Exercise Capacity and Physical Activity in Non-Cystic Fibrosis Bronchiectasis after a Pulmonary Rehabilitation Home-Based Programme: A Randomised Controlled Trial
Abstract
:1. Introduction
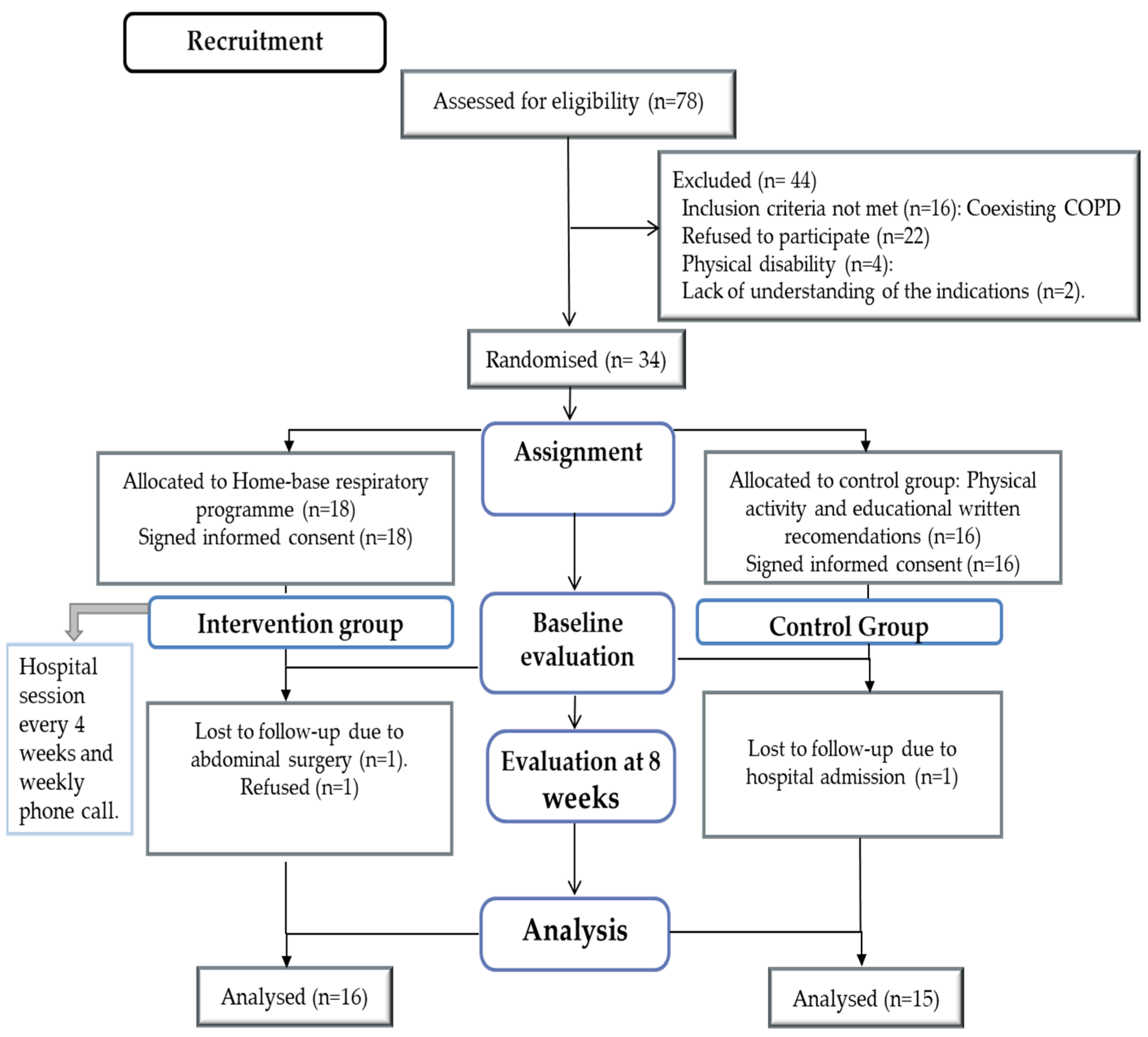
2. Materials and Methods
2.1. Design
2.2. Participants
2.2.1. Intervention
2.2.2. Strength Training
2.2.3. Endurance Training
2.2.4. Measurement of Variables
2.3. Statistical Analysis
3. Results
3.1. Dyspnoea
3.2. Six-Minute Walk Test
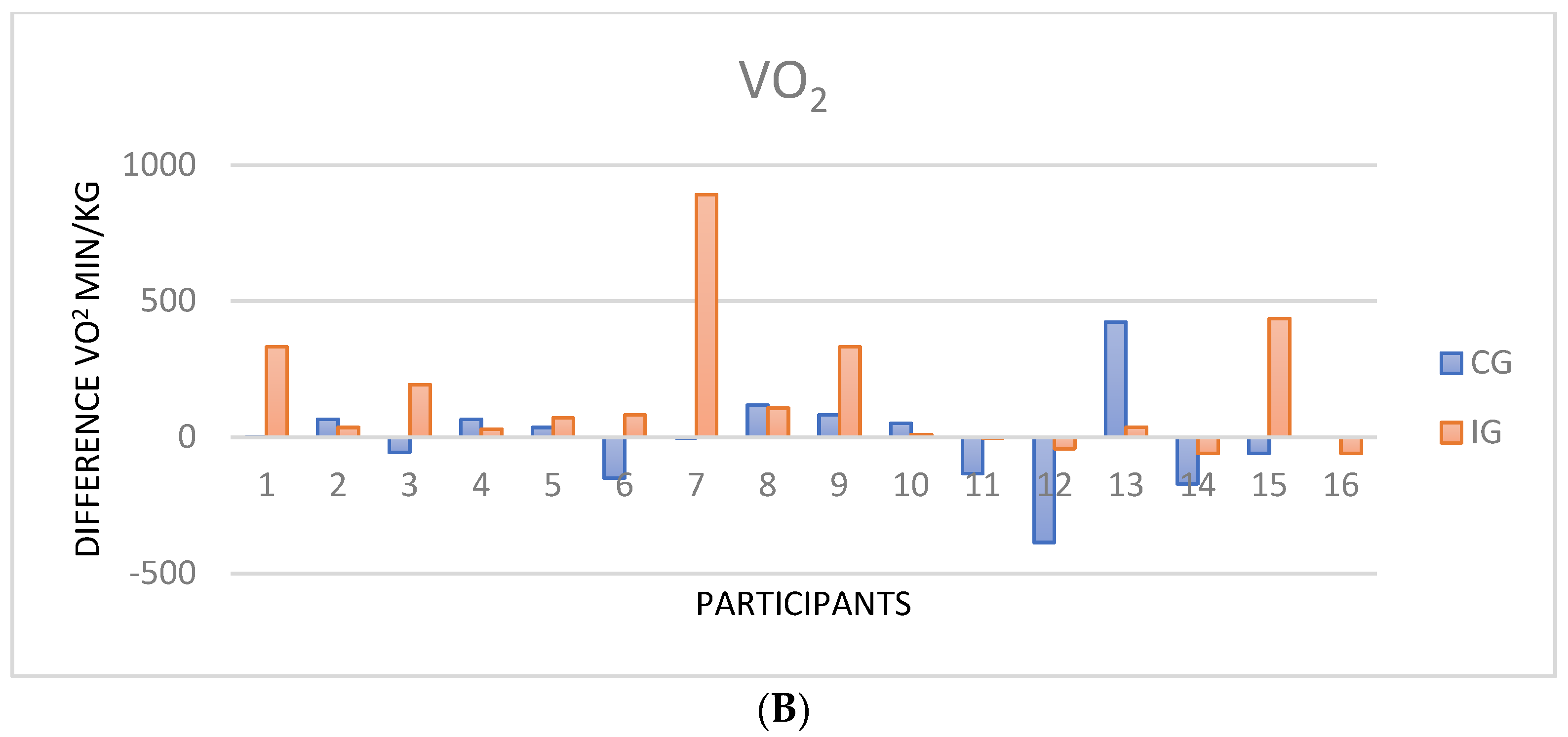
3.3. Cardiopulmonary Exercise Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martínez-García, M.Á.; Máiz, L.; Olveira, C.; Girón, R.M.; de la Rosa, D.; Blanco, M.; Cantón, R.; Vendrell, M.; Polverino, E.; de Gracia, J.; et al. Normativa sobre el tratamiento de las bronquiectasias en el adulto. Arch. Bronconeumol. 2018, 54, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.A.; Soler-Cataluña, J.-J.; Perpiñá-Tordera, M.; Román-Sánchez, P.; Soriano, J. Factors associated with lung function decline in adult patients with stable non-cystic fibrosis bronchiectasis. Chest 2007, 132, 1565–1572. Available online: http://www.ncbi.nlm.nih.gov/pubmed/17998359 (accessed on 7 June 2020). [CrossRef] [PubMed]
- Koulouris, N.G.G.; Retsou, S.; Kosmas, E.; Dimakou, K.; Malagari, K.; Mantzikopoulos, G.; Koutsoukou, A.; Milic-Emili, J.; Jordanoglou, J. Tidal expiratory flow limitation, dyspnoea and exercise capacity in patients with bilateral bronchiectasis. Eur. Respir. J. 2003, 21, 743–748. Available online: https://pubmed.ncbi.nlm.nih.gov/12765414/ (accessed on 18 February 2019). [CrossRef]
- Bar-Yoseph, R.; Ilivitzki, A.; Cooper, D.M.; Gur, M.; Mainzer, G.; Hakim, F.; Livnat, G.; Schnapp, Z.; Shalloufeh, G.; Zucker-Toledano, M.; et al. Exercise capacity in patients with cystic fibrosis vs. non-cystic fibrosis bronchiectasis. PLoS ONE 2019, 14, e0217491. Available online: https://dx.plos.org/10.1371/journal.pone.0217491 (accessed on 16 June 2020).
- Patel, S.; Cole, A.D.; Nolan, C.M.; Barker, R.E.; Jones, S.E.; Kon, S.; Cairn, J.; Loebinger, M.; Wilson, R.; Man, W.D.-C. Pulmonary rehabilitation in bronchiectasis: A propensity-matched study. Eur. Respir. J. 2019, 53, 1801264. Available online: http://ow.ly/fQua30mOlPw (accessed on 17 June 2019). [CrossRef]
- Ozalp, O.; Inal-Ince, D.; Calik, E.; Vardar-Yagli, N.; Saglam, M.; Savci, S.; Arikan, H.; Bosnak-Guclu, M.; Coplu, L. Extrapulmonary features of bronchiectasis: Muscle function, exercise capacity, fatigue, and health status. Multidiscip Respir. Med. 2012, 7, 3. Available online: http://mrmjournal.biomedcentral.com/articles/10.1186/2049-6958-7-3 (accessed on 19 June 2020). [CrossRef] [PubMed]
- Lee, A.L.; Gordon, C.S.; Osadnik, C.R. Exercise training for bronchiectasis. Cochrane Database Syst. Rev. 2021, 4, CD013110. [Google Scholar] [CrossRef]
- Lee, A.L.; Hill, C.J.; McDonald, C.F.; Holland, A.E. Pulmonary Rehabilitation in Individuals With Non–Cystic Fibrosis Bronchiectasis: A Systematic Review. Arch. Phys. Med. Rehabil. 2017, 98, 774–782.e1. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0003999316302465 (accessed on 19 June 2020). [CrossRef]
- Kumar, R.; Guleria, R.; Khilnani, G.C.; Mohan, A.; Madan, K.; Hadda, V.; Pandey, R.M. The effects of pulmonary rehabilitation in patients with non-cystic fibrosis bronchiectasis—A randomised controlled trial. Eur. Respir. J. 2017, 50 (Suppl. 61), OA307. Available online: https://erj.ersjournals.com/content/50/suppl_61/OA307 (accessed on 23 January 2022).
- Corso, S.D.; José, A.; Holland, A.E.; Selman, J.P.R.; Castro, R.A.S.; de Camargo, C.O.; Fonseca, D.S.; Athanazio, R.A.; Rached, S.Z.; Cukier, A. Home-based pulmonary rehabilitation in patients with bronchiectasis: A randomized controlled trial. Eur. Respir. J. 2017, 50 (Suppl. 61), OA4668. Available online: https://erj.ersjournals.com/content/50/suppl_61/OA4668 (accessed on 16 June 2021).
- Chalmers, J.D.; Crichton, M.L.; Brady, G.; Finch, S.; Lonergan, M.; Fardon, T.C. Pulmonary rehabilitation after exacerbation of bronchiectasis: A pilot randomized controlled trial. BMC Pulm. Med. 2019, 19, 85. Available online: https://bmcpulmmed.biomedcentral.com/articles/10.1186/s12890-019-0856-0 (accessed on 10 September 2021). [CrossRef] [PubMed]
- Lee, A.L.; Hill, C.J.; Cecins, N.; Jenkins, S.; Mcdonald, C.F.; Burge, A.T.; Rautela, L.; Stirling, R.G.; Thompson, P.J.; Holland, A.E. The short and long term effects of exercise training in non-cystic fibrosis bronchiectasis—A randomised controlled trial. Respir. Res. 2014, 15, 44. Available online: http://respiratory-research.com/content/15/1/44 (accessed on 14 July 2020). [CrossRef]
- Newall, C.; Stockley, R.A.; Hill, S.L. Exercise training and inspiratory muscle training in patients with bronchiectasis. Thorax 2005, 60, 943–948. Available online: http://www.scopus.com/inward/record.url?eid=2-s2.0-27744446821&partnerID=tZOtx3y1 (accessed on 5 September 2020). [CrossRef] [Green Version]
- Pehlivan, E.; Niksarlıoğlu, E.Y.; Balcı, A.; Kılıç, L. The effect of pulmonary rehabilitation on the physical activity level and general clinical status of patients with bronchiectasis. Turk. Thorac J. 2019, 20, 30–35. [Google Scholar] [CrossRef]
- José, A.; Holland, A.E.; Selman, J.P.R.R.; De Camargo, C.O.; Fonseca, D.S.; Athanazio, R.A.; Rached, S.Z.; Cukier, A.; Stelmach, R.; Corso, S.D. Home-based pulmonary rehabilitation in people with bronchiectasis: A randomised controlled trial. ERJ Open Res. 2021, 7, 00021-2021. [Google Scholar] [CrossRef] [PubMed]
- Troosters, T.; Gosselink, R.; Janssens, W.; Decramer, M. Exercise training and pulmonary rehabilitation: New insights and remaining challenges. Eur. Respir. Rev. 2010, 19, 24–29. [Google Scholar] [CrossRef]
- Cakmak, A.; Inal-Ince, D.; Sonbahar-Ulu, H.; Bozdemir-Ozel, C.; Ozalp, O.; Calik-Kutukcu, E.; Saglam, M.; Vardar-Yagli, N.; Arikan, H.; Selcuk, Z.T.; et al. Physical activity of patients with bronchiectasis compared with healthy counterparts: A cross-sectional study. Hear Lung 2020, 49, 99–104. [Google Scholar] [CrossRef]
- Bolton, C.E.; Bevan-Smith, E.F.; Blakey, J.D.; Crowe, P.; Elkin, S.L.; Garrod, R.; Greening, N.J.; Heslop, K.; Hull, J.H.; Man, W.D.-C.; et al. British Thoracic Society guideline on pulmonary rehabilitation in adults. Thorax 2013, 68 (Suppl. 2), ii1–ii30. Available online: http://thorax.bmj.com/lookup/doi/10.1136/thoraxjnl-2013-203808 (accessed on 18 June 2021). [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D.; Goodman, S.; Grunberg, S. CONSORT 2010 Statement Updated Guidelines for Reporting Parallel Group Randomized Trials Background to CONSORT. Mayo Clin Coll. Med. 2010, 115, 1097. Available online: www.consort-statement.org (accessed on 25 January 2021).
- Hebestreit, H.; Schmid, K.; Kieser, S.; Junge, S.; Ballmann, M.; Roth, K.; Hebestreit, A.; Schenk, T.; Schindler, C.; Posselt, H.-G.; et al. Quality of life is associated with physical activity and fitness in cystic fibrosis. BMC Pulm. Med. 2014, 14, 26. Available online: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3942299&tool=pmcentrez&rendertype=abstract (accessed on 31 August 2019). [CrossRef]
- Pritchard, A.; Burns, P.; Correia, J.; Jamieson, P.; Moxon, P.; Purvis, J.; Thomas, M.; Tighe, H.; Sylvester, K.P. ARTP statement on cardiopulmonary exercise testing 2021. BMJ Open Respir. Res. 2021, 8, e001122. [Google Scholar] [CrossRef]
- Koch, B.; Schäper, C.; Ittermann, T.; Spielhagen, T.; Dörr, M.; Völzke, H.; Opitz, C.F.; Ewert, R.; Gläser, D. Reference values for cardiopulmonary exercise testing in healthy volunteers: The SHIP study. Eur. Respir. J. 2009, 33, 389–397. Available online: https://erj.ersjournals.com/content/33/2/389 (accessed on 30 June 2022). [CrossRef]
- Mueller, S.; Winzer, E.B.; Duvinage, A.; Gevaert, A.B.; Edelmann, F.; Haller, B.; Pieske-Kraigher, E.; Beckers, P.; Bobenko, A.; Hommel, J.; et al. Effect of High-Intensity Interval Training, Moderate Continuous Training, or Guideline-Based Physical Activity Advice on Peak Oxygen Consumption in Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA 2021, 325, 542–551. Available online: https://jamanetwork.com/journals/jama/fullarticle/2776199 (accessed on 17 July 2022). [CrossRef]
- Crapo, R.O.; Casaburi, R.; Coates, A.L.; Enright, P.L.; MacIntyre, N.R.; McKay, R.T.; Johnson, D.; Wanger, J.S.; Zeballos, R.J.; Bittner, V.; et al. ATS Statement. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. Available online: http://www.atsjournals.org/doi/abs/10.1164/ajrccm.166.1.at1102 (accessed on 14 October 2020).
- Brooks, D.; Solway, S. ATS statement on six-minute walk test. Am. J. Respir. Crit. Care Med. 2003, 169, 1287. [Google Scholar] [CrossRef]
- Lee, A.L.; Hill, C.J.; Cecins, N.; Jenkins, S.; McDonald, C.F.; Burge, A.T.; Rautela, L.; Stirling, R.G.; Thompson, P.J.; Holland, A.E. Minimal important difference in field walking tests in non-cystic fibrosis bronchiectasis following exercise training. Respir. Med. 2014, 108, 1303–1309. [Google Scholar] [CrossRef]
- Pacheco, V.A. Cambios en actividad física tras un programa de rehabilitación respiratoria en EPOC. Rev. Esp. Patol. Torac. 2016, 28, 214–221. [Google Scholar]
- Tudor-Locke, C.; Craig, C.L.; Aoyagi, Y.; Bell, R.C.; Croteau, K.A.; De Bourdeaudhuij, I.; Ewald, B.; Gardner, A.W.; Hatano, Y.; Lutes, L.D.; et al. How many steps/day are enough? For older adults and special populations. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 80. [Google Scholar] [CrossRef]
- Martínez-Garcia, M.; Selma, M.J.; Navarro, C.; Martinez-Garcia, M.A.; Selma, M.J.; Navarro, C. Escalas multidimensionales en bronquiectasias. Med. Respir. 2015, 8, 31–38. [Google Scholar]
- Kiel, C. G*Power 3: A flexible statistical power analysis program for the social. Behav. Biomed. Sci. 2007, 39, 175–191. [Google Scholar]
- Maturana, F.M.; Soares, R.N.; Murias, J.M.; Schellhorn, P.; Erz, G.; Burgstahler, C.; Widmann, M.; Munz, B.; Thiel, A.; Nieß, A.M. Responders and non-responders to aerobic exercise training: Beyond the evaluation of|Enhanced Reader. Physiol. Rep. 2021, 9, e14951. [Google Scholar] [CrossRef]
- Thiel, A.; Sudeck, G.; Gropper, H.; Maturana, F.M.; Schubert, T.; Srismith, D.; Widmann, M.; Behrens, S.; Martus, P.; Munz, B.; et al. The iReAct study—A biopsychosocial analysis of the individual response to physical activity. Contemp. Clin. Trials Commun. 2020, 17, 100508. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; Rankinen, T. Individual differences in response to regular physical activity. Med. Sci. Sports Exerc. 2001, 33 (Suppl. 6), 446–451. [Google Scholar] [CrossRef] [PubMed]
- Fiuza-Luces, C.; Santos-Lozano, A.; Joyner, M.; Carrera-Bastos, P.; Picazo, O.; Zugaza, J.L.; Izquierdo, M.; Ruilope, L.M.; Lucia, A. Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nat. Rev. Cardiol. 2018, 15, 731–743. Available online: https://www.nature.com/articles/s41569-018-0065-1 (accessed on 15 June 2022). [CrossRef] [PubMed]
- Swaminathan, S.; Kuppurao, K.V.; Somu, N.; Vijayan, V.K. Reduced Exercise Capacity in Non-Cystic Fibrosis Bronchiectasis. Indian J. Pediatrics 2003, 70, 553–556. Available online: http://link.springer.com/10.1007/BF02723157 (accessed on 21 March 2022). [CrossRef]
- Pastré, J.; Prévotat, A.; Tardif, C.; Langlois, C.; Duhamel, A.; Wallaert, B. Determinants of exercise capacity in cystic fibrosis patients with mild-to-moderate lung disease. BMC Pulm. Med. 2014, 14, 74. Available online: http://bmcpulmmed.biomedcentral.com/articles/10.1186/1471-2466-14-74 (accessed on 27 July 2021). [CrossRef]
- de Camargo, A.A.; Boldorini, J.C.; Holland, A.E.; de Castro, R.A.S.; Lanza, F.d.C.; Athanazio, R.A.; Rached, S.Z.; Carvalho-Pinto, R.; Cukier, A.; Stelmach, R.; et al. Determinants of peripheral muscle strength and activity in daily life in people with bronchiectasis. Phys. Ther. 2018, 98, 153–161. [Google Scholar] [CrossRef]
- Yang, F.; Gao, L.; Wang, Q.; Deng, W.; Gao, W. Effect of exercise-based pulmonary rehabilitation in patients with bronchiectasis: A meta-analysis. Respir. Med. Res. 2022, 81, 100910. [Google Scholar] [CrossRef]
- Singh, S.J.; Puhan, M.A.; Andrianopoulos, V.; Hernandes, N.A.; Mitchell, K.E.; Hill, C.J.; Lee, A.L.; Camillo, C.A.; Troosters, T.; Spruit, M.A.; et al. An official systematic review of the European Respiratory Society/American Thoracic Society: Measurement properties of field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1447–1478. Available online: https://erj.ersjournals.com/content/44/6/1447 (accessed on 23 May 2018). [CrossRef]
- Lee Annemarie, L.; Cecins Nola, E.; Holland Anne, J.; Hill Catherine, F.; Mcdonald Christine, T.; Burge Angela, J.; Rautela, L.; Thompson, P.J.; Stirling, R.G.; Jenkins, S. Field Walking Tests Are Reliable and Responsive to Exercise Training in People With Non–Cystic Fibrosis Bronchiectasis. J. Cardiopulm. Rehabil. Prev. 2015, 35, 439–445. Available online: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=01273116-201511000-00009 (accessed on 5 March 2022).
- Gugg, K.; Zwick, R.H. Non-responders to outpatient pulmonary rehabilitation: A retrospective, controlled cohort study. Eur. Respir. J. 2020, 56 (Suppl. 64), 715. Available online: https://erj.ersjournals.com/content/56/suppl_64/715 (accessed on 7 July 2022).
- José, A.; Ramos, T.M.; de Castro, R.A.S.; de Oliveira, C.S.; de Camargo, A.A.; Athanazio, R.A.; Rached, S.Z.; Stelmac, R.; Corso, S.D. Reduced Physical Activity With Bronchiectasis. Respir. Care 2018, 63, 1498–1505. Available online: https://rc.rcjournal.com/content/63/12/1498 (accessed on 17 July 2021). [CrossRef] [PubMed]
- Bradley, J.; O’Neill, B.; Kent, L.; Hulzebos, E.H.J.; Arets, B.; Hebestreit, H.; on behalf of the Exercise Working Group European CF Society, for publication in Journal of CF. Physical activity assessment in cystic fibrosis: A position statement. J. Cyst. Fibros. 2015, 14, e25–e32. [Google Scholar] [CrossRef]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; Zu Wallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.-C.; et al. An official American thoracic society/European respiratory society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Groups | p | ||
|---|---|---|---|
| CG (n = 15) | IG (n = 16) | ||
| Aetiology | |||
| Post-infection | 4 (26.66%) | 6 (37.50%) | 0.61 1 |
| Idiopathic | 4 (26.66%) | 1 (6.25%) | |
| Rheumatoid arthritis | 1 (6.6%) | 2 (12.50%) | |
| Immunodeficiencies | 1 (6.6%) | 1 (6.25%) | |
| PCD * | 1 (6.6%) | 1 (6.25%) | |
| Post-measles | 1 (6.6%) | 2 (12.50%) | |
| Sarcoidosis | 1 (6.6%) | 1 (6.25%) | |
| ABPA ** | 1 (6.6%) | 1 (6.22%) | |
| Others | 1 (6.6%) | 1 (6.25%) | |
| General characteristics | |||
| Women | 10 (66.66%) | 13 (81.25%) | 0.30 1 |
| Age | 59.43 ± 93.00 | 63 ± 6.14 | 0.33 2 |
| BMI | 25.05 ± 2.52 | 26.35 ± 3.6 | 0.86 2 |
| Smoker | 1 (6.66%) | 2 (12.50%) | 0.47 2 |
| Former smoker | 2 (13.33%) | 4 (25.00%) | 0.29 2 |
| Never smoker | 12 (80.00%) | 10 (62.50%) | 0.16 2 |
| PYI | 2.70 ± 2.11 | 3.20 ± 1.83 | 0.45 2 |
| P. Aeruginosa | 5 (33.33%) | 6 (37.50%) | 0.10 2 |
| Dyspnoea | 2.07 ± 0.71 | 2.19 ± 0.63 | 0.28 2 |
| E-FACED | 3.33 ± 1.73 | 3.44 ± 1.91 | 0.56 1 |
| Mild | 7 (46.67%) | 8 (50.00%) | 0.54 1 |
| Moderate | 7 (46.67%) | 6 (37.50%) | |
| Severe | 1 (6.66%) | 2(12.50%) | |
| Lung function | |||
| FEV1 L | 1.84 ± 0.52 | 1.61 ± 0.44 | 0.56 1 |
| FEV1% | 75.41 ± 28.44 | 71.88 ± 20.72 | 0.83 1 |
| FVC L | 2.43 ± 0.71 | 2.17 ± 0.61 | 0.26 1 |
| FVC% | 79.13 ± 24.14 | 79.13 ± 22.00 | 0.99 1 |
| FEV1/FVC | 69.92 ± 14.73 | 74.33 ± 10.74 | 0.34 1 |
| Control Group | Intervention Group | |||||
|---|---|---|---|---|---|---|
| Before | After | p | Before | After | p | |
| Men ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||
| 6MWT | ||||||
| Walking distance (m) | 398.87 ± 67.10 | 433.13 ± 75.88 | 0.98 1 | 401.2 ± 71.60 | 451.19 ± 67.99 | 0.006 1 |
| SpO2 (%) | 95.4 ± 2.91 | 94.2 ± 3.24 | 0.42 1 | 95.4 ± 2.93 | 95.8 ± 3.20 | 0.34 1 |
| Borg Scale | 1.82 ± 2.3 | 2.33 ± 3.2 | 0.38 2 | 2.45 ± 3.8 | 2.88 ± 1.31 | 0.30 2 |
| Physical activity | ||||||
| Total steps per day (steps) | 4.793 ± 3.236 | 4.824 ± 3.113 | 0.94 1 | 4.578 ± 3.424 | 6.591 ± 3.482 | 0.007 1 |
| METS * | 1.4 ± 0.3 | 1.26 ± 0.36 | 0.15 1 | 1.42 ± 0.35 | 1.57 ± 0.35 | 0.47 1 |
| Lying time | 9.03 ± 1.42 | 8.52 ± 1.02 | 0.01 1 | 8.86 ± 1.44 | 7.91 ± 1.17 | 0.02 1 |
| Sleep time (hour) | 6.79 ± 1.37 | 6.73 ± 0.9 | 0.03 1 | 6.55 ± 1.06 | 6.85 ± 1.01 | 0.23 2 |
| Wearing time (hour) | 22.34 ± 2.58 | 22.64 ± 1.85 | 0.75 1 | 23.32 ± 0.28 | 22.85 ± 1.71 | 0.22 1 |
| Dyspnoea (mMRC) | 2.07 ± 0.70 | 2.13 ± 0.64 | 0.06 | 2.19 ± 0.57 | 1.72 ± 0.05 | 0.04 1 |
| Control Group | Intervention Group | |||||
|---|---|---|---|---|---|---|
| Before | After | p | Before | After | p | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||
| W peak (%) | 62.7 ± 21.46 | 63.1 ± 17.34 | 0.242 | 67.2 ± 16.3 | 67.4 ± 11.9 | 0.92 1 |
| VO2 max (mL/min/kg) | 13 ± 2.9 | 13.3 ± 3.4 | 0.57 1 | 14.4 ± 3.2 | 14.9 ± 3.0 | 0.17 1 |
| VO2 peak (%) | 59.8 ± 14.6 | 62.2 ± 14.14 | 0.30 1 | 62.9 ± 15.8 | 66.8 ± 15.5 | 0.001 2 |
| HR (bpm) | 117.13 ± 13.88 | 102.87 ± 29.04 | 0.041 | 116.25 ± 14.31 | 123.69 ± 20.5 | 0.051 |
| HR(%) | 73.6 ± 8.1 | 61.3 ± 26.9 | 0.22 2 | 68.4 ± 10.1 | 61.7 ± 20.5 | 0.49 2 |
| O2/HR(%) | 79.2 ± 12.2 | 81 ± 16.9 | 0.59 1 | 83.4 ± 15.8 | 83.4 ± 15.8 | 0.51 1 |
| VE Max (L/min) | 34.5 ± 7.2 | 33.5 ± 11.2 | 0.65 1 | 39.8 ± 16.6 | 41.5 ± 18.4 | 0.46 1 |
| EqCO2 | 31.2 ± 3.0 | 31.5 ± 4.5 | 0.73 2 | 32.4 ± 4.6 | 32.1 ± 4.1 | 0.37 2 |
| RER | 0.99 ± 0.1 | 0.92 ± 0.1 | 0.09 1 | 1.03 ± 0.01 | 1.01 ± 0.1 | 0.19 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cedeño de Jesús, S.; Almadana Pacheco, V.; Valido Morales, A.; Muñíz Rodríguez, A.M.; Ayerbe García, R.; Arnedillo-Muñoz, A. Exercise Capacity and Physical Activity in Non-Cystic Fibrosis Bronchiectasis after a Pulmonary Rehabilitation Home-Based Programme: A Randomised Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 11039. https://doi.org/10.3390/ijerph191711039
Cedeño de Jesús S, Almadana Pacheco V, Valido Morales A, Muñíz Rodríguez AM, Ayerbe García R, Arnedillo-Muñoz A. Exercise Capacity and Physical Activity in Non-Cystic Fibrosis Bronchiectasis after a Pulmonary Rehabilitation Home-Based Programme: A Randomised Controlled Trial. International Journal of Environmental Research and Public Health. 2022; 19(17):11039. https://doi.org/10.3390/ijerph191711039
Chicago/Turabian StyleCedeño de Jesús, Sindy, Virginia Almadana Pacheco, Agustín Valido Morales, Ana Miriam Muñíz Rodríguez, Rut Ayerbe García, and Aurelio Arnedillo-Muñoz. 2022. "Exercise Capacity and Physical Activity in Non-Cystic Fibrosis Bronchiectasis after a Pulmonary Rehabilitation Home-Based Programme: A Randomised Controlled Trial" International Journal of Environmental Research and Public Health 19, no. 17: 11039. https://doi.org/10.3390/ijerph191711039





