Associations between Advanced Glycation End Products, Body Composition and Mediterranean Diet Adherence in Kidney Transplant Recipients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Body Composition and Anthropometric Measurement
2.2. Mediterranean Diet Serving Score
2.3. Medical History, Clinical and Laboratory Parameters
2.4. Advanced Glycation End Products (AGE) Measurement
2.5. Statistical Analyses
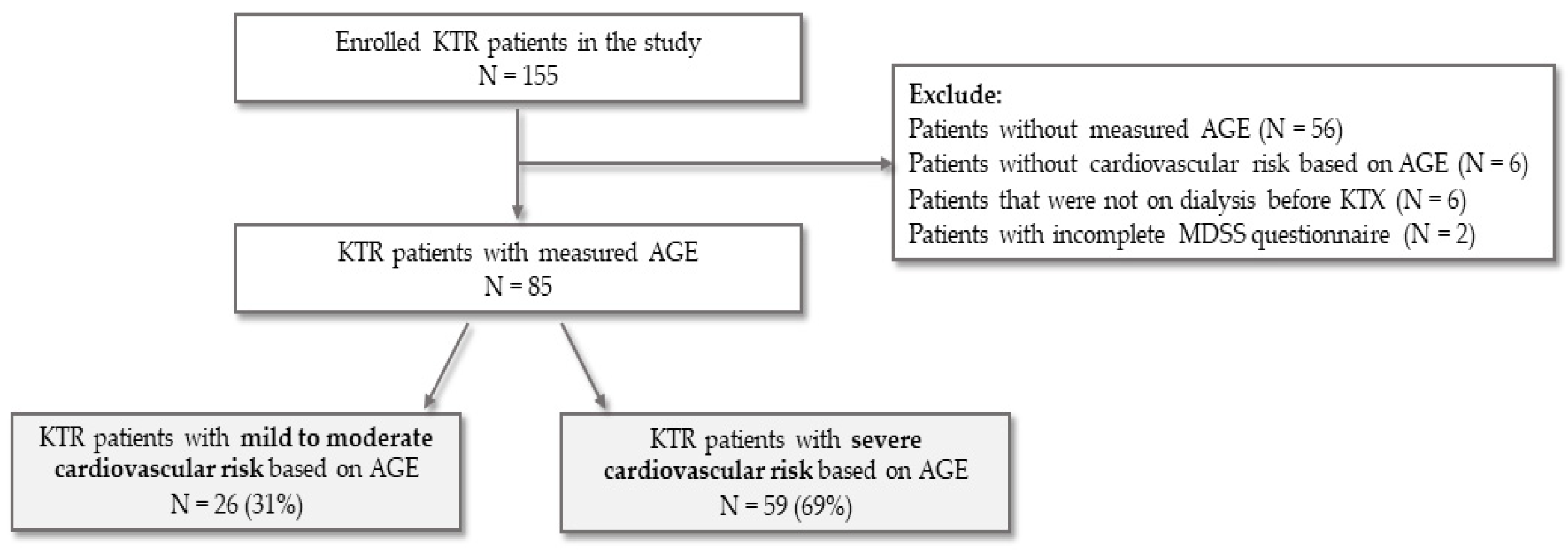
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Devine, P.A.; Courtney, A.E.; Maxwell, A.P. Cardiovascular risk in renal transplant recipients. J. Nephrol. 2019, 32, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Shirali, A.C.; Bia, M.J. Management of Cardiovascular Disease in Renal Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2008, 3, 491–504. [Google Scholar] [CrossRef]
- Neale, J. Cardiovascular risk factors following renal transplant. World J. Transplant. 2015, 5, 183. [Google Scholar] [CrossRef]
- Gill, J.S. Cardiovascular Disease in Transplant Recipients: Current and Future Treatment Strategies. Clin. J. Am. Soc. Nephrol. 2008, 3, S29–S37. [Google Scholar] [CrossRef]
- Chan, M.; Chadban, S. Nutritional management of kidney transplantation. In Nutritional Management of Renal Disease; Elsevier: Amsterdam, The Netherlands, 2022; pp. 607–627. [Google Scholar]
- de Vries, A.P.J.; Bakker, S.J.L.; van Son, W.J.; van der Heide, J.J.H.; Ploeg, R.J.; The, H.T.; de Jong, P.E.; Gans, R.O.B. Metabolic Syndrome Is Associated with Impaired Long-term Renal Allograft Function; Not All Component criteria Contribute Equally. Am. J. Transplant. 2004, 4, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Calviño, J.; Cigarran, S.; Gonzalez-Tabares, L.; Menendez, N.; Latorre, J.; Cillero, S.; Millan, B.; Cobelo, C.; Sanjurjo-Amado, A.; Quispe, J.; et al. Advanced glycation end products (AGEs) estimated by skin autofluorescence are related with cardiovascular risk in renal transplant. PLoS ONE 2018, 13, e0201118. [Google Scholar] [CrossRef] [PubMed]
- Sotomayor, C.G.; Gomes-Neto, A.W.; van Londen, M.; Gans, R.O.B.; Nolte, I.M.; Berger, S.P.; Navis, G.J.; Rodrigo, R.; Leuvenink, H.G.D.; Schalkwijk, C.G.; et al. Circulating Advanced Glycation Endproducts and Long-Term Risk of Cardiovascular Mortality in Kidney Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2019, 14, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, K.; Wang, Z.; Liu, C.; Han, Z.; Tao, J.; Lu, P.; Wang, J.; Wu, B.; Huang, Z.; et al. Advanced glycation end products accelerate arteriosclerosis after renal transplantation through the AGE/RAGE/ILK pathway. Exp. Mol. Pathol. 2015, 99, 312–319. [Google Scholar] [CrossRef]
- Hartog, J.W.; Smit, A.J.; van Son, W.J.; Navis, G.; Gans, R.O.; Wolffenbuttel, B.H.; de Jong, P.E. Advanced glycation end products in kidney transplant patients: A putative role in the development of chronic renal transplant dysfunction. Am. J. Kidney Dis. 2004, 43, 966–975. [Google Scholar] [CrossRef]
- Slagter, J.S.; Outmani, L.; Tran, K.T.C.K.; Ijzermans, J.N.M.; Minnee, R.C. Robot-assisted kidney transplantation as a minimally invasive approach for kidney transplant recipients: A systematic review and meta-analyses. Int. J. Surg. 2022, 99, 106264. [Google Scholar] [CrossRef]
- Serni, S.; Pecoraro, A.; Sessa, F.; Gemma, L.; Greco, I.; Barzaghi, P.; Grosso, A.A.; Corti, F.; Mormile, N.; Spatafora, P.; et al. Robot-Assisted Laparoscopic Living Donor Nephrectomy: The University of Florence Technique. Front. Surg. 2021, 7, 588215. [Google Scholar] [CrossRef]
- Vignolini, G.; Greco, I.; Sessa, F.; Gemma, L.; Pecoraro, A.; Barzaghi, P.; Grosso, A.; Corti, F.; Mormile, N.; Martiriggiano, M.; et al. The University of Florence Technique for Robot-Assisted Kidney Transplantation: 3-Year Experience. Front. Surg. 2020, 7, 583798. [Google Scholar] [CrossRef] [PubMed]
- Segev, D.L.; Simpkins, C.E.; Thompson, R.E.; Locke, J.E.; Warren, D.S.; Montgomery, R.A. Obesity Impacts Access to Kidney Transplantation. J. Am. Soc. Nephrol. 2008, 19, 349–355. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, C.W.; Harrison, L.E.A.; Eldehni, M.T.; Jefferies, H.J.; Szeto, C.-C.; John, S.G.; Sigrist, M.K.; Burton, J.O.; Hothi, D.; Korsheed, S.; et al. Circulating Endotoxemia: A Novel Factor in Systemic Inflammation and Cardiovascular Disease in Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Shardlow, A.; McIntyre, N.J.; Kolhe, N.V.; Nellums, L.B.; Fluck, R.J.; McIntyre, C.W.; Taal, M.W. The association of skin autofluorescence with cardiovascular events and all-cause mortality in persons with chronic kidney disease stage 3: A prospective cohort study. PLOS Med. 2020, 17, e1003163. [Google Scholar] [CrossRef]
- Wang, A.Y.-M.; Wong, C.-K.; Yau, Y.-Y.; Wong, S.; Chan, I.H.-S.; Lam, C.W.-K. Skin Autofluorescence Associates With Vascular Calcification in Chronic Kidney Disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1784–1790. [Google Scholar] [CrossRef]
- Mulder, D.J.; Water, T.; Van De Lutgers, H.L.; Graaff, R.; Gans, R.O.; Zijlstra, F.; Smit, A.J. Skin Autofluorescence, a Novel Marker for Glycemic and Oxidative Stress-Derived Advanced Glycation Endproducts: An Overview of Current Clinical Studies, Evidence, and Limitations. Diabetes Technol. Ther. 2006, 8, 523–535. [Google Scholar] [CrossRef]
- Gomes-Neto, A.W.; Osté, M.C.J.; Sotomayor, C.G.; van den Berg, E.; Geleijnse, J.M.; Berger, S.P.; Gans, R.O.B.; Bakker, S.J.L.; Navis, G.J. Mediterranean style diet and kidney function loss in kidney transplant recipients. Clin. J. Am. Soc. Nephrol. 2020, 15, 238–246. [Google Scholar] [CrossRef]
- Goldfarb Cyrino, L.; Galpern, J.; Moore, L.; Borgi, L.; Riella, L.V. A Narrative Review of Dietary Approaches for Kidney Transplant Patients. Kidney Int. Reports 2021, 6, 1764–1774. [Google Scholar] [CrossRef]
- Osté, M.C.J.; Corpeleijn, E.; Navis, G.J.; Keyzer, C.A.; Soedamah-Muthu, S.S.; van den Berg, E.; Postmus, D.; de Borst, M.H.; Kromhout, D.; Bakker, S.J.L. Mediterranean style diet is associated with low risk of new-onset diabetes after renal transplantation. BMJ Open Diabetes Res. Care 2017, 5, e000283. [Google Scholar] [CrossRef] [Green Version]
- Vučković, M.; Radić, J.; Gelemanović, A.; Raos, H.; Bučan Nenadić, D.; Kolak, E.; Radić, M. Mediterranean Diet Adherence and Nutritional Status in Dalmatian Kidney Transplant Recipients—Are They Related? Nutrients 2021, 13, 3246. [Google Scholar] [CrossRef]
- Nafar, M.; Noori, N.; Jalali-Farahani, S.; Hosseinpanah, F.; Poorrezagholi, F.; Ahmadpoor, P.; Samadian, F.; Firouzan, A.; Einollahi, B. Mediterranean diets are associated with a lower incidence of metabolic syndrome one year following renal transplantation. Kidney Int. 2009, 76, 1199–1206. [Google Scholar] [CrossRef]
- Vučković, M.; Radić, J.; Gelemanović, A.; Bučan Nenadić, D.; Kolak, E.; Radić, M. Associations between Depression, Nutritional Status and Mediterranean Diet in Dalmatian Kidney Transplant Recipients. Nutrients 2021, 13, 4479. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, E.; Betriu, À.; Salas-Salvadó, J.; Pamplona, R.; Barbé, F.; Purroy, F.; Farràs, C.; Fernández, E.; López-Cano, C.; Mizab, C.; et al. Mediterranean diet, physical activity and subcutaneous advanced glycation end-products’ accumulation: A cross-sectional analysis in the ILERVAS project. Eur. J. Nutr. 2020, 59, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Mariscal, F.M.; Cardelo, M.P.; la Cruz, S.; Alcala-Diaz, J.F.; Roncero-Ramos, I.; Guler, I.; Vals-Delgado, C.; López-Moreno, A.; Luque, R.M.; Delgado-Lista, J.; et al. Reduction in Circulating Advanced Glycation End Products by Mediterranean Diet Is Associated with Increased Likelihood of Type 2 Diabetes Remission in Patients with Coronary Heart Disease: From the Cordioprev Study. Mol. Nutr. Food Res. 2021, 65, 1901290. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.M.; Leiva Balich, L.; Concha, M.J.; Mizon, C.; Bunout Barnett, D.; Barrera Acevedo, G.; Hirsch Birn, S.; Jimenez Jaime, T.; Henriquez, S.; Uribarri, J.; et al. Reduction of serum advanced glycation end-products with a low calorie Mediterranean diet. Nutr Hosp. 2015, 31, 2511–2517. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Arab, L.; Sun, K.; Nicklett, E.J.; Ferrucci, L. Fat Mass Is Inversely Associated with Serum Carboxymethyl-Lysine, An Advanced Glycation End Product, in Adults. J. Nutr. 2011, 141, 1726–1730. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Kubo, A.; Sugioka, Y.; Mitsui, R.; Fukuhara, N.; Nihei, F.; Takeda, Y. Relationship between advanced glycation end-product accumulation and low skeletal muscle mass in Japanese men and women. Geriatr. Gerontol. Int. 2017, 17, 785–790. [Google Scholar] [CrossRef]
- Eble, A.S.; Thorpe, S.R.; Baynes, J.W. Nonenzymatic glucosylation and glucose-dependent cross-linking of protein. J. Biol. Chem. 1983, 258, 9406–9412. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Du Yan, S.; Wautier, J.-L.; Stern, D. Activation of Receptor for Advanced Glycation End Products. Circ. Res. 1999, 84, 489–497. [Google Scholar] [CrossRef] [Green Version]
- URIBARRI, J.; CAI, W.; SANDU, O.; PEPPA, M.; GOLDBERG, T.; VLASSARA, H. Diet-Derived Advanced Glycation End Products Are Major Contributors to the Body’s AGE Pool and Induce Inflammation in Healthy Subjects. Ann. N. Y. Acad. Sci. 2005, 1043, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Keogh, J.B.; Deo, P.; Clifton, P.M. Differential Effects of Dietary Patterns on Advanced Glycation end Products: A Randomized Crossover Study. Nutrients 2020, 12, 1767. [Google Scholar] [CrossRef] [PubMed]
- Van Hecke, T.; Ho, P.L.; Goethals, S.; De Smet, S. The potential of herbs and spices to reduce lipid oxidation during heating and gastrointestinal digestion of a beef product. Food Res. Int. 2017, 102, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Henning, S.M.; Zhang, Y.; Zerlin, A.; Li, L.; Gao, K.; Lee, R.-P.; Karp, H.; Thames, G.; Bowerman, S.; et al. Antioxidant-rich spice added to hamburger meat during cooking results in reduced meat, plasma, and urine malondialdehyde concentrations. Am. J. Clin. Nutr. 2010, 91, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.J.; Lim, B.O.; Park, G.B.; Joo, S.T. Effects of Various Fiber Additions on Lipid Digestion during In Vitro Digestion of Beef Patties. J. Food Sci. 2009, 74, C653–C657. [Google Scholar] [CrossRef]
- Gorelik, S.; Ligumsky, M.; Kohen, R.; Kanner, J. The Stomach as a “Bioreactor”: When Red Meat Meets Red Wine. J. Agric. Food Chem. 2008, 56, 5002–5007. [Google Scholar] [CrossRef]
- Vulcain, E.; Goupy, P.; Caris-Veyrat, C.; Dangles, O. Inhibition of the metmyoglobin-induced peroxidation of linoleic acid by dietary antioxidants: Action in the aqueous vs. lipid phase. Free Radic. Res. 2005, 39, 547–563. [Google Scholar] [CrossRef]
- Pierre, F. Meat and cancer: Haemoglobin and haemin in a low-calcium diet promote colorectal carcinogenesis at the aberrant crypt stage in rats. Carcinogenesis 2003, 24, 1683–1690. [Google Scholar] [CrossRef]
- Baye, E.; Kiriakova, V.; Uribarri, J.; Moran, L.J.; de Courten, B. Consumption of diets with low advanced glycation end products improves cardiometabolic parameters: Meta-analysis of randomised controlled trials. Sci. Rep. 2017, 7, 2266. [Google Scholar] [CrossRef]
- Bettiga, A.; Fiorio, F.; Di Marco, F.; Trevisani, F.; Romani, A.; Porrini, E.; Salonia, A.; Montorsi, F.; Vago, R. The Modern Western Diet Rich in Advanced Glycation End-Products (AGEs): An Overview of Its Impact on Obesity and Early Progression of Renal Pathology. Nutrients 2019, 11, 1748. [Google Scholar] [CrossRef] [Green Version]
- Mc780—User Manual. Available online: https://tanita.eu/media/wysiwyg/manuals/medical-approved-body-composition-monitors/mc-780-portable-instruction-manual.pdf (accessed on 20 June 2018).
- Monteagudo, C.; Mariscal-Arcas, M.; Rivas, A.; Lorenzo-Tovar, M.L.; Tur, J.A.; Olea-Serrano, F. Proposal of a mediterranean diet serving score. PLoS ONE 2015, 10, 1–13. [Google Scholar] [CrossRef]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Almengló, C.; Rodriguez-Ruiz, E.; Alvarez, E.; López-Lago, A.; González-Juanatey, J.R.; Garcia-Allut, J.L. Minimal invasive fluorescence methods to quantify advanced glycation end products (AGEs) in skin and plasma of humans. Methods 2021, 203, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Diagnoptics. Available online: https://www.diagnoptics.com/agereaderapp/ (accessed on 23 June 2019).
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2020. Available online: https://Www.R-Project.Org/ (accessed on 24 April 2020).
- Friedman, J.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Tibshirani, R.; Tibshirani, R.; Taylor, J.; Loftus, S.R.J. SelectiveInference: Tools for Post-Selection Inference. 2019. Available online: https://cran.r-project.org/web/packages/selectiveInference/ (accessed on 1 March 2022).
- Viramontes Hörner, D.; Selby, N.M.; Taal, M.W. Skin autofluorescence and malnutrition as predictors of mortality in persons receiving dialysis: A prospective cohort study. J. Hum. Nutr. Diet. 2020, 33, 852–861. [Google Scholar] [CrossRef]
- Cavero-Redondo, I.; Soriano-Cano, A.; Álvarez-Bueno, C.; Cunha, P.G.; Martínez-Hortelano, J.A.; Garrido-Miguel, M.; Berlanga-Macías, C.; Martínez-Vizcaíno, V. Skin Autofluorescence–Indicated Advanced Glycation End Products as Predictors of Cardiovascular and All-Cause Mortality in High-Risk Subjects: A Systematic Review and Meta-analysis. J. Am. Heart Assoc. 2018, 7, e009833. [Google Scholar] [CrossRef]
- Arsov, S.; Graaff, R.; van Oeveren, W.; Stegmayr, B.; Sikole, A.; Rakhorst, G.; Smit, A.J. Advanced glycation end-products and skin autofluorescence in end-stage renal disease: A review. Clin. Chem. Lab. Med. 2014, 52, 11–20. [Google Scholar] [CrossRef]
- Ueno, H.; Koyama, H.; Fukumoto, S.; Tanaka, S.; Shoji, T.; Shoji, T.; Emoto, M.; Tahara, H.; Inaba, M.; Kakiya, R.; et al. Advanced glycation end products, carotid atherosclerosis, and circulating endothelial progenitor cells in patients with end-stage renal disease. Metabolism 2011, 60, 453–459. [Google Scholar] [CrossRef]
- Hartog, J.W.L.; de Vries, A.P.J.; Bakker, S.J.L.; Graaff, R.; van Son, W.J.; van der Heide, J.J.H.; Gans, R.O.B.; Wolffenbuttel, B.H.R.; de Jong, P.E.; Smit, A.J. Risk factors for chronic transplant dysfunction and cardiovascular disease are related to accumulation of advanced glycation end-products in renal transplant recipients. Nephrol. Dial. Transplant. 2006, 21, 2263–2269. [Google Scholar] [CrossRef]
- Indyk, D.; Bronowicka-Szydełko, A.; Gamian, A.; Kuzan, A. Advanced glycation end products and their receptors in serum of patients with type 2 diabetes. Sci. Rep. 2021, 11, 13264. [Google Scholar] [CrossRef]
- Connolly, G.M.; Cunningham, R.; McNamee, P.T.; Young, I.S.; Maxwell, A.P. Elevated Serum Phosphate Predicts Mortality in Renal Transplant Recipients. Transplantation 2009, 87, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Giachelli, C.M. The emerging role of phosphate in vascular calcification. Kidney Int. 2009, 75, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Ermak, G.; Davies, K.J. Calcium and oxidative stress: From cell signaling to cell death. Mol. Immunol. 2002, 38, 713–721. [Google Scholar] [CrossRef]
- Silva, D.M.; Queiroz, N.P.; Freitas, A.T.V.S.; Passarelli, M.; Corgosinho1, F.C.; Peixoto, M. do R.G. Serum advanced glycation end products are not associated with muscle strength in hemodialysis patients. Eur. J. Clin. Nutr. 2019, 73, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Anderson, J.E. Cardiovascular and Survival Paradoxes in Dialysis Patients: Reverse Epidemiology in Patients with Chronic Kidney Disease Who Are Not Yet on Dialysis. Semin. Dial. 2007, 20, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Chavalitdhamrong, D.; Danovitch, G.M.; Bunnapradist, S. Cardiovascular and survival paradoxes in dialysis patients: Is There a Reversal of Reverse Epidemiology in Renal Transplant Recipients? Semin. Dial. 2007, 20, 544–548. [Google Scholar] [CrossRef]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced Glycation End Products in Foods and a Practical Guide to Their Reduction in the Diet. J. Am. Diet. Assoc. 2010, 110, 911–916.e12. [Google Scholar] [CrossRef]
- DeChristopher, L.R. Perspective: The Paradox in Dietary Advanced Glycation End Products Research—The Source of the Serum and Urinary Advanced Glycation End Products Is the Intestines, Not the Food. Adv. Nutr. An Int. Rev. J. 2017, 8, 679–683. [Google Scholar] [CrossRef]
- Kosoku, A.; Uchida, J.; Nishide, S.; Kabei, K.; Shimada, H.; Iwai, T.; Maeda, K.; Hanayama, Y.; Ishihara, T.; Naganuma, T.; et al. Association of sarcopenia with phase angle and body mass index in kidney transplant recipients. Sci. Rep. 2020, 10, 266. [Google Scholar] [CrossRef]
- Kaya, E.; Bakir, A.; Koseoglu, Y.K.; Velidedeoglu, M.; Trabulus, S.; Seyahi, N. Association of Nutritional Assessment by Phase Angle With Mortality in Kidney Transplant Patients in an 8-Year Follow-Up. Prog. Transplant. 2019, 29, 321–326. [Google Scholar] [CrossRef]
| Total (N = 85) | Mild to Moderate CV Risk Based on AGE (N = 26) | Severe CV Risk Based on AGE (N = 59) | p * | |
|---|---|---|---|---|
| Age (years), median (IQR) | 63 (15) | 54 (28.5) | 67 (12) | <0.001 |
| Sex, N (%) | ||||
| Women | 35 (41.18) | 12 (46.15) | 23 (38.98) | 0.704 |
| Men | 50 (58.82) | 14 (53.85) | 36 (61.02) | |
| AGE, median (IQR) | 3.3 (1.2) | 2.6 (0.28) | 3.6 (0.95) | <0.001 |
| Time since kidney transplantation (years), median (IQR) | 8 (9) | 4.5 (8.75) | 8 (10.5) | 0.109 |
| Dialysis duration (years), median (IQR) | 2 (3.75) | 2 (1.98) | 2.5 (4.69) | 0.082 |
| Dialysis type, N (%) | ||||
| PD | 27 (31.76) | 15 (57.69) | 12 (20.34) | 0.003 |
| HD | 43 (50.59) | 8 (30.77) | 35 (59.32) | |
| PD + HD | 15 (17.65) | 3 (11.54) | 12 (20.34) | |
| Smoking status, N (%) | ||||
| Never | 35 (41.18) | 14 (63.64) | 21 (52.50) | 0.353 |
| Ex | 15 (17.65) | 3 (13.64) | 12 (30.00) | |
| Current | 12 (14.11) | 5 (22.73) | 7 (17.50) | |
| COMORBIDITIES | ||||
| Presence of arterial hypertension, N (%) | ||||
| No | 5 (5.88) | 2 (7.69) | 3 (5.08) | 1.000 |
| Yes | 80 (94.12) | 24 (92.31) | 56 (94.92) | |
| Presence of diabetes mellitus, N (%) | ||||
| No | 65 (76.47) | 22 (84.62) | 43 (72.88) | 0.369 |
| Yes | 20 (23.53) | 4 (15.38) | 16 (27.12) | |
| Presence of chronic kidney disease, N (%) | ||||
| eGFR > 60 mL/min/1.73 m2 | 25 (29.41) | 14 (53.85) | 11 (18.64) | 0.002 |
| eGFR < 60 mL/min/1.73 m2 | 60 (70.59) | 12 (46.15) | 48 (81.36) | |
| Presence of CVD, N (%) | ||||
| No | 60 (70.59) | 21 (80.77) | 39 (66.1) | 0.267 |
| Yes | 25 (29.41) | 5 (19.23) | 20 (33.9) | |
| Presence of CVA, N (%) | ||||
| No | 74 (87.06) | 23 (88.46) | 51 (86.44) | 1.000 |
| Yes | 11 (12.94) | 3 (11.54) | 8 (13.56) | |
| LABORATORY PARAMETERS | ||||
| Alb (g/L), mean (SD) | 41.76 (2.93) | 42.18 (2.77) | 41.59 (3) | 0.420 |
| Ca (mmol/L), median (IQR) | 2.42 (0.16) | 2.41 (0.15) | 2.42 (0.17) | 0.682 |
| CRP (mg/L), median (IQR) | 2.15 (4) | 1.55 (2.67) | 2.65 (4.42) | 0.243 |
| E, mean (SD) | 4.69 (0.69) | 4.7 (0.72) | 4.69 (0.68) | 0.987 |
| Glucose (mmol/L), median (IQR) | 5.2 (0.9) | 5.15 (0.95) | 5.3 (1) | 0.834 |
| Hb (g/L), median (IQR) | 132.38 (16.65) | 132.35 (16.19) | 132.39 (16.98) | 0.991 |
| K (mmol/L), median (IQR) | 4.1 (0.6) | 4.1 (0.5) | 4 (0.7) | 0.924 |
| Total cholesterol (mmol/L), mean (SD) | 5.39 (1.06) | 5.39 (1.17) | 5.39 (1.02) | 0.992 |
| Creatinine (mmol/L), median (IQR) | 131 (52) | 115.5 (61.5) | 135 (46.5) | 0.063 |
| LDL cholesterol (mmol/L), mean (SD) | 3.13 (0.95) | 3.05 (1.08) | 3.16 (0.9) | 0.672 |
| MCV (fL), median (IQR) | 87.25 (7.1) | 87.2 (6.9) | 87.3 (7.15) | 0.765 |
| Na (mmol/L), median (IQR) | 140.7 (2.44) | 140.32 (2.01) | 140.87 (2.61) | 0.355 |
| P (mmol/L), median (IQR) | 1.03 (0.23) | 1.04 (0.24) | 1 (0.2) | 0.264 |
| Tgl (mmol/L), median (IQR) | 1.8 (1.1) | 1.9 (1) | 1.71 (1.07) | 0.370 |
| Uric acid (mmol/L), mean (SD) | 390.34 (91.2) | 377.12 (100.86) | 396.03 (87.02) | 0.389 |
| Urea (mmol/L), median (IQR) | 9.7 (5.7) | 8 (3.47) | 10.8 (4.85) | 0.002 |
| eGFR (ml/min/1.73 m2), median (IQR) | 43 (28.6) | 61.95 (34.43) | 39.7 (16.55) | 0.009 |
| Total (N = 85) | Mild to Moderate CV Risk Based on AGE (N = 26) | Severe CV Risk Based on AGE (N = 59) | p * | |
|---|---|---|---|---|
| ANTHROPOMETRIC PARAMETERS | ||||
| Height (cm), mean (SD) | 174.2 (9.99) | 174.92 (10.81) | 173.89 (9.71) | 0.677 |
| Weight (kg), mean (SD) | 81.26 (15.59) | 84.31 (17.34) | 79.98 (14.76) | 0.256 |
| BMI (kg/m2), mean (SD) | 26.68 (4.09) | 27.52 (4.76) | 26.33 (3.76) | 0.236 |
| BMI (kg/m2) as categories, N (%) | ||||
| <25, normal weight | 28 (34.57) | 8 (33.33) | 20 (35.09) | 0.114 |
| 25–30, overweight | 37 (45.68) | 8 (33.33) | 29 (50.88) | |
| >30, obese | 16 (19.75) | 8 (33.33) | 8 (14.04) | |
| Middle upper arm circumference (cm), median (IQR) | 31 (5.25) | 31 (3) | 31 (5.5) | 0.705 |
| Waist circumference (cm), mean (SD) | 99.48 (12.1) | 99.9 (13.77) | 99.31 (11.52) | 0.851 |
| WHtR, mean (SD) | 0.57 (0.07) | 0.57 (0.07) | 0.57 (0.07) | 0.916 |
| BODY COMPOSITION | ||||
| Fat mass (kg), mean (SD) | 20.5 (8.71) | 23.36 (8.59) | 19.28 (8.54) | 0.054 |
| Fat mass (%), mean (SD) | 24.7 (8.87) | 27.31 (7.27) | 23.59 (9.31) | 0.086 |
| Fat-free mass (kg), mean (SD) | 60.96 (12) | 60.96 (12.35) | 60.96 (11.96) | 1.000 |
| Visceral fat (level), mean (SD) | 9.83 (3.85) | 9 (4.4) | 10.2 (3.55) | 0.204 |
| Metabolic age (year), mean (SD) | 51.14 (13.16) | 46.38 (16.07) | 53.18 (11.25) | 0.033 |
| Muscle mass (kg), mean (SD) | 57.92 (11.44) | 57.92 (11.77) | 57.92 (11.4) | 0.998 |
| Skeletal muscle mass (kg), median (IQR) | 31.8 (12) | 30.5 (12.92) | 32.1 (11.67) | 0.975 |
| Skeletal muscle mass (%), median (IQR) | 40.26 (6.29) | 38.82 (5.22) | 40.88 (6.64) | 0.182 |
| Phase angle (°), median (IQR) | 5.09 (0.75) | 5.36 (0.67) | 4.98 (0.76) | 0.036 |
| Trunk fat mass (kg), median (IQR) | 10.45 (5.72) | 12.55 (5.85) | 10.3 (5.45) | 0.067 |
| Mediterranean Diet Serving Score (MDSS) | ||||
| Total MDSS points, median (IQR) | 8 (4) | 8.5 (3) | 8 (5.5) | 0.882 |
| Adherence to MeDi, N (%) | ||||
| MDSS < 14 points | 75 (88.24) | 24 (92.31) | 51 (86.44) | 0.683 |
| MDSS ≥ 14 points | 10 (11.76) | 2 (7.69) | 8 (13.56) | |
| Adherence to specific food or food group | ||||
| Fruits (no), N (%) | 56 (65.88) | 18 (69.23) | 38 (64.41) | 0.854 |
| Fruits (yes), N (%) | 29 (34.12) | 8 (30.77) | 21 (35.59) | |
| Vegetable (no), N (%) | 68 (80) | 22 (84.62) | 46 (77.97) | 0.680 |
| Vegetable (yes), N (%) | 17 (20) | 4 (15.38) | 13 (22.03) | |
| Cereals (no), N (%) | 45 (52.94) | 10 (38.46) | 35 (59.32) | 0.124 |
| Cereals (yes), N (%) | 40 (47.06) | 16 (61.54) | 24 (40.68) | |
| Potato (no), N (%) | 18 (21.18) | 4 (15.38) | 14 (23.73) | 0.562 |
| Potato (yes), N (%) | 67 (78.82) | 22 (84.62) | 45 (76.27) | |
| Olive oil (no), N (%) | 60 (70.59) | 17 (65.38) | 43 (72.88) | 0.659 |
| Olive oil (yes), N (%) | 25 (29.41) | 9 (34.62) | 16 (27.12) | |
| Nuts (no), N (%) | 72 (84.71) | 24 (92.31) | 48 (81.36) | 0.334 |
| Nuts (yes), N (%) | 13 (15.29) | 2 (7.69) | 11 (18.64) | |
| Dairy (no), N (%) | 28 (32.94) | 10 (38.46) | 18 (30.51) | 0.639 |
| Dairy (yes), N (%) | 57 (67.06) | 16 (61.54) | 41 (69.49) | |
| Beans (no), N (%) | 57 (67.06) | 16 (61.54) | 41 (69.49) | 0.639 |
| Beans (yes), N (%) | 28 (32.94) | 10 (38.46) | 18 (30.51) | |
| Eggs (no), N (%) | 57 (67.06) | 16 (61.54) | 41 (69.49) | 0.639 |
| Eggs (yes), N (%) | 28 (32.94) | 10 (38.46) | 18 (30.51) | |
| Fish (no), N (%) | 50 (58.82) | 15 (57.69) | 35 (59.32) | 1.000 |
| Fish (yes), N (%) | 35 (41.18) | 11 (42.31) | 24 (40.68) | |
| White meat (no), N (%) | 59 (69.41) | 16 (61.54) | 43 (72.88) | 0.429 |
| White meat (yes), N (%) | 26 (30.59) | 10 (38.46) | 16 (27.12) | |
| Red meat (no), N (%) | 52 (61.18) | 19 (73.08) | 33 (55.93) | 0.210 |
| Red meat (yes), N (%) | 33 (38.82) | 7 (26.92) | 26 (44.07) | |
| Sweets (no), N (%) | 37 (43.53) | 17 (65.38) | 20 (33.9) | 0.014 |
| Sweets (yes), N (%) | 48 (56.47) | 9 (34.62) | 39 (66.1) | |
| Alcohol (no), N (%) | 71 (83.53) | 22 (84.62) | 49 (83.05) | 1.000 |
| Alcohol (yes), N (%) | 14 (16.47) | 4 (15.38) | 10 (16.95) | |
| Consumption frequency of each food or food group ** | ||||
| Fruits, median (IQR) | 2 (1) | 2 (1) | 2 (1) | 0.653 |
| Vegetable, median (IQR) | 2 (1) | 2 (1) | 2 (1) | 0.689 |
| Cereals, median (IQR) | 2 (1) | 1 (1) | 2 (1) | 0.186 |
| Potato, median (IQR) | 3 (1) | 3 (1) | 3 (1) | 0.931 |
| Olive oil, median (IQR) | 2 (2) | 2 (2) | 2 (2) | 0.794 |
| Nuts, median (IQR) | 5 (3) | 4 (2.75) | 5 (3) | 0.368 |
| Dairy, median (IQR) | 2 (1) | 2 (1) | 2 (1) | 0.485 |
| Beans, median (IQR) | 5 (1) | 5 (1.75) | 5 (1) | 0.374 |
| Eggs, median (IQR) | 5 (1) | 5 (1) | 5 (1) | 0.365 |
| Fish, median (IQR) | 5 (1) | 5 (1) | 5 (1) | 0.808 |
| White meat, median (IQR) | 4 (1) | 4 (1) | 4 (2) | 0.623 |
| Red meat, median (IQR) | 4 (2) | 4 (1.75) | 4 (2) | 0.184 |
| Sweets, median (IQR) | 4 (4) | 3 (3) | 5 (3) | 0.018 |
| Alcohol, median (IQR) | 7 (2.5) | 6.5 (2) | 7 (3) | 0.215 |
| LASSO Regression (Severe vs. Mild to Moderate CV Risk Based on AGE) | LASSO Regression with AGE | |||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | Lower CI | Upper CI | p * | Coefficient | Lower CI | Upper CI | p * | |
| Age (years) | 0.098 | 0.041 | 0.260 | 0.001 | 0.018 | 0.005 | 0.032 | 0.005 |
| Dialysis (type) | 1.621 | −2.210 | 2.418 | 0.273 | 0.095 | −0.45 | 0.322 | 0.411 |
| Dialysis (years) | / | 0.030 | −0.036 | 0.074 | 0.171 | |||
| Comorbidities | ||||||||
| CV disease (yes) | / | 0.154 | −0.629 | 0.493 | 0.383 | |||
| CKD (yes) | 1.670 | 0.403 | 40.596 | 0.017 | 0.435 | 0.028 | 0.799 | 0.018 |
| Laboratory parameters | ||||||||
| Ca (mmol/L) | −1.322 | −27.967 | 17.838 | 0.371 | / | |||
| CRP (mg/L) | 0.075 | −2.912 | 0.370 | 0.828 | 0.029 | −0.021 | 0.064 | 0.113 |
| P (mmol/L) | / | −0.293 | −1.039 | 1.889 | 0.479 | |||
| Uric acid (mmol/L) | / | 0.002 | 0 | 0.004 | 0.008 | |||
| Urea (mmol/L) | 0.002 | −7.341 | 0.116 | 0.950 | / | |||
| Body composition parameters | ||||||||
| Skeletal muscle mass (%) | 0.092 | −0.348 | 1.033 | 0.221 | / | |||
| Trunk fat mass (kg) | −0.021 | −0.942 | 0.798 | 0.451 | −0.046 | −0.075 | −0.015 | 0.002 |
| Mediterranean diet | ||||||||
| Cereals (yes) | −1.017 | −2.528 | 1.646 | 0.218 | / | |||
| Nuts (yes) | / | 0.454 | −0.028 | 0.866 | 0.031 | |||
| Red meat (yes) | 0.851 | −4.441 | 1.983 | 0.490 | / | |||
| Sweets (yes) | 0.926 | −1.365 | 9.593 | 0.112 | / | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radić, J.; Vučković, M.; Gelemanović, A.; Kolak, E.; Bučan Nenadić, D.; Begović, M.; Radić, M. Associations between Advanced Glycation End Products, Body Composition and Mediterranean Diet Adherence in Kidney Transplant Recipients. Int. J. Environ. Res. Public Health 2022, 19, 11060. https://doi.org/10.3390/ijerph191711060
Radić J, Vučković M, Gelemanović A, Kolak E, Bučan Nenadić D, Begović M, Radić M. Associations between Advanced Glycation End Products, Body Composition and Mediterranean Diet Adherence in Kidney Transplant Recipients. International Journal of Environmental Research and Public Health. 2022; 19(17):11060. https://doi.org/10.3390/ijerph191711060
Chicago/Turabian StyleRadić, Josipa, Marijana Vučković, Andrea Gelemanović, Ela Kolak, Dora Bučan Nenadić, Mirna Begović, and Mislav Radić. 2022. "Associations between Advanced Glycation End Products, Body Composition and Mediterranean Diet Adherence in Kidney Transplant Recipients" International Journal of Environmental Research and Public Health 19, no. 17: 11060. https://doi.org/10.3390/ijerph191711060
APA StyleRadić, J., Vučković, M., Gelemanović, A., Kolak, E., Bučan Nenadić, D., Begović, M., & Radić, M. (2022). Associations between Advanced Glycation End Products, Body Composition and Mediterranean Diet Adherence in Kidney Transplant Recipients. International Journal of Environmental Research and Public Health, 19(17), 11060. https://doi.org/10.3390/ijerph191711060







