google Scholar] [CrossRef]
- Shih, Y.J.; Liu, C.H.; Lan, W.C.; Huang, Y.H. A novel chemical oxo-precipitation (COP) process for efficient remediation of boron wastewater at room temperature. Chemosphere 2014, 111, 232–237. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Shih, Y.-J.; Chen, P.-Y.; Huang, Y.-H. Precipitation recovery of boron from aqueous solution by chemical oxo-precipitation at room temperature. Appl. Energy 2016, 164, 1052–1058. [Google Scholar] [CrossRef]
- Yilmaz, A.E.; Boncukcuoglu, R.; Kocakerim, M.M. A quantitative comparison between electrocoagulation and chemical coagulation for boron removal from boron-containing solution. J. Hazard. Mater. 2007, 149, 475–481. [Google Scholar] [CrossRef]
- Wided, B.; Khawla, M.; Eya, B.K.; Béchir, H. Evaluation of boron removal by coagulation-flocculation and electrocoagulation. Int. J. Eng. Res. Technol. 2014, 3, 2923–2928. [Google Scholar] [CrossRef]
- Bektaş, N.; Öncel, S.; Akbulut, H.Y.; Dimoglo, A. Removal of boron by electrocoagulation. Environ. Chem. Lett. 2004, 2, 51–54. [Google Scholar] [CrossRef]
- Dolati, M.; Aghapour, A.A.; Khorsandi, H.; Karimzade, S. Boron removal from aqueous solutions by electrocoagulation at low concentrations. J. Environ. Chem. Eng. 2017, 5, 5150–5156. [Google Scholar] [CrossRef]
- Chen, M.; Dollar, O.; Shafer-Peltier, K.; Randtke, S.; Waseem, S.; Peltier, E. Boron removal by electrocoagulation: Removal mechanism, adsorption models and factors influencing removal. Water Res. 2020, 170, 115362. [Google Scholar] [CrossRef] [PubMed]
- Güven, E.D.; Güler, E.; Akıncı, G.; Bölükbaş, A. Influencing factors in the removal of high concentrations of boron by electrocoagulation. J. Hazard. Toxic Radioact. Waste 2018, 22, 04017031. [Google Scholar] [CrossRef]
- Kartikaningsih, D.; Huang, Y.H.; Shih, Y.J. Electro-oxidation and characterization of nickel foam electrode for removing boron. Chemosphere 2017, 166, 184–191. [Google Scholar] [CrossRef]
- Widhiastuti, F.; Lin, J.-Y.; Shih, Y.-J.; Huang, Y.-H. Electrocoagulation of boron by electrochemically co-precipitated spinel ferrites. Chem. Eng. J. 2018, 350, 893–901. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Eiband, M.M.S.G.; de Melo, J.V.; Martínez-Huitle, C.A. Electrocoagulation and advanced electrocoagulation processes: A general review about the fundamentals, emerging applications and its association with other technologies. J. Electroanal. Chem. 2017, 801, 267–299. [Google Scholar] [CrossRef]
- da Silva, D.M.L.; Carneiro, M.T.W.D.; Ribeiro, J. Boron removal from mining and synthetic effluents by electrocoagulation using aluminum electrodes. Sci. World J. 2019, 5, 3746964. [Google Scholar] [CrossRef]
- Lin, J.Y.; Raharjo, A.; Hsu, L.H.; Shih, Y.J.; Huang, Y.H. Electrocoagulation of tetrafluoroborate (BF4-) and the derived boron and fluorine using aluminum electrodes. Water Res. 2019, 155, 362–371. [Google Scholar] [CrossRef]
- Zerze, H.; Karagoz, B.; Ozbelge, H.O.; Bicak, N.; Aydogan, N.; Yilmaz, L. Imino-bis-propane diol functional polymer for efficient boron removal from aqueous solutions via continuous PEUF process. Desalination 2013, 310, 158–168. [Google Scholar] [CrossRef]
- Can, B.Z.; Boncukcuoğlu, R.; Bayar, S.; Bayhan, Y.K. Influence of operating parameters on the arsenic and boron removal by electrocoagulation. J. Chem. Soc. Pak. 2016, 38, 843–849. [Google Scholar] [CrossRef]
- Zeboudji, B.; Drouiche, N.; Lounici, H.; Mameri, N.; Ghaffour, N. The Influence of parameters affecting boron removal by electrocoagulation process. Sep. Sci. Technol. 2013, 48, 1280–1288. [Google Scholar] [CrossRef]
- Ezechi, E.H.; Isa, M.H.; Muda, K.; Kutty, S.R.M. A comparative evaluation of two electrode systems on continuous electrocoagulation of boron from produced water and mass transfer resistance. J. Water Process. Eng. 2020, 34, 101133. [Google Scholar] [CrossRef]
- Massara, T.M.; Yilmaz, A.E.; Cengiz, I.; Malamis, S.; Yilmaz, M.T.; Komesli, O.T.; Stanchev, P.; Inglezakis, V.J.; Katsou, E. The effect of initial pH and retention time on boron removal by continuous electrocoagulation process. Desalin. Water Treat. 2018, 112, 99–105. [Google Scholar] [CrossRef]
- Can, B.Z.; Boncukcuoğlu, R.; Yılmaz, A.E.; Fil, B.A. Arsenic and boron removal by electrocoagulation with aluminum electrodes. Arab. J. Sci. Eng. 2015, 41, 2229–2237. [Google Scholar] [CrossRef]
- Li, S. Study on Pretreatment of Seawater and Boron Removal from Seawater. Master’s Thesis, Tianjin University of Science & Technology, Tianjin, China, 2012. [Google Scholar]
- Isa, M.H.; Ezechi, E.H.; Ahmed, Z.; Magram, S.F.; Kutty, S.R. Boron removal by electrocoagulation and recovery. Water Res. 2014, 51, 113–123. [Google Scholar] [CrossRef]
- Ayers, P.; Dudeney, A.W.L.; Kaftraman, F. Solvent extraction of boron with 2-ethyl-1,3-hexanediol and 2-chloro-4-(1,1,3,3-tetramethylbutyl)-6-methylol-phenol. J. Inorg. Nucl. Chem. 1981, 43, 2097–2100. [Google Scholar] [CrossRef]
- Balinski, A.; Recksiek, V.; Kelly, N. Solvent extraction of boric acid: Comparison of five different monohydric alcohols and equilibrium modeling with numerical methods. Processes 2021, 9, 398. [Google Scholar] [CrossRef]
- Fortuny, A.; Coll, M.T.; Kedari, C.S.; Sastre, A.M. Effect of phase modifiers on boron removal by solvent extraction using 1,3 diolic compounds. J. Chem. Technol. Biotechnol. 2014, 89, 858–865. [Google Scholar] [CrossRef]
- Karakaplan, M.; Tural, S.; Tural, B.; Turgut, Y.; Hoşgören, H. The solvent extraction of boron with synthesized aliphatic 1,3-diols: Stripping and extraction behavior of boron by 2,2,5-trimethyl-1,3-hexanediol. Solvent Extr. Ion. Exch. 2004, 22, 897–911. [Google Scholar] [CrossRef]
- Lv, J.H.; Liu, J.D.; Sun, Y.J.; Li, C.L. Kinetics of forward extraction of boric acid from salt lake brine by 2-ethyl-1,3-hexanediol in toluene using single drop technique. Chin. J. Chem. Eng. 2014, 22, 496–502. [Google Scholar] [CrossRef]
- Matsumoto, M.; Kondo, K.; Hirata, M.; Kokubu, S.; Hano, T.; Takada, T. Recovery of boric acid from wastewater by solvent extraction. Sep. Sci. Technol. 1997, 32, 983–991. [Google Scholar] [CrossRef]
- Peng, X.W.; Li, L.J.; Shi, D.; Zhang, L.C.; Li, H.F.; Nie, F.; Song, F.G. Recovery of boric acid from salt lake brines by solvent extraction with 2-butyl-1-n-octanol. Hydrometallurgy 2018, 177, 161–167. [Google Scholar] [CrossRef]
- Yáñez-Fernández, A.; Inestrosa-Izurieta, M.J.; Urzúa, J.I. Concurrent magnesium and boron extraction from natural lithium brine and its optimization by response surface methodology. Desalination 2021, 517, 115269. [Google Scholar] [CrossRef]
- Zhang, R.; Xie, Y.M.; Song, J.F.; Xing, L.X.; Kong, D.F.; Li, X.-M.; He, T. Extraction of boron from salt lake brine using 2-ethylhexanol. Hydrometallurgy 2016, 160, 129–136. [Google Scholar] [CrossRef]
- Biçak, N.; Gazi, M.; Bulutcu, N. N,N-bis(2,3-dihydroxypropyl) octadecylamine for liquid-liquid extraction of boric acid. Sep. Sci. Technol. 2007, 38, 165–177. [Google Scholar] [CrossRef]
- Avraham, E.; Noked, M.; Soffer, A.; Aurbach, D. The feasibility of boron removal from water by capacitive deionization. Electrochim. Acta 2011, 56, 6312–6317. [Google Scholar] [CrossRef]
- Cetinkaya, A.Y. Effect of operating parameters on boron removal using a combined system. Mater. Res. Express 2019, 6, 075509. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, X.; Zhao, X.; Li, F. Removal of high level boron in aqueous solutions using continuous electrodeionization (CEDI). Sep. Purif. Technol. 2018, 192, 297–301. [Google Scholar] [CrossRef]
- Sarıçiçek, E.N.; Tuğaç, M.M.; Özdemir, V.T.; İpek, İ.Y.; Arar, Ö. Removal of boron by boron selective resin-filled electrodeionization. Environ. Technol. Innov. 2021, 23, 101742. [Google Scholar] [CrossRef]
- Arar, Ö.; Yüksel, Ü.; Kabay, N.; Yüksel, M. Application of electrodeionization (EDI) for removal of boron and silica from reverse osmosis (RO) permeate of geothermal water. Desalination 2013, 310, 25–33. [Google Scholar] [CrossRef]
- Darwish, N.B.; Kochkodan, V.; Hilal, N. Microfiltration of micro-sized suspensions of boron-selective resin with PVDF membranes. Desalination 2017, 403, 161–171. [Google Scholar] [CrossRef]
- Alharati, A.; Swesi, Y.; Fiaty, K.; Charcosset, C. Boron removal in water using a hybrid membrane process of ion exchange resin and microfiltration without continuous resin addition. J. Water Process. Eng. 2017, 17, 32–39. [Google Scholar] [CrossRef]
- Neo, J.G.; Japip, S.; Luo, L.; Chung, T.-S.; Weber, M.; Maletzko, C. Hydroxyl-terminated poly(ethyleneimine) polymer enhanced ultrafiltration for boron removal. Sep. Purif. Technol. 2019, 222, 214–220. [Google Scholar] [CrossRef]
- Tortora, F.; Innocenzi, V.; Mazziotti di Celso, G.; Vegliò, F.; Capocelli, M.; Piemonte, V.; Prisciandaro, M. Application of micellar-enhanced ultrafiltration in the pre-treatment of seawater for boron removal. Desalination 2018, 428, 21–28. [Google Scholar] [CrossRef]
- Alharati, A.; Valour, J.-P.; Urbaniak, S.; Swesi, Y.; Fiaty, K.; Charcosset, C. Boron removal from seawater using a hybrid sorption/microfiltration process without continuous addition of resin. Chem. Eng. Process. 2018, 131, 227–233. [Google Scholar] [CrossRef]
- Liu, J. Study on Nanofiltration Seawater Desalination and Its Performance of Boron Removal. Ph.D. Thesis, Tianjin University, Tianjin, China, 2010. [Google Scholar]
- Landsman, M.R.; Lawler, D.F.; Katz, L.E. Application of electrodialysis pretreatment to enhance boron removal and reduce fouling during desalination by nanofiltration/reverse osmosis. Desalination 2020, 491, 114563. [Google Scholar] [CrossRef]
- Ban, S.-H.; Im, S.-J.; Cho, J.; Jang, A. Comparative performance of FO-RO hybrid and two-pass SWRO desalination processes: Boron removal. Desalination 2019, 471, 114114. [Google Scholar] [CrossRef]
- Kayaci, S.; Tantekin-Ersolmaz, S.B.; Ahunbay, M.G.; Krantz, W.B. Technical and economic feasibility of the concurrent desalination and boron removal (CDBR) process. Desalination 2020, 486, 114474. [Google Scholar] [CrossRef]
- Wu, S.B.; Pan, X.H.; Chu, X.Z. Research advance of boron removal in reverse osmosis desalination. J. Environ. Health 2008, 25, 928–930. [Google Scholar] [CrossRef]
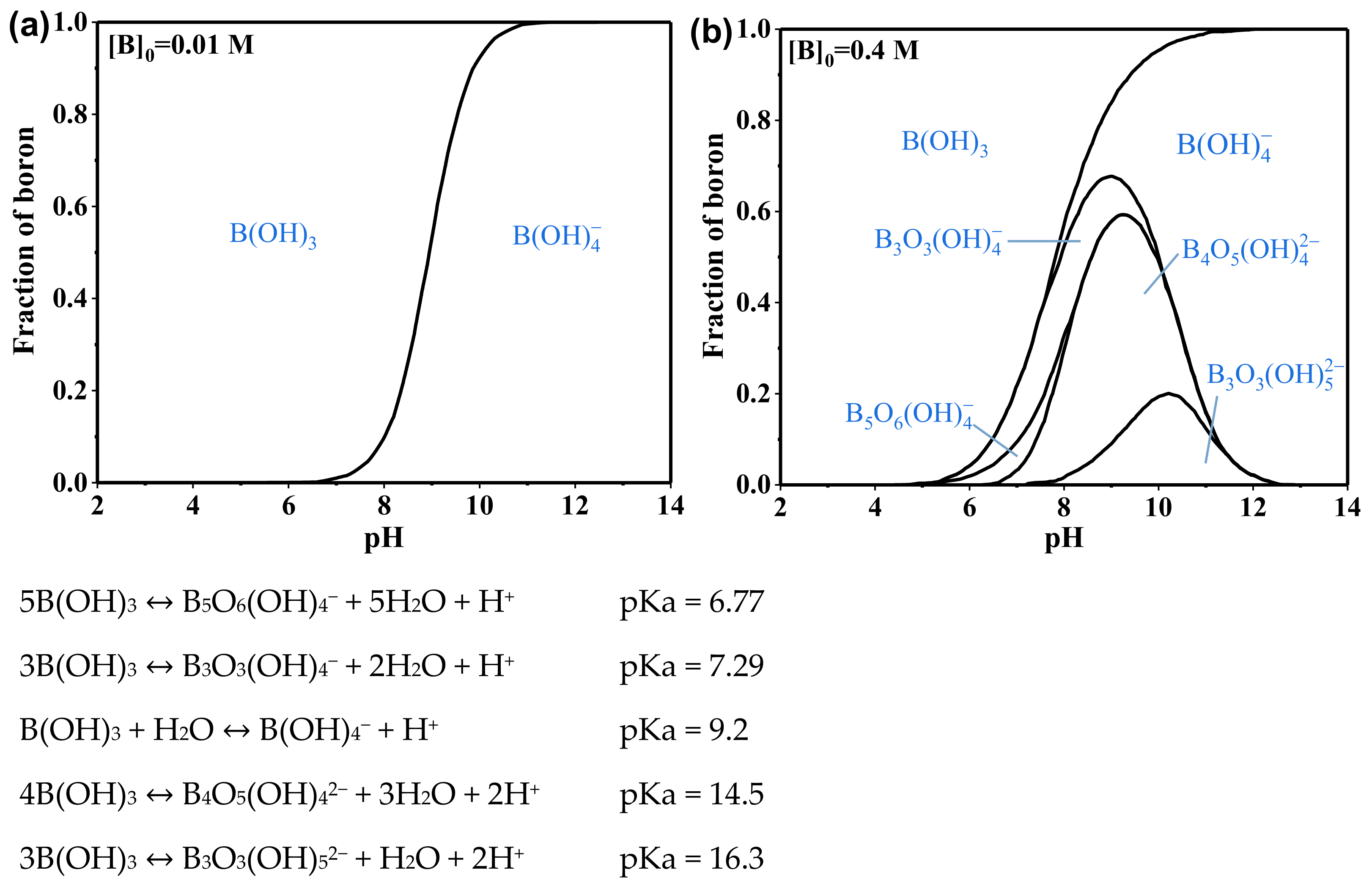

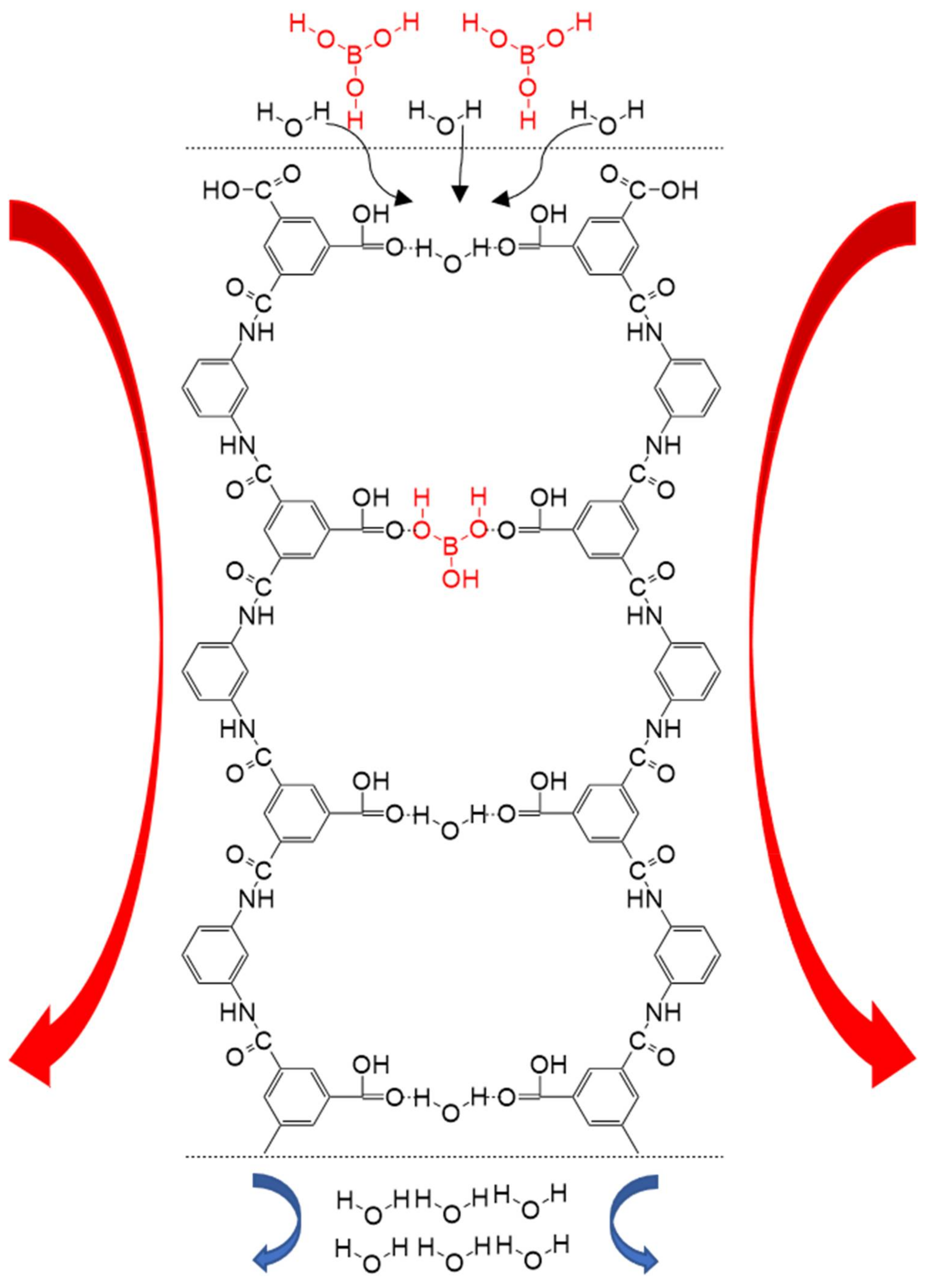

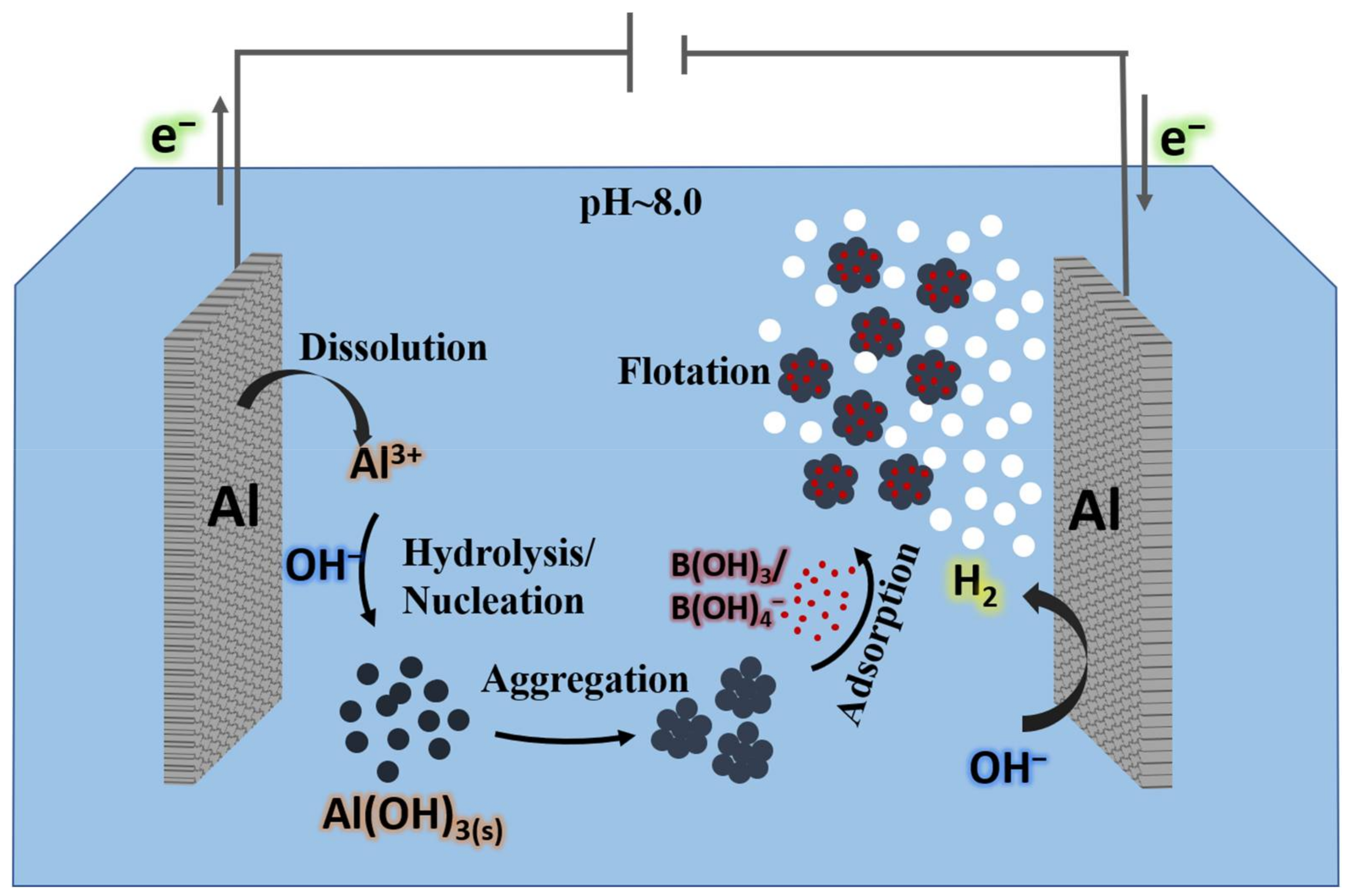
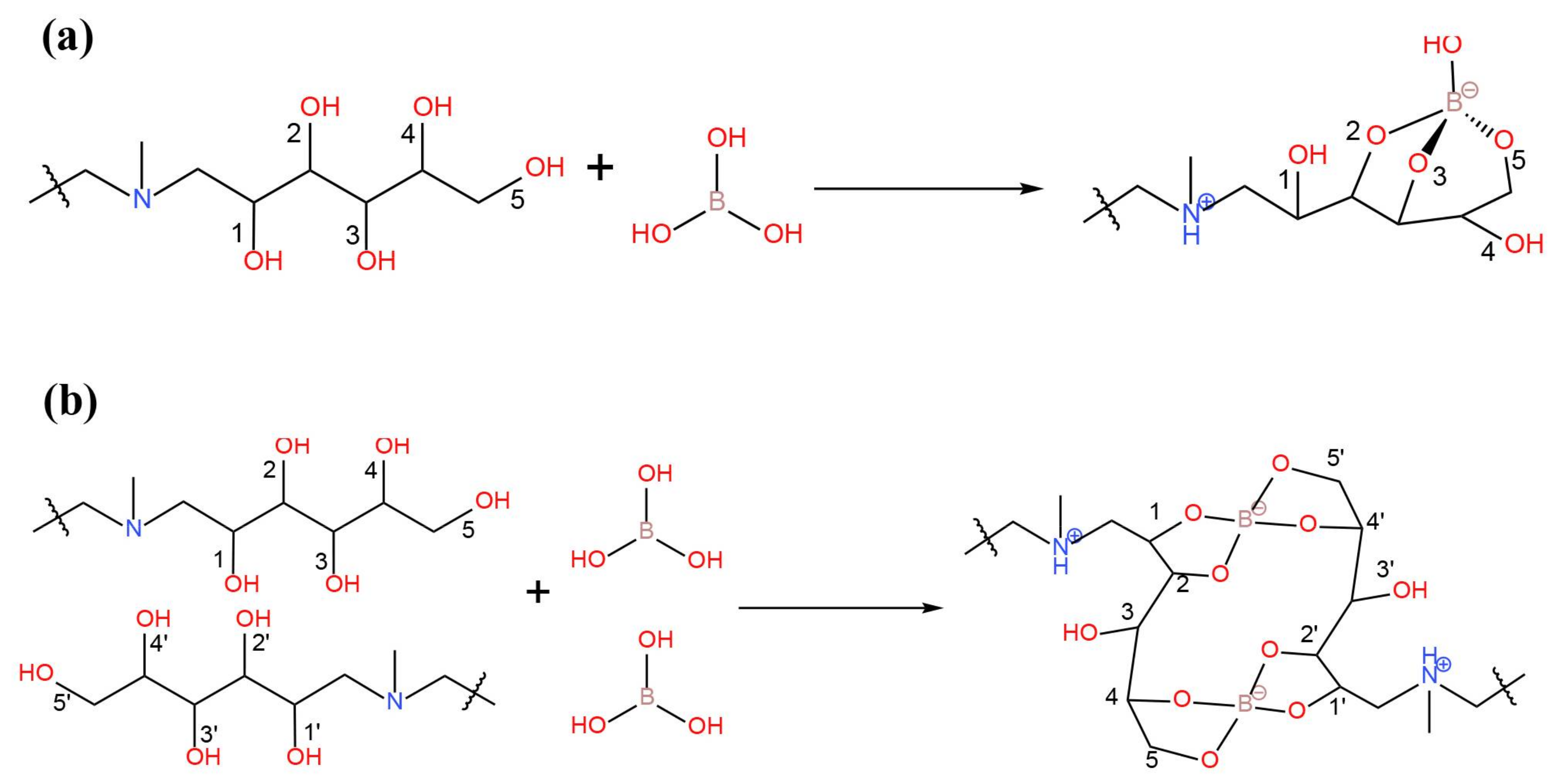

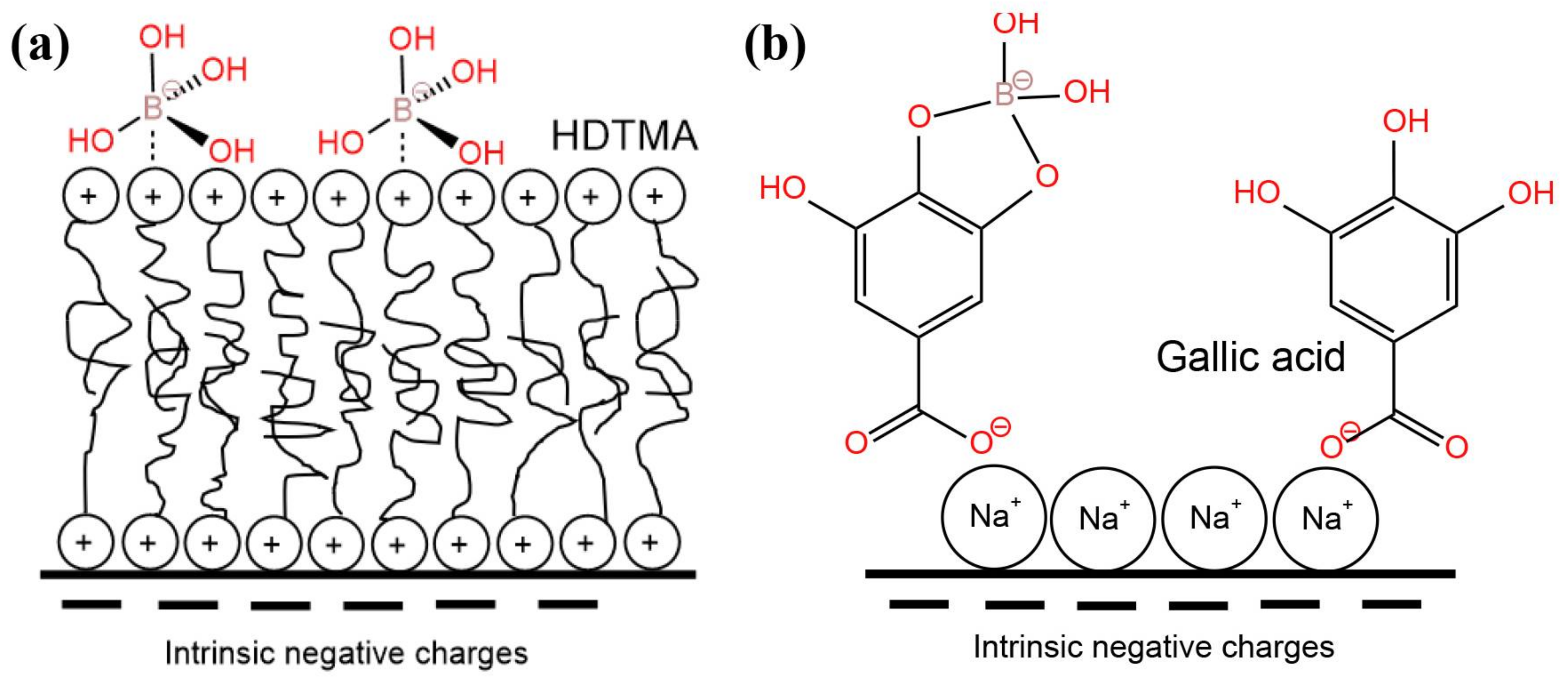
| Temperature (°C) | Solubility (Molar) | Temperature (°C) | Solubility (Molar) |
|---|---|---|---|
| 0 | 0.4304 | 60 | 2.3961 |
| 10 | 0.5776 | 70 | 2.7067 |
| 20 | 0.8154 | 80 | 3.8424 |
| 30 | 1.0678 | 90 | 4.9151 |
| 40 | 1.4108 | 100 | 6.5119 |
| 50 | 1.8670 |
| Polyol | k1 (L·mol−1) | k2 (L2·mol−2) |
|---|---|---|
| 1,2-ethylene glycol | 2.15 | 1.15 |
| 1,3-propanediol | 1.27 | 0.11 |
| Glycerin | 16.0 | 41.2 |
| Catechol | 7.8 × 103 | 1.42 × 104 |
| D-mannitol | 1.10 × 102 | 1.37 × 105 |
| D-glucose | 1.5 × 103 | 7.60 × 103 |
| Sorbitol | / | 4.4 × 105 |
| D-ribose | / | 1.57 × 107 |
| Animals | Dose (mg·kg−1) | Toxicological Effect |
|---|---|---|
| Mouse | 79 | Slow growth |
| Rabbit | 44 | Fetal deformities |
| Dog | 29 | Testicular atrophy |
| Rat | 26 | Sperm inhibition |
| Rat | 52 | Testicular atrophy |
| Rat | 13 | Decreased fetus body size |
| Countries/ Organizations | Drinking Water (mg·L−1) | Industrial Effluent (mg·L−1) | Countries | Drinking Water (mg·L−1) | Industrial Effluent (mg·L−1) |
|---|---|---|---|---|---|
| WHO | 2.4 | - | China | 0.5 | 5.0 (Shanghai) 2.0 (Beijing) |
| EU | 1.0 | - | Malaysia | 0.5 | 4.0 |
| USA | 1.0 (California) 0.9 (Wisconsin) 0.63 (Florida) 0.6 (Minnesota) | - | India | 0.5 | 2.0 |
| Canada | 5.0 | - | Morocco | 0.3 | - |
| New Zealand | 1.4 | - | Egypt | 0.5 | - |
| Australia | 4.0 | - | Kuwait | 0.5 | - |
| South Korea | 1.0 | - | Saudi Arabia | 0.5 | - |
| Japan | 1.0 | 10 | Iraq | 0.1 | - |
| Singapore | 2.4 | 5.0 | Jordan | 1.0 | - |
| Israel | 0.3 | 1.5 | Brazil | - | 5.0 |
| Membrane Processes | Conditions | Removal Rate | Refs. | |
|---|---|---|---|---|
| RO | UiO-66 + RO | 55 bar, 25 °C, pH = 8, [B]0 = 5 ppm | 91.2% | [37] |
| RO | NBS + RO | 55 bar, 25 °C, pH = 8, [B]0 = 5 ppm | 93.1% | [13] |
| RO | PIB/MPD/TMC + RO | 1.55 MPa, 25 °C, [PIB] = 0.30%, [B]0 = 5 ppm | 93.12% | [39] |
| RO | EDBSA/TMC + RO | 1.2 MPa, 25 °C, [EDBSA] = 1%, [TMC] = 0.15%, [B]0 = 5 ppm | 90.6% | [40] |
| FO | FTS H2OTM membrane | [FS] a: pH = 10, [B]0 = 50 mg·L−1, [DS] b = 1 M MgCl2 | 90% | [43] |
| FO | PSU membrane | [FS]: pH = 10, [B]0 = 50 mg·L−1, [DS] = 1 M MgCl2 | 84% | [43] |
| FO | Aquaporin Inside™ membrane | [FS]: pH = 10, [B]0 = 50 mg·L−1, [DS] = 1 M MgCl2 | 76% | [43] |
| FO | - | [FS]: pH = 8, [B]0 = 10 mg·L−1, [DS]: 0.2 M NaCl, pH = 12.5 | 94% | [44] |
| ED | M50-QGO1 membranes | 30 V, 3 h, pH = 9.14, [B]0 = 1000 mg·L−1 | 76.6% | [46] |
| ED | BPED | 12.5 V, 60 min, pH = 9.2, [B]0 = 100 mg·L−1 | 90.2% | [48] |
| DD | AFN | [B]0 = 66 mg·L−1, pH = 11.6, [Cl−1] = 0.5 mg·L−1 | 88.8% | [50] |
| MD | PVDF membrane | 205 kPa,18 h, 59 °C, pH = 7.48, [B]0 = 5.37 mg·L−1 | 91.25% | [27] |
| MD | PVDF membrane | 180 kPa, 250 h, 50 °C, pH = 7.7, [B]0 = 12.7 mg·L−1 | 99.8% | [27,52] |
| Commercial Resin/Fiber | Manufacturer | Adsorption Capacity | Refs. |
|---|---|---|---|
| Diaion CRB01 | Mitsubishi Chemical Corporation | ≥1.2 eq·L−1 | [7] |
| Diaion CRB02 | Mitsubishi Chemical Corporation | 7.46 mg·g−1 | [8] |
| Diaion CRB03 | Mitsubishi Chemical Corporation | ≥0.7 eq·L−1 | [65] |
| Diaion CRB05 | Mitsubishi Chemical Corporation | ≥0.95 eq·L−1 | [65] |
| Dowex 2 × 8 | Dow Chemical Company | 17.0 mg·g−1 | [66] |
| Dowex XUS 43594.00 | Dow Chemical Company | 3.35 mg·g−1 | [8] |
| DowexTM BSR-1 | Dow Chemical Company | 0.7 eq·L−1 | [7] |
| Amberlite IRA-743 | Rohm & Haas Company | 7.46 mg·g−1 | [8] |
| Amberlite PWA10 | Rohm & Haas Company | ≥0.7 eq·L−1 | [7] |
| Purolite S108 | Purolite Company | 6.27 mg·g−1 | [8] |
| Purolite S110 | Purolite Company | 0.8 eq·L−1 | [7] |
| Chelest fiber GRY-HW | Chelest Company | 12.07 mg·g−1 | [67] |
| Modified Resins | Functional Monomer | Saturated Adsorption Capacity | Refs. |
|---|---|---|---|
| Glycidyl methacrylate-NMDG | NMDG | 20.00 mg·g−1 | [75] |
| PAF-1-NMDG P2-NMDG | NMDG | 18.38 mg·g−1 16.86 mg·g−1 | [72] [72] |
| CA@KH-550@EPH@ NMDG(CKEN) | NMDG | 15.35 mg·g−1 | [74] |
| 3DOM CLPGMA-NMDG-6 | NMDG | 24.00 mg·g−1 | [78] |
| Adsorbent | Conditions | Equilibrium a/Maximum b Adsorption Capacity | Refs. |
|---|---|---|---|
| CQDs/LDHs | [B]0 = 25 mg·L−1, 3 h, pH = 8.5, adsorbent dose = 2 g·L−1, 25 °C | a 19.5 mg·g−1 | [79] |
| Perlite-HDTMA | [B]0 = 8000 mg·L−1, 4 h, pH = 4, 25 °C | b 833.3 mg·g−1 | [80] |
| Perlite-GA | [B]0 = 8000 mg·L−1, 15 h, pH = 7–9, 25 °C | b 2500 mg·g−1 | [80] |
| FHT | [B]0 = 25 mg·L−1, 1.5 h, adsorbent dose = 4 g·L−1, 25 °C | a 3.1 mg·g−1 | [81] |
| I-LDH | [B]0 = 1000 mg·L−1, 24 h, pH = 7, adsorbent dose = 7.5 g·L−1, 25 °C | b 21.62 mg·g−1 | [82] |
| I-CLDH | [B]0 = 1000 mg·L−1, 24 h, pH = 7, adsorbent dose = 7.5 g·L−1, 25 °C | b 77.83 mg·g−1 | [82] |
| Adsorbent | Conditions | Equilibrium a/Maximum b Adsorption Capacity | Refs. |
|---|---|---|---|
| Fly ash zeolite | [B]0 = 50 mg·L−1, 0.5 h, pH = 7, adsorbent dose = 20 g·L−1, 25 °C | a 2.3 mg·g−1 | [84] |
| Waste tire rubber | [B]0 = 17.5 mg·L−1, 48 h, pH = 2, adsorbent dose = 1 g·L−1, 21 °C | a 16.72 mg·g−1 | [85] |
| Waste concrete | [B]0 = 10 mg·L−1, 24 h, pH = 12, adsorbent dose = 66.7 g·L−1 | a 0.117 mg·g−1 | [56] |
| Non-activated waste sepiolite | [B]0 = 600 mg·L−1, 24 h, pH = 10, adsorbent dose = 2 g·L−1, 20 °C | b 96.15 mg·g−1 | [86] |
| Activated waste sepiolite | [B]0 = 600 mg·L−1, 24 h, pH = 10, adsorbent dose = 2 g·L−1, 20 °C | b 178.57 mg·g−1 | [86] |
| Steelmaking slag | [B]0 = 500 mg·L−1, 24 h, adsorbent dose = 2 g·L−1, 25 °C | b 145 mg·g−1 | [87] |
| Adsorbent | Conditions | Equilibrium a/Maximum b Adsorption Capacity | Refs. |
|---|---|---|---|
| Bentonite | [B]0 = 120 mg·L−1, pH = 9, 24 h, [CaCl2] = 0.1 M, adsorbent dose = 50 g·L−1 | b 0.51 mg·g−1 | [88] |
| Bentonite-FeCl3 | [B]0 = 120 mg·L−1, pH = 9, 24 h, [CaCl2] = 0.1 M, adsorbent dose = 50 g·L−1 | b 0.83 mg·g−1 | [88] |
| Kaolinite | [B]0 = 120 mg·L−1, pH = 9, 24 h, [CaCl2] = 0.1 M, adsorbent dose = 50 g·L−1 | b 0.60 mg·g−1 | [88] |
| Kaolinite-FeCl3 | [B]0 = 120 mg·L−1, pH = 9, 24 h, [CaCl2] = 0.1 M, adsorbent dose = 50 g·L−1 | b 0.80 mg·g−1 | [88] |
| Waste calcite | [B]0 = 120 mg·L−1, pH = 9, 24 h, [CaCl2] = 0.1 M, adsorbent dose = 50 g·L−1 | b 1.05 mg·g−1 | [88] |
| Waste calcite-FeCl3 | [B]0 = 120 mg·L−1, pH = 9, 24 h, [CaCl2] = 0.1 M, adsorbent dose = 50 g·L−1 | b 1.60 mg·g−1 | [88] |
| Zeolite | [B]0 = 120 mg·L−1, pH = 9, 24 h, [CaCl2] = 0.1 M, adsorbent dose = 50 g·L−1 | b 0.53 mg·g−1 | [88] |
| Zeolite-FeCl3 | [B]0 = 120 mg·L−1, pH = 9, 24 h, [CaCl2] = 0.1 M, adsorbent dose = 50 g·L−1 | b 0.76 mg·g−1 | [88] |
| Rice residue | [B]0 = 120 mg·L−1, pH = 7, 48 h, [CaCl2] = 0.1 M, adsorbent dose = 2 g·L−1 | b 9.26 mg·g−1 | [88] |
| Rice residue-FeCl3 | [B]0 = 120 mg·L−1, pH = 7, 48 h, [CaCl2] = 0.1 M, adsorbent dose = 2 g·L−1 | b 9.17 mg·g−1 | [88] |
| Walnut shell residue | [B]0 = 120 mg·L−1, pH = 7, 48 h, [CaCl2] = 0.1 M, adsorbent dose = 2 g·L−1 | b 7.04 mg·g−1 | [88] |
| Walnut shell residue-FeCl3 | [B]0 = 120 mg·L−1, pH = 7, 48 h, [CaCl2] = 0.1 M, adsorbent dose = 2 g·L−1 | b 7.58 mg·g−1 | [88] |
| Wheat residue | [B]0 = 120 mg·L−1, pH = 7, 48 h, [CaCl2] = 0.1 M, adsorbent dose = 2 g·L−1 | b 5.59 mg·g−1 | [88] |
| Wheat residue-FeCl3 | [B]0 = 120 mg·L−1, pH = 7, 48 h, [CaCl2] = 0.1 M, adsorbent dose = 2 g·L−1 | b 6.06 mg·g−1 | [88] |
| Magnesite and bentonite clay composite | [B]0 = 20 mg·L−1, 30 min, pH = 11, adsorbent dose = 2 g·L−1, 26 °C | b 4 mg·g−1 | [89] |
| Vermiculite-HDTMA | [B]0 = 7205 mg·L−1, pH = 11, 15 h, 56.5 °C | a 258.13 mg·g−1 | [90] |
| Vermiculite-GA | [B]0 = 7181.3 mg·L−1, pH = 8.48, 2 h, 40.8 °C | a 152.4 mg·g−1 | [90] |
| Perlite-HDTMA | [B]0 = 8000 mg·L−1, 4 h, pH = 4, 25 °C | b 833.3 mg·g−1 | [80] |
| Perlite-GA | [B]0 = 8000 mg·L−1, 15 h, pH = 7–9, 25 °C | b 2500.0 mg·g−1 | [80] |
| CWES | [B]0 = 50 mg·L−1, pH = 4, 48 h, adsorbent dose = 1 g·L−1, 25 °C | b 31.06 mg·g−1 | [57] |
| ESM | [B]0 = 50 mg·L−1, pH = 8, 48 h, adsorbent dose = 1 g·L−1, 25 °C | b 33.3 mg·g−1 | [91] |
| MESM | [B]0 = 50 mg·L−1, pH = 4, 48 h, adsorbent dose = 1 g·L−1, 25 °C | b 33.3 mg·g−1 | [91] |
| Adsorbent | Conditions | Maximum Adsorption Capacity | Refs. |
|---|---|---|---|
| ZIF-8 | [B]0 = 0.5 M, 12 h, pH = 4.43, adsorbent dose = 5 g·L−1, 45 °C | 247.44 mg·g−1 | [92] |
| ZIF-67 | [B]0 = 0.5 M, 24 h, pH = 4, adsorbent dose = 3 g·L−1, 35 °C | 579.80 mg·g−1 | [93] |
| UiO-66 | [B]0 = 0.7 M, 12 h, adsorbent dose = 5 g·L−1, 35 °C | 140.53 mg·g−1 | [94] |
| PAF-1-NMDG | [B]0 = 19.4 mM·L−1, 1 h, adsorbent dose = 5 g·L−1 | 18.38 mg·g−1 | [72] |
| P2-NMDG | [B]0 = 19.4 mM·L−1, 1 h, adsorbent dose = 5 g·L−1 | 16.86 mg·g−1 | [72] |
| 3DOM CLPGMA-NMDG-6 | [B]0 = 500 mg·L−1, pH = 8, 24 h, adsorbent dose = 5 g·L−1, 25 °C | 24.00 mg·g−1 | [78] |
| CTS-NMDG | [B]0 = 2000 mg·L−1, pH = 7, 10 h, adsorbent dose = 10 g·L−1, 25 °C | 20.36 mg·g−1 | [95] |
| Adsorbent | Size (μm) | T (°C) | pH | Maximum Adsorption Capacity | Refs. |
|---|---|---|---|---|---|
| MgO | - | 30 | 10 | 216 mg·g−1 | [96] |
| Al2O3 | - | - | 9 | 6.4 mg·g−1 | [98] |
| CAAl | 800 | - | 9 | 56.3 mg·g−1 | [98] |
| TiO2-CTS | 450 | 25 | 4 | 4.35 mg·g−1 | [99] |
| Cr2O3-CTS | 450 | 25 | 4 | 3.52 mg·g−1 | [99] |
| Fe3O4-CTS | 450 | 25 | 4 | 4.42 mg·g−1 | [99] |
| Adsorbent | Conditions | Equilibrium a/Maximum b Adsorption Capacity | Refs. |
|---|---|---|---|
| M-NMDG | [B]0 = 32 mg·L−1, 30 min, pH = 8.2 adsorbent dose = 1.2 g·L−1, 25 °C | b 6.68 mg·g−1 | [100] |
| M-TACA | [B]0 = 32 mg·L−1, 30 min, pH = 8.2 adsorbent dose = 1.2 g·L−1, 25 °C | b 13.44 mg·g−1 | [100] |
| poly(β-CD-(NH2)7-TCL)@gluconolactone | [B]0 = 300 mg·L−1, pH = 9.2 adsorbent dose = 2 g·L−1, 25 °C | a 26.3 ± 5.9 mg·g−1 | [101] |
| poly(β-CD-(NH2)7-TFN)@gluconolactone | [B]0 = 300 mg·L−1, pH = 9.2 adsorbent dose = 2 g·L−1, 25 °C | a 44.0 ± 1.8 mg·g−1 | [101] |
| β-CD-9PGMA-NMDG | [B]0 = 1000 mg·L−1, 1 h, pH = 8, adsorbent dose = 5 g·L−1, 20 °C | b 31.1 mg·g−1 | [55] |
| β-CD-9PGMA-EN-PG | [B]0 = 1000 mg·L−1, 1 h, pH = 8, adsorbent dose = 5 g·L−1, 20 °C | b 20.5 mg·g−1 | [55] |
| P(GMA-co-TRIM)-EN-PG | [B]0 = 1000 mg·L−1, pH = 9, adsorbent dose = 5 g·L−1, 30 °C | a 29.2 mg·g−1 | [102] |
| P(GMA-co-TRIM)-TETA-PG | [B]0 = 1000 mg·L−1, pH = 8, adsorbent dose = 5 g·L−1, 30 °C | a 23.3 mg·g−1 | [102] |
| Zr-CTS | [B]0 = 500 mg·L−1,48 h, pH = 7, adsorbent dose = 100 g·L−1, 25 °C | a 24.5 mg·g−1 | [58] |
| HSGUM | [B]0 = 25 M, 24 h, 25 °C, adsorbent dose = 5 g·L−1 | b 44.32 mg·g−1 | [103] |
| CKEN | [B]0 = 100 M,15 h, pH = 9.5, 25 °C adsorbent dose = 12 g·L−1 | b 31.8 mg·g−1 | [74] |
| Processes | Conditions | Removal Rate | Refs. | |
|---|---|---|---|---|
| CP | Lime milk | [B]0 = 31.5 g·L−1, pH = 10, lime milk dosage = 30 g·L−1 | 71.4% | [107] |
| COP | H2O2 + Ba(OH)2 | [B]0 = 1000 mg·L−1, 4 h, pH = 10.5 | 99.7% | [110] |
| CC | PACSM | [B]0 = 5 mg·L−1, pH = 10.5, 15 °C, [PACSM] = 0.5 mg·L−1 | 87.5% | [128] |
| CC | PAFCS | [B]0 = 5 mg·L−1, pH = 11, 20 °C, [PAFCS] = 3 mg·L−1 | 93.6% | [128] |
| EC | EC-Al | [B]0 = 100 mg·L−1, D * = 10 mm, pH = 8, 60 min, CD = 5.5 mA·cm−2 | 70% | [114] |
| EC | EC-Al | [B]0 = 9.3 mM, [NaCl] = 10 mM, pH = 8.0, CD = 5 mA·cm−2 | 74.1% | [121] |
| EC | EC-Al | [B]0 = 500 mg·L−1, pH = 8.5, 90 min, CD = 10 mA·cm−2 | 55% | [116] |
| EC | EC-Ni | [B]0 = 10 mg·L−1, pH = 8, 2 h, CD = 1.25 mA·cm−2 | 99.2% | [117] |
| EC | EC-Fe/Ni | [B]0 = 10 mg·L−1, pH = 8, 60 min, CD = 3.75 mA·cm−2 | 95% | [118] |
| EC | EC-Al | [B]0 = 15 mg·L−1, pH = 8, 150 min, CD = 6 mA·cm−2 | 96% | [124] |
| EC | EC-Al | [B]0 = 10.4 mg·L−1, pH = 6.3, 89 min, CD = 17.4 mA·cm−2 | 99.7% | [129] |
| EC | EC-Al | [B]0 = 5 mg·L−1, 45 min, D = 5 mm, pH = 7.84, CD = 12.5 mA·cm−2 | 88% | [125] |
| EC | EC-Fe | [B]0 = 5 mg·L−1, 45 min, D = 5 mm, pH = 7.84, CD = 12.5 mA·cm−2 | 78% | [125] |
| Extractant | Conditions | Extraction Rate | Refs. |
|---|---|---|---|
| EHD/CTMP | 0.25 M EHD/0.25 M CTMP, pH = 9.2 | 90.0% | [130] |
| BPO | [E] * = 0.1 M, [B]0 = 0.1 M | 63.1% | [139] |
| 4,5-Dimethyl-2,4-hexanediol | [B]0 = 0.01 M, [E] = 0.5 M, 25 °C, pH = 2 | 85.9% | [133] |
| 4,6-Dimethyl-2,4-heptanediol | [B]0 = 0.01 M, [E] = 0.5 M, 25 °C, pH = 2 | 71.2% | [133] |
| 4,7-Dimethyl-2,4-octanediol | [B]0 = 0.01 M, [E] = 0.5 M, 25 °C, pH = 2 | 68.8% | [133] |
| 2,2,4-Trimethyl-1,3-pentanediol | [B]0 = 0.01 M, [E] = 0.5 M, 25 °C, pH = 2 | 96.9% | [133] |
| 2,2,5-Trimethyl-1,3-hexanediol | [B]0 = 0.01 M, [E] = 0.5 M, 25 °C, pH = 2 | 96.8% | [133] |
| 2,2,6-Trimethyl-1,3-heptanediol | [B]0 = 0.01 M, [E] = 0.5 M, 25 °C, pH = 2 | 91.4% | [133] |
| 2,3,4-Trimethyl-1,3-pentanediol | [B]0 = 0.01 M, [E] = 0.5 M, 25 °C, pH = 2 | 96.3% | [133] |
| 2,3,5-Trimethyl-1,3-hexanediol | [B]0 = 0.01 M, [E] = 0.5 M, 25 °C, pH = 2 | 96.2% | [133] |
| 2,3,6-Trimethyl-1,3-heptanediol | [B]0 = 0.01 M, [E] = 0.5 M, 25 °C, pH = 2 | 94.8% | [133] |
| 2-butyl-2-ethyl-l,3-propanediol | [B]0 = 0.03 M, [E] = 1.1 M | 78.0% | [135] |
| 2-ethylhexanol | - | 99.5% | [138] |
| 2-butyl-1-n-octanol | [B]0 = 14.84 g·L−1, [E] = 0.2 M, O/A = 1 | 99.4% | [136] |
| 2-ethyl-1,3-hexanediol in toluene | [B]0 = 0.1748 M, 45 °C, pH = 1, | 93.5% | [134] |
| BEPD in 25% decanol/Kerosene | [B]0 = 148 mg·L−1, [E] = 0.6 M | 85.0% | [132] |
| TMPD in 25% decanol/Kerosene | [B]0 = 148 mg·L−1, [E] = 0.6 M | 93.0% | [132] |
| 2-ethyl-1-hexanol in kerosene | [B]0 = 2 g·L−1, [E] = 70% | 98.3% | [131] |
| 2-ethylhexanol in kerosene | [B]0 = 7.29 g·L−1, pH = 1.54, [E] = 50%, O/A = 4, | 98.8% | [137] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Xu, C.; Chen, P.; Li, K.; Zhou, Q.; Ye, M.; Zhang, L.; Lu, Y. Advances in Technologies for Boron Removal from Water: A Comprehensive Review. Int. J. Environ. Res. Public Health 2022, 19, 10671. https://doi.org/10.3390/ijerph191710671
Liu X, Xu C, Chen P, Li K, Zhou Q, Ye M, Zhang L, Lu Y. Advances in Technologies for Boron Removal from Water: A Comprehensive Review. International Journal of Environmental Research and Public Health. 2022; 19(17):10671. https://doi.org/10.3390/ijerph191710671
Chicago/Turabian StyleLiu, Xiaowei, Congjin Xu, Peng Chen, Kexin Li, Qikun Zhou, Miaomaio Ye, Liang Zhang, and Ye Lu. 2022. "Advances in Technologies for Boron Removal from Water: A Comprehensive Review" International Journal of Environmental Research and Public Health 19, no. 17: 10671. https://doi.org/10.3390/ijerph191710671
APA StyleLiu, X., Xu, C., Chen, P., Li, K., Zhou, Q., Ye, M., Zhang, L., & Lu, Y. (2022). Advances in Technologies for Boron Removal from Water: A Comprehensive Review. International Journal of Environmental Research and Public Health, 19(17), 10671. https://doi.org/10.3390/ijerph191710671








