Toxic Metals and Metalloids in Infant Formulas Marketed in Brazil, and Child Health Risks According to the Target Hazard Quotients and Target Cancer Risk
Abstract
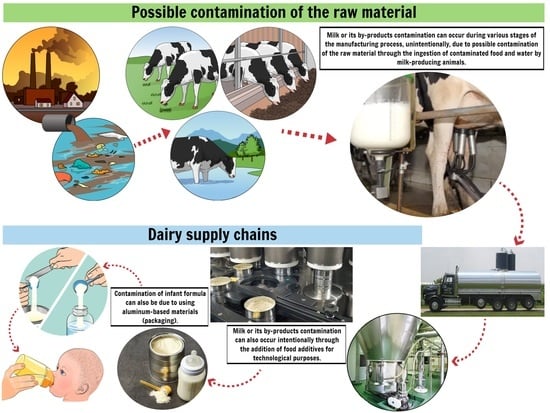
:1. Introduction
2. Material and Methods
2.1. Sample Selection
2.2. Reagents
2.3. Sample Preparation and Metal and Metalloid Determinations
2.4. Infant Potential Health Risk
2.4.1. Target Hazard Quotient
2.4.2. Target Cancer Risk
2.5. Statistical Analyses
3. Results and Discussion
3.1. Method Accuracy and Precision
3.2. Metal and Metalloid Contents in Infant Formulas
3.3. Metal and Metalloid Contents in Different Infant Formula Batches from the Same Manufacturer
3.4. Estimating Infant Health Risk for Toxic Metals and Metalloids Found in Infant Formulas
3.4.1. Target Hazard Quotient
3.4.2. Target Cancer Risk
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. 2006. Available online: https://www.who.int/publications/i/item/924154693X (accessed on 11 January 2022).
- Ministry of Health Brazil (BRAZIL). Saúde da Criança: Aleitamento Materno e Alimentação Complementar. Ministério da Saúde, Secretaria de Atenção à Saúde. 2015. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/saude_crianca_aleitamento_materno_cab23.pdf (accessed on 10 January 2022).
- Almeida, C.C.; Mendonça Pereira, B.F.; Leandro, K.C.; Costa, M.P.; Spisso, B.F.; Conte-Junior, C.A. Bioactive compounds in infant formula and their effects on infant nutrition and health: A systematic literature review. Int. J. Food Sci. 2021, 2021, 8850080. [Google Scholar] [CrossRef]
- American Academy of Pediatrics (APP). Policy statement: Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef]
- Almeida, C.C.; Baião, D.; Leandro, K.C.; Paschoalin, V.; Costa, M.; Conte-Junior, C.A. Protein quality in infant formulas marketed in Brazil: Assessments on biodigestibility, essential amino acid content and proteins of biological importance. Nutrients 2021, 13, 3933. [Google Scholar] [CrossRef]
- Brazilian Health Regulatory Agency (Anvisa) (BRAZIL). RDC n° 44, de 19 de Setembro de 2011. Regulamento Técnico para Fórmulas Infantis de Seguimento para Lactentes e Crianças de Primeira Infância. Diário Oficial da União. 20 set. 2011; Seção 1. 2011. Available online: http://www.ibfan.org.br/site/wp-content/uploads/2014/06/Resolucao_RDC_n_43_de_19_de_setembro_de_2011.pdf (accessed on 10 January 2022).
- Codex Alimentarius. Standard for Infant Formula and Formulas for Special Medical Purposes Intended for Infants. 2007. Available online: https://www.ibfan.org/wp-content/uploads/2019/05/standard-for-infant-formula-and-formulas-for-special-medical-purposes-intended-for-infants.pdf (accessed on 10 January 2022).
- European Commission. Commission Directive 2006/141/EC of 22 December 2006 on Infant Formula and Follow-on Formulae and Amending Directive 1999/21/EC. 2006. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006L0141&from=EN (accessed on 10 January 2022).
- de Mendonça Pereira, B.F.; de Almeida, C.C.; Leandro, K.C.; da Costa, M.P.; Conte-Junior, C.A.; Spisso, B.F. Occurrence, sources, and pathways of chemical contaminants in infant formulas. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1378–1396. [Google Scholar] [CrossRef]
- Năstăsescu, V.; Mititelu, M.; Goumenou, M.; Docea, A.O.; Renieri, E.; Udeanu, D.I.; Oprea, E.; Arsene, A.L.; Dinu-Pîrvu, C.E.; Ghica, M. Heavy metal and pesticide levels in dairy products: Evaluation of human health risk. Food Chem. Toxicol. 2020, 146, 111844. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metals Toxicity and the Environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef]
- Codex Alimentarius. General Standard for Contaminants and Toxins in Food and Feed. 2019. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B193-1995%252FCXS_193e.pdf (accessed on 10 June 2022).
- United States Environmental Protection Agency (U.S. EPA). Method 6020B (SW-846): Inductively Coupled Plasma-Mass Spectrometry, Revision 2. Washington, DC. 2014. Available online: https://www.epa.gov/sites/default/files/2015-12/documents/6020b.pdf (accessed on 6 September 2021).
- Almeida, C.C.; Baião, D.; Rodrigues, P.A.; Saint’Pierre, T.D.; Hauser-Davis, R.A.; Leandro, K.C.; Paschoalin, V.; da Costa, M.P.; Conte-Junior, C.A. Macrominerals and trace minerals in commercial infant formulas marketed in Brazil: Compliance with established minimum and maximum requirements, label statements, and estimated daily intake. Front. Nutr. 2022, 9, 857698. [Google Scholar] [CrossRef]
- National Institute of Metrology, Standardization and Industrial Quality (INMETRO) (BRAZIL). Guidance on validation of analytical methods: Guidance document. DOQ-CGCRE-008. 2020. Available online: http://www.inmetro.gov.br/Sidoq/Arquivos/Cgcre/DOQ/DOQ-Cgcre-8_08.pdf (accessed on 5 December 2021).
- United States Environmental Protection Agency (U.S. EPA). Risk-Assessment Guidance for Superfund. Volume 1. Human Health Evaluation Manual. Part A. Interim Report (Final). 2009. Available online: https://www.epa.gov/sites/default/files/2015-09/documents/partf_200901_final.pdf (accessed on 11 November 2021).
- United States Environmental Protection Agency (U.S. EPA). Regional Screening Level (RSL) Subchronic Toxicity Supporting. 2022. Available online: https://semspub.epa.gov/work/HQ/402403.pdf (accessed on 4 July 2022).
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Tin; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2005. Available online: https://www.atsdr.cdc.gov/ToxProfiles/tp55-c8.pdf (accessed on 2 May 2021).
- Antoine, J.; Fung, L.; Grant, C.N. Assessment of the potential health risks associated with the aluminium, arsenic, cadmium and lead content in selected fruits and vegetables grown in Jamaica. Toxicol. Rep. 2017, 4, 181–187. [Google Scholar] [CrossRef]
- Office Environmental Health Hazard Assessment (OEHHA). Technical Support Document for Cancer Potency Factors Appendix H. 2009. Available online: https://oehha.ca.gov/media/downloads/crnr/appendixhexposure.pdf (accessed on 1 July 2022).
- Ishak, I.; Rosli, F.D.; Mohamed, J.; Ismail, M.F.M. Comparison of digestion methods for the determination of trace elements and heavy metals in human hair and nails. Malays. J. Med. Sci. 2015, 22, 11–20. [Google Scholar]
- Magnusson, B.; Örnemark, U. Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics, 2nd ed. 2014. Available online: https://www.eurachem.org/images/stories/Guides/pdf/MV_guide_2nd_ed_EN.pdf (accessed on 30 September 2021).
- Kazi, T.G.; Jalbani, N.; Baig, J.A.; Afridi, H.I.; Kandhro, G.A.; Arain, M.B.; Jamali, M.K.; Shah, A.Q. Determination of toxic elements in infant formulae by using electrothermal atomic absorption spectrometer. Food Chem. Toxicol. 2009, 47, 1425–1429. [Google Scholar] [CrossRef]
- Narin, I.; Tuzen, M.; Soylak, M. Aluminium determination in environmental samples by graphite furnace atomic absorption spectrometry after solid phase extraction on amberlite XAD-1180/pyrocatechol violet chelating resin. Talanta 2004, 63, 411–418. [Google Scholar] [CrossRef]
- Igweze, Z.N.; Ekhator, O.C.; Nwaogazie, I.; Orisakwe, O.E. Public health and paediatric risk assessment of aluminium, arsenic and mercury in infant formulas marketed in Nigeria. Sultan Qaboos Univ. Med. J. 2020, 20, e63–e70. [Google Scholar] [CrossRef]
- Domínguez, A.; Paz, S.; Rubio, C.; Gutiérrez, A.; González-Weller, D.; Revert, C.; Hardisson, A. Essential and toxic metals in infant formula from the European community. Open Access J. Toxicol. 2017, 2, 555585. [Google Scholar] [CrossRef]
- Ljung, K.; Palm, B.; Grandér, M.; Vahter, M. High concentrations of essential and toxic elements in infant formula and infant foods—A matter of concern. Food Chem. 2011, 127, 943–951. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum. 2017. Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 10 January 2022).
- Bohrer, D.; Nascimento, P.C.; Binotto, R.; Becker, E. Influence of the glass packing on the contamination of pharmaceutical products by aluminium. Part III: Interaction container-chemicals during the heating for sterilisation. J. Trace Elem. Med. Biol. 2003, 17, 107–115. [Google Scholar] [CrossRef]
- Campbell, A.; Becaria, A.; Lahiri, D.K.; Sharman, K.; Bondy, S.C. Chronic exposure to aluminum in drinking water increases inflammatory parameters selectively in the brain. J. Neurosci. Res. 2004, 75, 565–572. [Google Scholar] [CrossRef]
- Trzcinka-Ochocka, M.; Jakubowski, M.; Razniewska, G.; Halatek, T.; Gazewski, A. The effects of environmental cadmium exposure on kidney function: The possible influence of age. Environ. Res. 2004, 95, 143–150. [Google Scholar] [CrossRef]
- Tuzen, M.; Soylak, M. Evaluation of trace element contents in canned foods marketed from Turkey. Food Chem. 2007, 102, 1089–1095. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Crisponi, G.; Bertolasi, V.; Faa, G.; Remelli, M. Aluminium-dependent human diseases and chelating properties of aluminium chelators for biomedical applications. Met. Ions Neurol. Syst. 2012, 10, 103–123. [Google Scholar] [CrossRef]
- Goyer, R.A. Toxic and essential metal interactions. Annu. Rev. Nutr. 1997, 17, 37–50. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (U.S. EPA). Department of Health and Human Services. Reference Dose (RfD): Description and Use in Health Risk Assessments. Background Document 1A. 1993. Available online: https://www.epa.gov/iris/reference-dose-rfd-description-and-use-health-risk-assessments (accessed on 15 June 2022).
- Rogers, S.H.; Rardin, L.R.; Lawlor, K.; Chen, C.Y.; Borsuk, M.E. Communicating Arsenic’s Risks. Int. J. Environ. Res. Public Health 2019, 16, 3436. [Google Scholar] [CrossRef]
- Schuhmacher–Wolz, U.; Dieter, H.H.; Klein, D.; Schneider, K. Oral exposure to inorganic arsenic: Evaluation of its carcinogenic and non-carcinogenic effects. Crit. Rev. Toxicol. 2009, 39, 271–298. [Google Scholar] [CrossRef]
- Gundert-Remy, U.; Damm, G.; Foth, H.; Freyberger, A.; Gebel, T.; Golka, K.; Röhl, C.; Schupp, T.; Wollin, K.M.; Hengstler, J.G. High exposure to inorganic arsenic by food: The need for risk reduction. Arch. Toxicol. 2015, 89, 2219–2227. [Google Scholar] [CrossRef]
- Shibata, T.; Meng, C.; Umoren, J.; West, H. Risk Assessment of Arsenic in Rice Cereal and Other Dietary Sources for Infants and Toddlers in the U.S. Int. J. Environ. Res. Public Health 2016, 13, 361. [Google Scholar] [CrossRef] [Green Version]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Aluminum; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2008. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp22.pdf (accessed on 2 May 2021).
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Tin and Tin Compounds. U.S. Department of Health and Human Services. 2005. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp55.pdf (accessed on 25 May 2022).
| ICP-MS Condition | Value |
|---|---|
| Radiofrequency power | 1100 W |
| Plasma flow | 17.0 L·min−1 |
| Auxiliary gas flow | 1.2 L·min−1 |
| Carrier gas flow | 0.98 L·min−1 |
| Skimmer composition | Pt |
| Dwell time | 50 ms per isotope |
| Scanning mode | Peak hopping |
| Resolution | 0.7 uma (u) |
| Scans by reading | 5 |
| Toxic Elements | Reference Materials (mg·kg−1) | |||||
|---|---|---|---|---|---|---|
| Skimmed Milk Powder BD150® | Non-Fat Milk Powder 1549® | |||||
| Experimental | Reference | Recovery | Experimental | Reference | Recovery | |
| Hg | 0.072 ± 0.035 | 0.06 ± 0.007 | 120% | - | - | - |
| Cd | 0.010 ± 0.005 | 0.0114 ± 0.0029 | 87.1% | 0.0006 ± 0.011 | 0.0005 ± 0.0002 | 120% |
| Pb | 0.018 ± 0.870 | 0.019 ± 0.004 | 94.7% | 0.019 ± 0.016 | 0.019 ± 0.03 | 100% |
| IFs | Toxic Elements (mg·kg−1) | ||||||
|---|---|---|---|---|---|---|---|
| Al | As | Cd | Sn | Hg | Pb | U | |
| ME1 | 0.724 ± 0.141 b,c | 0.016 ± 0.004 b | 0.004 ± 0.001 a | 0.007 ± 0.003 c | <LOQ | <LOD | 0.005 ± 0.002 b,c |
| NC1 | 0.459 ± 0.177 c,d | <LOQ | 0.005 ± 0.002 a | 0.081 ± 0.022 a | <LOQ | <LOD | <LOD |
| NN1 | 0.504 ± 0.099 c | <LOQ | 0.004 ± 0.002 a | 0.054 ± 0.023 a,b | <LOQ | 0.036 ± 0.041 | <LOD |
| DM1 | 0.432 ± 0.049 d | 0.031 ± 0.003 a | 0.005 ± 0.002 a | 0.068 ± 0.022 a,b | <LOD | 0.016 ± 0.021 | 0.009 ± 0.001 a |
| DA1 | 0.746 ± 0.189 a,b,c | 0.020 ± 0.009 a,b | 0.005 ± 0.002 a | 0.040 ± 0.011 b | <LOD | <LOD | 0.011 ± 0.001 a |
| ME2 | 0.673 ± 0.215 b,c,d | 0.021 ± 0.007 a,b | <LOQ | 0.010 ± 0.002 c | <LOD | 0.023 ± 0.033 | 0.007 ± 0.003 a,b |
| NC2 | 0.942 ± 0.200 a,b | 0.012 ± 0.009 b | 0.008 ± 0.003 a | 0.095 ± 0.024 a | <LOD | 0.011 ± 0.001 | 0.003 ± 0.001 c |
| NN2 | 0.494 ± 0.138 c,d | 0.015 ± 0.005 b | 0.004 ± 0.002 a | 0.075 ± 0.021 a | <LOD | <LOD | 0.002 ± 0.001 c |
| DM2 | <LOQ | 0.034 ± 0.006 a | 0.004 ± 0.001 a | 0.033 ± 0.011 b | <LOD | <LOD | 0.009 ± 0.002 a,b |
| DA2 | 1.241 ± 0.113 a | 0.024 ± 0.008 a,b | 0.004 ± 0.002 a | 0.035 ± 0.012 b | <LOD | <LOD | 0.016 ± 0.007 a |
| IFs (Batches) | Toxic Elements (mg·kg−1) | ||||||
|---|---|---|---|---|---|---|---|
| Al | As | Cd | Sn | Hg | Pb | U | |
| Phase 1 infant formulas | |||||||
| ME1A | 0.561 ± 0.048 b | 0.013 ± 0.005 a | <LOQ | 0.007 ± 0.003 a | <LOQ | <LOD | 0.002 ± 0.001 b |
| ME1B | 0.806 ± 0.017 a | 0.021 ± 0.008 a | 0.002 ± 0.001 a | 0.013 ± 0.004 a | <LOQ | <LOD | 0.006 ± 0.003 a,b |
| ME1C | 0.804 ± 0.080 a | 0.015 ± 0.001 a | 0.003 ± 0.001 a | <LOQ | <LOQ | <LOD | 0.006 ± 0.001 a |
| NC1A | <LOQ | <LOQ | 0.004 ± 0.001 a | 0.076 ± 0.012 a,b | <LOQ | <LOD | <LOD |
| NC1B | <LOQ | 0.014 ± 0.001 | 0.006 ± 0.004 a | 0.105 ± 0.035 a | <LOQ | <LOD | <LOD |
| NC1C | 1.008 ± 0.178 | <LOQ | 0.004 ± 0.001 a | 0.061 ± 0.006 b | <LOQ | <LOD | <LOD |
| NN1A | <LOQ | <LOQ | 0.002 ± 0.001 a | 0.033 ± 0.013 b | <LOQ | <LOD | <LOD |
| NN1B | 0.602 ± 0.038 a | <LOQ | 0.007 ± 0.004 a | 0.101 ± 0.025 a | <LOD | 0.036 ± 0.041 | <LOD |
| NN1C | 0.481 ± 0.054 b | 0.011 ± 0.002 | 0.003 ± 0.001 a | 0.028 ± 0.006 b | <LOQ | <LOD | <LOD |
| DM1A | <LOQ | 0.028 ± 0.004 a | 0.003 ± 0.001 a | 0.051 ± 0.023 a | <LOD | <LOD | 0.009 ± 0.001 a |
| DM1B | 0.441 ± 0.097 a | 0.032 ± 0.001 a | 0.006 ± 0.003 a | 0.093 ± 0.026 a | <LOD | 0.016 ± 0.021 | 0.008 ± 0.001 a |
| DM1C | 0.476 ± 0.158 a | 0.033 ± 0.004 a | 0.005 ± 0.001 a | 0.062 ± 0.016 a | <LOD | <LOD | 0.009 ± 0.001 a |
| DA1A | 0.904 ± 0.225 a | 0.028 ± 0.004 a | 0.005 ± 0.001 a | 0.030 ± 0.005 b | <LOD | <LOQ | 0.010 ± 0.001 a |
| DA1B | <LOQ | 0.023 ± 0.005 a | 0.007 ± 0.003 a | 0.039 ± 0.005 b | <LOD | <LOD | 0.011 ± 0.001 a |
| DA1C | 1.031 ± 0.096 a | <LOQ | 0.003 ± 0.001 a | 0.052 ± 0.006 a | <LOD | <LOD | 0.012 ± 0.001 a |
| Phase 2 infant formulas | |||||||
| ME2A | 0.916 ± 0.047 a | 0.022 ± 0.001 a | <LOQ | <LOQ | <LOD | <LOQ | 0.008 ± 0.001 a |
| ME2B | 0.507 ± 0.021 b | 0.025 ± 0.005 a | <LOQ | <LOQ | <LOD | 0.023 ± 0.033 | 0.007 ± 0.001 a,b |
| ME2C | 0.596 ± 0.060 b | 0.015 ± 0.006 a | 0.002 ± 0.001 | 0.023 ± 0.014 | <LOD | <LOQ | 0.005 ± 0.001 b |
| NC2A | 0.742 ± 0.258 a | <LOQ | 0.007 ± 0.002 a | 0.073 ± 0.011 b | <LOD | 0.011 ± 0.001 | 0.002 ± 0.001 a |
| NC2B | 1.142 ± 0.221 a | 0.012 ± 0.001 a | 0.010 ± 0.001 a | 0.120 ± 0.015 a | <LOD | <LOQ | 0.004 ± 0.001 a |
| NC2C | 0.942 ± 0.354 a | 0.014 ± 0.003 a | 0.008 ± 0.002 a | 0.093 ± 0.024 a,b | <LOD | <LOQ | 0.003 ± 0.001 a |
| NN2A | 0.570 ± 0.108 a | 0.014 ± 0.007 a | 0.003 ± 0.001 a | 0.059 ± 0.005 b | <LOD | <LOQ | 0.002 ± 0.001 a |
| NN2B | 0.578 ± 0.081 a | 0.014 ± 0.008 a | 0.006 ± 0.002 a | 0.098 ± 0.033 a | <LOD | <LOQ | 0.002 ± 0.001 a |
| NN2C | <LOQ | 0.018 ± 0.003 a | 0.004 ± 0.001 a | 0.068 ± 0.014ª,b | <LOD | <LOD | 0.001 ± 0.001 a |
| DM2A | 0.434 ± 0.058 | 0.039 ± 0.007 a | 0.005 ± 0.002 a | 0.046 ± 0.017 a | <LOD | <LOD | 0.009 ± 0.001 a,b |
| DM2B | <LOQ | 0.032 ± 0.004 a | 0.004 ± 0.001 a | 0.030 ± 0.001 a | <LOD | <LOD | 0.011 ± 0.001 a |
| DM2C | <LOQ | 0.032 ± 0.002 a | 0.004 ± 0.001 a | 0.024 ± 0.005 a | <LOD | <LOD | 0.008 ± 0.001 b |
| DA2A | 1.294 ± 0.146 a | 0.022 ± 0.003 a | 0.005 ± 0.001 a | 0.037 ± 0.006 a | <LOD | <LOD | 0.020 ± 0.002 a |
| DA2B | 1.111 ± 0.129 a | 0.026 ± 0.002 a | 0.004 ± 0.001 a | 0.032 ± 0.004 a | <LOD | <LOD | 0.008 ± 0.001 b |
| DA2C | 1.316 ± 0.132 a | 0.023 ± 0.003 a | 0.004 ± 0.001 a | 0.035 ± 0.003 a | <LOD | <LOD | 0.020 ± 0.001 a |
| IFs | THQ (mg·kg·day−1) | TCR (mg·kg·day−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| iAs | Hg | Cd | Pb | Al | Sn | U | Al | As | Pb | |
| Phase 1 infant formulas | ||||||||||
| ME1A | 0.8628 | n.d. | n.d. | n.d. | 0.0109 | 0.0002 | 0.0157 | 0.0002 | 0.0004 | n.d. |
| ME1B | 1.3339 | n.d. | 0.0384 | n.d. | 0.0156 | 0.0004 | 0.0398 | 0.0003 | 0.0006 | n.d. |
| ME1C | 0.9720 | n.d. | 0.0550 | n.d. | 0.0156 | n.d. | 0.0361 | 0.0003 | 0.0004 | n.d. |
| NC1A | n.d. | n.d. | 0.0803 | n.d. | n.d. | 0.0026 | n.d. | n.d. | n.d. | n.d. |
| NC1B | 0.9361 | n.d. | 0.1318 | n.d. | n.d. | 0.0036 | n.d. | n.d. | 0.0004 | n.d. |
| NC1C | n.d. | n.d. | 0.0809 | n.d. | 0.0206 | 0.0021 | n.d. | 0.0004 | n.d. | n.d. |
| NN1A | n.d. | n.d. | 0.0463 | n.d. | n.d. | 0.0011 | n.d. | n.d. | n.d. | n.d. |
| NN1B | n.d. | n.d. | 0.1351 | 0.2138 | 0.0124 | 0.0035 | n.d. | 0.0002 | n.d. | 0.000006 |
| NN1C | 0.7714 | n.d. | 0.0562 | n.d. | 0.0099 | 0.0010 | n.d. | 0.0002 | 0.0003 | n.d. |
| DM1A | 1.7117 | n.d. | 0.0642 | n.d. | n.d. | 0.0016 | 0.0528 | n.d. | 0.0008 | n.d. |
| DM1B | 1.9464 | n.d. | 0.1150 | 0.0846 | 0.0082 | 0.0029 | 0.0501 | 0.0002 | 0.0009 | 0.000003 |
| DM1C | 2.0472 | n.d. | 0.0907 | n.d. | 0.0088 | 0.0019 | 0.0564 | 0.0002 | 0.0009 | n.d. |
| DA1A | 1.8317 | n.d. | 0.1012 | n.d. | 0.0167 | 0.0010 | 0.0645 | 0.0004 | 0.0008 | n.d. |
| DA1B | 1.4701 | n.d. | 0.1286 | n.d. | n.d. | 0.0012 | 0.0681 | n.d. | 0.0006 | n.d. |
| DA1C | n.d. | n.d. | 0.0558 | n.d. | 0.0200 | 0.0017 | 0.0800 | 0.0004 | n.d. | n.d. |
| Phase 2 infant formulas | ||||||||||
| ME2A | 1.0969 | n.d. | n.d. | n.d. | 0.0138 | n.d. | 0.0393 | 0.0003 | 0.0005 | n.d. |
| ME2B | 1.2374 | n.d. | n.d. | 0.1003 | 0.0076 | n.d. | 0.0334 | 0.0001 | 0.0006 | 0.000003 |
| ME2C | 0.7569 | n.d. | 0.0304 | n.d. | 0.0090 | 0.0006 | 0.0264 | 0.0002 | 0.0003 | n.d. |
| NC2A | n.d. | n.d. | 0.1075 | 0.0461 | 0.0107 | 0.0018 | 0.0097 | 0.0002 | n.d. | 0.000001 |
| NC2B | 0.5848 | n.d. | 0.1392 | n.d. | 0.0165 | 0.0029 | 0.0208 | 0.0003 | 0.0002 | n.d. |
| NC2C | 0.6875 | n.d. | 0.1131 | n.d. | 0.0136 | 0.0022 | 0.0124 | 0.0003 | 0.0003 | n.d. |
| NN2A | 0.5212 | n.d. | 0.0363 | n.d. | 0.0062 | 0.0011 | 0.0063 | 0.0001 | 0.0003 | n.d. |
| NN2B | 0.4957 | n.d. | 0.0611 | n.d. | 0.0063 | 0.0018 | 0.0062 | 0.0001 | 0.0002 | n.d. |
| NN2C | 0.6598 | n.d. | 0.0392 | n.d. | n.d. | 0.0012 | 0.0050 | n.d. | 0.0003 | n.d. |
| DM2A | 1.8267 | n.d. | 0.0680 | n.d. | 0.0061 | 0.0011 | 0.0411 | 0.0001 | 0.0006 | n.d. |
| DM2B | 1.5085 | n.d. | 0.0566 | n.d. | n.d. | 0.0007 | 0.0516 | n.d. | 0.0005 | n.d. |
| DM2C | 1.5130 | n.d. | 0.0542 | n.d. | n.d. | 0.0006 | 0.0363 | n.d. | 0.0005 | n.d. |
| DA2A | 1.0955 | n.d. | 0.0679 | n.d. | 0.0195 | 0.0009 | 0.1013 | 0.0004 | 0.0003 | n.d. |
| DA2B | 1.3109 | n.d. | 0.0544 | n.d. | 0.0167 | 0.0008 | 0.0386 | 0.0003 | 0.0006 | n.d. |
| DA2C | 1.1523 | n.d. | 0.0646 | n.d. | 0.0198 | 0.0009 | 0.0981 | 0.0004 | 0.0005 | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Almeida, C.C.; Baião, D.d.S.; Rodrigues, P.d.A.; Saint’Pierre, T.D.; Hauser-Davis, R.A.; Leandro, K.C.; Paschoalin, V.M.F.; da Costa, M.P.; Conte-Junior, C.A. Toxic Metals and Metalloids in Infant Formulas Marketed in Brazil, and Child Health Risks According to the Target Hazard Quotients and Target Cancer Risk. Int. J. Environ. Res. Public Health 2022, 19, 11178. https://doi.org/10.3390/ijerph191811178
de Almeida CC, Baião DdS, Rodrigues PdA, Saint’Pierre TD, Hauser-Davis RA, Leandro KC, Paschoalin VMF, da Costa MP, Conte-Junior CA. Toxic Metals and Metalloids in Infant Formulas Marketed in Brazil, and Child Health Risks According to the Target Hazard Quotients and Target Cancer Risk. International Journal of Environmental Research and Public Health. 2022; 19(18):11178. https://doi.org/10.3390/ijerph191811178
Chicago/Turabian Stylede Almeida, Cristine Couto, Diego dos Santos Baião, Paloma de Almeida Rodrigues, Tatiana Dillenburg Saint’Pierre, Rachel Ann Hauser-Davis, Katia Christina Leandro, Vania Margaret Flosi Paschoalin, Marion Pereira da Costa, and Carlos Adam Conte-Junior. 2022. "Toxic Metals and Metalloids in Infant Formulas Marketed in Brazil, and Child Health Risks According to the Target Hazard Quotients and Target Cancer Risk" International Journal of Environmental Research and Public Health 19, no. 18: 11178. https://doi.org/10.3390/ijerph191811178
APA Stylede Almeida, C. C., Baião, D. d. S., Rodrigues, P. d. A., Saint’Pierre, T. D., Hauser-Davis, R. A., Leandro, K. C., Paschoalin, V. M. F., da Costa, M. P., & Conte-Junior, C. A. (2022). Toxic Metals and Metalloids in Infant Formulas Marketed in Brazil, and Child Health Risks According to the Target Hazard Quotients and Target Cancer Risk. International Journal of Environmental Research and Public Health, 19(18), 11178. https://doi.org/10.3390/ijerph191811178