Fluorescence-Guided Surgery and Novel Innovative Technologies for Improved Visualization in Pediatric Urology
Abstract
:1. Introduction
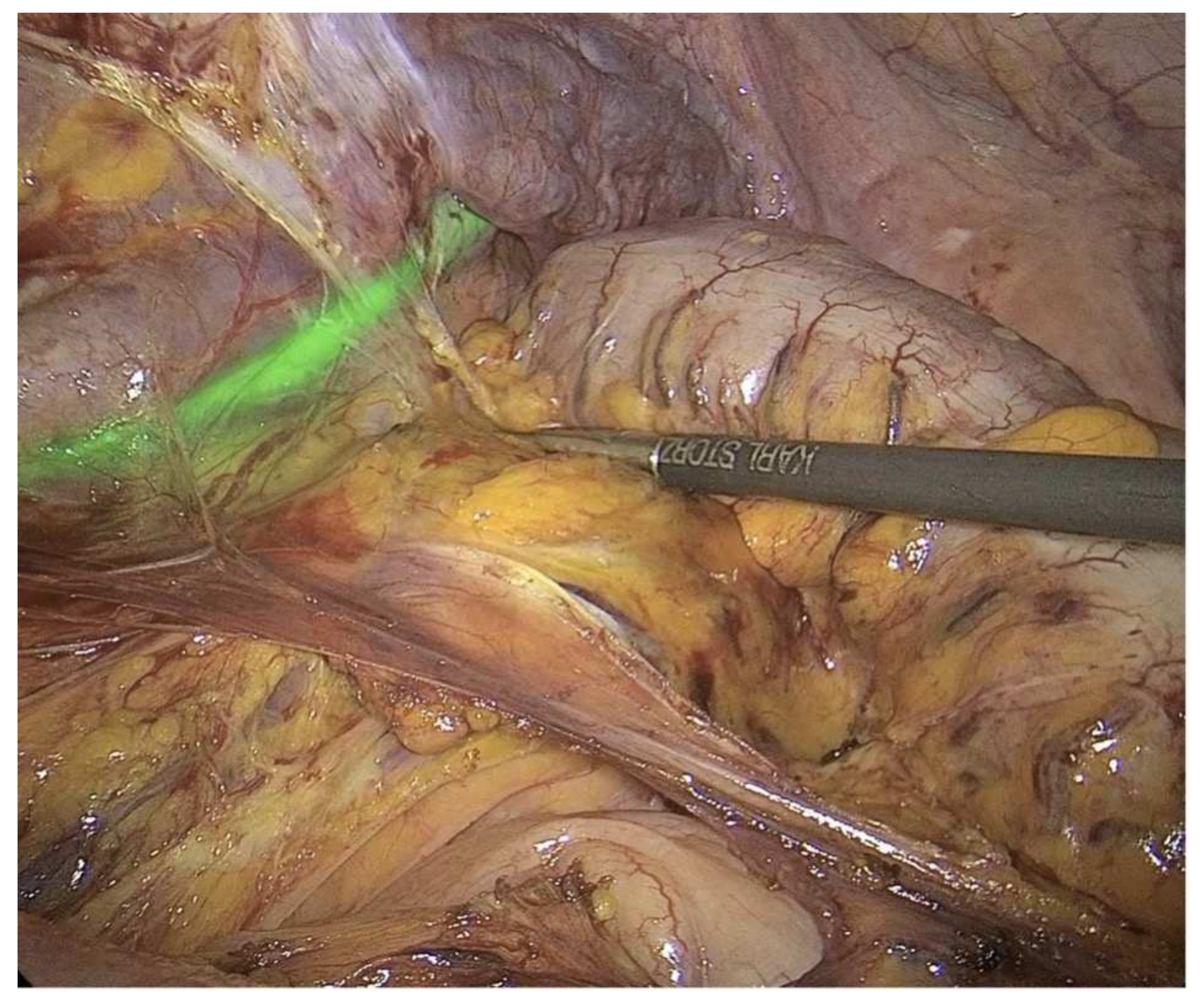
2. Fluorescence-Guided Surgery (FSG)
3. Three-Dimensional (3D) Reconstructions and Printing Technologies
4. Augmented Reality (AR)
5. Contrast-Enhanced Ultrasound (CEUS)
6. Intraoperative Magnetic Resonance Imaging (iMRI)
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Autorino, R.; Porpiglia, F.; Dasgupta, P.; Rassweiler, J.; Catto, J.; Hampton, L.; Lima, E.; Mirone, V.; Derweesh, I.; Debruyne, F. Precision surgery and genitourinary cancers. Eur. J. Surg. Oncol. (EJSO) 2017, 43, 893–908. [Google Scholar] [CrossRef] [PubMed]
- Paraboschi, I.; Farneti, F.; Jannello, L.; Manzoni, G.; Berrettini, A.; Mantica, G. Narrative review on applications of fluorescence-guided surgery in adult and paediatric urology. AME Med. J. 2021, 7, 15. [Google Scholar] [CrossRef]
- van den Berg, N.S.; van Leeuwen, F.W.B.; van der Poel, H.G. Fluorescence guidance in urologic surgery. Curr. Opin. Urol. 2012, 22, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Amparore, D.; Pecoraro, A.; Checcucci, E.; DE Cillis, S.; Piramide, F.; Volpi, G.; Piana, A.; Verri, P.; Granato, S.; Sica, M.; et al. 3D imaging technologies in minimally invasive kidney and prostate cancer surgery: Which is the urologists’ perception? Minerva Urol. Nephrol. 2022, 74, 178–185. [Google Scholar] [CrossRef]
- Porpiglia, F.; Bertolo, R.; Checcucci, E.; Amparore, D.; Autorino, R.; Dasgupta, P.; Wiklund, P.; Tewari, A.; Liatsikos, E.; Fiori, C.; et al. Development and validation of 3D printed virtual models for robot-assisted radical prostatectomy and partial nephrectomy: Urologists’ and patients’ perception. World J. Urol. 2018, 36, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Porpiglia, F.; Checcucci, E.; Amparore, D.; Piramide, F.; Volpi, G.; Granato, S.; Verri, P.; Manfredi, M.; Bellin, A.; Piazzolla, P.; et al. Three-dimensional Augmented Reality Robot-assisted Partial Nephrectomy in Case of Complex Tumours (PADUA ≥ 10): A New Intraoperative Tool Overcoming the Ultrasound Guidance. Eur. Urol. 2020, 78, 229–238. [Google Scholar] [CrossRef]
- Bertolo, R.; Hung, A.; Porpiglia, F.; Bove, P.; Schleicher, M.; Dasgupta, P. Systematic review of augmented reality in urological interventions: The evidences of an impact on surgical outcomes are yet to come. World J. Urol. 2020, 38, 2167–2176. [Google Scholar] [CrossRef] [PubMed]
- Wildeboer, R.R.; de la Rosette, J.; Mischi, M.; Wijkstra, H. Contrast-enhanced Ultrasound in Urology. In Smith’s Textbook of Endourology; Smith, A.D., Preminger, G.M., Kavoussi, L.R., Badlani, G.H., Rastinehad, A.R., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 1605–1615. [Google Scholar] [CrossRef]
- D’Amico, A.; Tempany, C.; Cormack, R.; Hata, N.; Jinzaki, M.; Tuncali, K.; Weinstein, M.; Richie, J. Transperineal Magnetic Resonance Image Guided Prostate Biopsy. J. Urol. 2000, 164, 385–387. [Google Scholar] [CrossRef]
- Privitera, L.; Paraboschi, I.; Cross, K.; Giuliani, S. Above and Beyond Robotic Surgery and 3D Modelling in Paediatric Cancer Surgery. Front. Pediatr. 2021, 9, 777840. [Google Scholar] [CrossRef] [PubMed]
- Privitera, L.; Paraboschi, I.; Dixit, D.; Arthurs, O.J.; Giuliani, S. Image-guided surgery and novel intraoperative devices for enhanced visualisation in general and paediatric surgery: A review. Innov. Surg. Sci. 2022, 6, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Paraboschi, I.; De Coppi, P.; Stoyanov, D.; Anderson, J.; Giuliani, S. Fluorescence imaging in pediatric surgery: State-of-the-art and future perspectives. J. Pediatr. Surg. 2021, 56, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Paraboschi, I.; Privitera, L.; Kramer-Marek, G.; Anderson, J.; Giuliani, S. Novel Treatments and Technologies Applied to the Cure of Neuroblastoma. Children 2021, 8, 482. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, T.; Nakamura, Y.A.; Choyke, P.L.; Kobayashi, H. Fluorescence-Guided Surgery. Front. Oncol. 2017, 7, 314. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.T.; Tsien, R.Y. Fluorescence-guided surgery with live molecular navigation—A new cutting edge. Nat. Rev. Cancer 2013, 13, 653–662. [Google Scholar] [CrossRef]
- Lau, C.T.; Au, D.M.; Wong, K.K.Y. Application of indocyanine green in pediatric surgery. Pediatr. Surg. Int. 2019, 35, 1035–1041. [Google Scholar] [CrossRef]
- Herz, D.; DaJusta, D.; Ching, C.; McLeod, D. Segmental arterial mapping during pediatric robot-assisted laparoscopic heminephrectomy: A descriptive series. J. Pediatr. Urol. 2016, 12, 266.e1–266.e6. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bautista, B.; Mata, D.P.; Parente, A.; Pérez-Caballero, R.; De Agustín, J.C. First Experience with Fluorescence in Pediatric Laparoscopy. Eur. J. Pediatr. Surg. Rep. 2019, 7, e43–e46. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.; Turrà, F.; Del Conte, F.; Izzo, S.; Gargiulo, F.; Farina, A.; Severino, G.; Cerulo, M.; Escolino, M. Indocyanine Green Fluorescence Lymphography: A New Technique to Perform Lymphatic Sparing Laparoscopic Palomo Varicocelectomy in Children. J. Laparoendosc. Adv. Surg. Tech. A 2019, 29, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.; Del Conte, F.; Cerulo, M.; Gargiulo, F.; Izzo, S.; Esposito, G.; Spagnuolo, M.I.; Escolino, M. Clinical application and technical standardization of indocyanine green (ICG) fluorescence imaging in pediatric minimally invasive surgery. Pediatr. Surg. Int. 2019, 35, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.; Coppola, V.; Del Conte, F.; Cerulo, M.; Esposito, G.; Farina, A.; Crocetto, F.; Castagnetti, M.; Settimi, A.; Escolino, M. Near-Infrared fluorescence imaging using indocyanine green (ICG): Emerging applications in pediatric urology. J. Pediatr. Urol. 2020, 16, 700–707. [Google Scholar] [CrossRef]
- Esposito, C.; Autorino, G.; Coppola, V.; Esposito, G.; Paternoster, M.; Castagnetti, M.; Cardone, R.; Cerulo, M.; Borgogni, R.; Cortese, G.; et al. Technical standardization of ICG near-infrared fluorescence (NIRF) laparoscopic partial nephrectomy for duplex kidney in pediatric patients. World J. Urol. 2021, 39, 4167–4173. [Google Scholar] [CrossRef]
- Carty, K.N.; Hwang, A.; Gordon, A.; Locke, R.; DeMarco, R.T.; Bayne, C.E. Indocyanine green (ICG) assessment of ureteral perfusion during pediatric robotic surgery. J. Pediatr. Surg. Case Rep. 2021, 74, 102058. [Google Scholar] [CrossRef]
- Abdelhafeez, A.H.; Murphy, A.J.; Brennan, R.; Santiago, T.C.; Lu, Z.; Krasin, M.J.; Bissler, J.J.; Gleason, J.M.; Davidoff, A.M. Indocyanine Green–Guided Nephron-Sparing Surgery for Pediatric Renal Tumors. J. Pediatr. Surg. 2021, 57, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Indocyanine Green (ICG) Guided Tumor Resection. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04084067 (accessed on 1 September 2022).
- Cheung, C.L.; Looi, T.; Lendvay, T.S.; Drake, J.M.; Farhat, W.A. Use of 3-Dimensional Printing Technology and Silicone Modeling in Surgical Simulation: Development and Face Validation in Pediatric Laparoscopic Pyeloplasty. J. Surg. Educ. 2014, 71, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Imizcoz, F.L.; Falcioni, G.; Weller, S.; Corbetta, J.P.; Bailez, M.M. Developing Simulation Models for Minimally Invasive Surgery in Pediatric Urology. Videoscopy 2020, 30, vor.2020.0678. [Google Scholar] [CrossRef]
- Baughman, S.M.; Richardson, R.R.; Podberesky, D.J.; Dalrymple, N.C.; Yerkes, E.B. 3-Dimensional Magnetic Resonance Genitography: A Different Look at Cloacal Malformations. J. Urol. 2007, 178, 1675–1679. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.N. Use of rotational fluoroscopy and 3-D reconstruction for pre-operative imaging of complex cloacal malformations. Semin. Pediatric Surg. 2016, 25, 96–101. [Google Scholar] [CrossRef]
- Jarboe, M.D.; Teitelbaum, D.H.; Dillman, J.R. Combined 3D rotational fluoroscopic-MRI cloacagram procedure defines luminal and extraluminal pelvic anatomy prior to surgical reconstruction of cloacal and other complex pelvic malformations. Pediatr. Surg. Int. 2012, 28, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.J.; Shnorhavorian, M.; Oelschlager, A.-M.E.A.; Ripley, B.; Shivaram, G.M.; Avansino, J.R.; Merguerian, P.A. Use of 3D reconstruction cloacagrams and 3D printing in cloacal malformations. J. Pediatric Urol. 2017, 13, 395.e1–395.e6. [Google Scholar] [CrossRef] [PubMed]
- Gasior, A.C.; Reck, C.; Lane, V.; Wood, R.J.; Patterson, J.; Strouse, R.; Lin, S.; Cooper, J.; Bates, D.G.; Levitt, M.A. Transcending Dimensions: A Comparative Analysis of Cloaca Imaging in Advancing the Surgeon’s Understanding of Complex Anatomy. J. Digit. Imaging 2019, 32, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Krois, W.; Schmölz, L.; Wagner, M.; Gröpel, P.; Unger, E.; Berger, A.; Metzelder, M.; Reck, C.A. Cysto-Vaginoscopy of a 3D-Printed Cloaca Model: A Step toward Personalized Noninvasive Preoperative Assessment in Patients with Complex Anorectal Malformations. Eur. J. Pediatric Surg. 2021, 32, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Tekes, A.; Ertan, G.; Solaiyappan, M.; Stec, A.; Sponseller, P.; Huisman, T.; Gearhart, J. 2D and 3D MRI features of classic bladder exstrophy. Clin. Radiol. 2014, 69, e223–e229. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.K.; Falkert, A.; Germer, U.; Rösch, W.H. Biometry of the pubovisceral muscle and levator hiatus assessed by three-dimensional ultrasound in females with bladder exstrophy-epispadias complex after functional reconstruction. Ultrasound Obs. Gynecol. 2009, 34, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.K.; Falkert, A.; Brandl, R.; Hirschfelder, H.; Koller, M.; Rãsch, W.H. Pelvic-floor imaging using three-dimensional ultrasonography and magnetic resonance imaging in the long term follow-up of the bladder-exstrophy-epispadias complex. BJU Int. 2010, 105, 248–253. [Google Scholar] [CrossRef]
- Siapno, A.E.; Yi, B.C.; Daniels, D.; Bolagani, A.; Kwan, L.; Walker, D.; Aninwene, G.E.; Eleswarapu, S.; Joshi, S.H.; Sturm, R.M. Measurement accuracy of 3-Dimensional mapping technologies versus standard goniometry for angle assessment. J. Pediatric Urol. 2020, 16, 547–554. [Google Scholar] [CrossRef]
- Ezer, M.; Aydoğan, T.B.; Huri, E. Urologic Surgery in Digital Era: Foresights and Futuristic Approach. Balk. Med. J. 2021, 38, 324–330. [Google Scholar] [CrossRef]
- Paraboschi, I.; Gnech, M.; De Marco, E.A.; Minoli, D.G.; Bebi, C.; Zanetti, S.P.; Manzoni, G.; Montanari, E.; Berrettini, A. Pediatric Urolithiasis: Current Surgical Strategies and Future Perspectives. Front. Pediatr. 2022, 10, 886425. [Google Scholar] [CrossRef]
- Souzaki, R.; Kawakubo, N.; Matsuura, T.; Yoshimaru, K.; Koga, Y.; Takemoto, J.; Shibui, Y.; Kohashi, K.; Hayashida, M.; Oda, Y.; et al. Navigation surgery using indocyanine green fluorescent imaging for hepatoblastoma patients. Pediatr. Surg. Int. 2019, 35, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Wellens, L.M.; Meulstee, J.; van de Ven, C.P.; van Scheltinga, C.E.J.T.; Littooij, A.S.; van den Heuvel-Eibrink, M.M.; Fiocco, M.; Rios, A.C.; Maal, T.; Wijnen, M.H.W.A. Comparison of 3-Dimensional and Augmented Reality Kidney Models with Conventional Imaging Data in the Preoperative Assessment of Children with Wilms Tumors. JAMA Netw. Open 2019, 2, e192633. [Google Scholar] [CrossRef] [Green Version]
- Chaussy, Y.; Vieille, L.; Lacroix, E.; Lenoir, M.; Marie, F.; Corbat, L.; Henriet, J.; Auber, F. 3D reconstruction of Wilms’ tumor and kidneys in children: Variability, usefulness and constraints. J. Pediatric Urol. 2020, 16, 830.e1–830.e8. [Google Scholar] [CrossRef]
- Berrettini, A.; Sampogna, G.; Zanetti, S.P.; Gallioli, A.; Gnech, M.; De Marco, E.A.; Minoli, D.G.; Longo, F.; Manzoni, G.; Montanari, E. Semi-closed-circuit vacuum-assisted MiniPCNL system in pediatric patients. J. Pediatric Urol. 2021, 17, 275–276. [Google Scholar] [CrossRef] [PubMed]
- Gallioli, A.; Berrettini, A.; Sampogna, G.; Llorens, E.; Quiróz, Y.; Gnech, M.; DE Lorenzis, E.; Albo, G.; Palou, J.; Manzoni, G.; et al. Semi-closed-circuit vacuum-assisted mini percutaneous nephrolithotomy in the pediatric population: The initial experience of two tertiary referral centers. Minerva Urol. Nephrol. 2022, 74, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Rassweiler, J.J.; Müller, M.; Fangerau, M.; Klein, J.; Goezen, A.S.; Pereira, P.; Meinzer, H.-P.; Teber, D. Ipad-Assisted Percutaneous Access to the Kidney Using Marker-Based Navigation: Initial Clinical Experience. Eur. Urol. 2012, 61, 628–631. [Google Scholar] [CrossRef]
- Müller, M.; Rassweiler, M.-C.; Klein, J.; Seitel, A.; Gondan, M.; Baumhauer, M.; Teber, D.; Rassweiler, J.J.; Meinzer, H.-P.; Maier-Hein, L. Mobile augmented reality for computer-assisted percutaneous nephrolithotomy. Int. J. Comput. Assist. Radiol. Surg. 2013, 8, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Duran, C.; Beltrán, V.P.; González, A.; Gómez, C.; Del Riego, J. Contrast-enhanced Voiding Urosonography for Vesicoureteral Reflux Diagnosis in Children. Radio Graphics. 2017, 37, 1854–1869. [Google Scholar] [CrossRef] [PubMed]
- Yousefifard, M.; Toloui, A.; Alavi, S.N.R.; Neishaboori, A.M.; Ahmadzadeh, K.; Ghelichkhani, P.; Safari, S.; Abbasi, A.; Ataei, N.; Hosseini, M. Contrast-enhanced voiding urosonography, a possible candidate for the diagnosis of vesicoureteral reflux in children and adolescents; a systematic review and meta-analysis. J. Pediatric Urol. 2022, 18, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Darge, K.; Papadopoulou, F.; Ntoulia, A.; Bulas, D.I.; Coley, B.; Fordham, L.; Paltiel, H.J.; McCarville, M.B.; Volberg, F.M.; Cosgrove, D.O.; et al. Safety of contrast-enhanced ultrasound in children for non-cardiac applications: A review by the Society for Pediatric Radiology (SPR) and the International Contrast Ultrasound Society (ICUS). Pediatr. Radiol. 2013, 43, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Čizmarević, U.; Hanžič, N.; Žerdin, M. The Use of Contrast-enhanced Ultrasound in Pediatrics: A Case Series. Cureus 2019, 11, e6215. [Google Scholar] [CrossRef] [PubMed]
- Bosio, M.; Manzoni, G.A. Detection of posterior urethral valves with voiding cystourethrosonography with echo contrast. J. Urol. 2002, 168, 1711–1715, discussion 1715. [Google Scholar] [CrossRef]
- Fischer, K.M.; Bowen, D.K.; Kovell, R.C.; Back, S.J.; Darge, K.; Weiss, D.A. Intraoperative contrast enhanced sonourethrography to characterize urethral stricture in a pediatric patient. Urol. Case Rep. 2020, 32, 101223. [Google Scholar] [CrossRef]
- Schwarze, V.; Rübenthaler, J.; Marschner, C.; Fabritius, M.P.; Rueckel, J.; Fink, N.; Puhr-Westerheide, D.; Gresser, E.; Froelich, M.F.; Schnitzer, M.L.; et al. Advanced Fusion Imaging and Contrast-Enhanced Imaging (CT/MRI–CEUS) in Oncology. Cancers 2020, 12, 2821. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, H.N.; Maruf, M.; Massanyi, E.Z.; Shah, B.; Tekes, A.; Gearhart, J.P. 3-Dimensional Magnetic Resonance Imaging Guided Pelvic Floor Dissection for Bladder Exstrophy: A Single Arm Trial. J. Urol. 2019, 202, 406–412. [Google Scholar] [CrossRef]
- Dunn, E.A.; Kasprenski, M.; Facciola, J.; Benz, K.; Maruf, M.; Zaman, M.H.; Gearhart, J.; Di Carlo, H.; Tekes, A. Anatomy of Classic Bladder Exstrophy: MRI Findings and Surgical Correlation. Curr. Urol. Rep. 2019, 20, 48. [Google Scholar] [CrossRef]

| Author, Year | Disease, Number of Patients | Type of Surgery | Dye (Administration Route and Dosage) | Imaging System | IDEAL Framework Stage |
|---|---|---|---|---|---|
| Herz et al. [17], 2016 | Duplex kidney (n = 6) | Robot-assisted laparoscopic heminephrectomy | ICG (iv, 1.25–2.5 mg, 30–60 s prior to surgery) | FireflyTM system, da Vinci (Intuitive Surgical, Inc., Sunnyvale, CA, USA) | 2a |
| Fernàndez-Bautista et al. [18], 2019 | Varicocele (n = 1); Non-functioning kidney (n = 2) | Laparoscopic Palomo varicocelectomy; Laparoscopic nephrectomy | ICG (iv, nd, at the time of the laparoscopic Palomo varicocelectomy; iv, 0.2 mg/kg, at time of thelaparoscopic nephrectomy) | 1488 HD 3-Chip camera system, Stryker | 1 |
| Esposito et al. [19], 2019 | Varicocele (n = 25) | Laparoscopic Palomo varicocelectomy | ICG (intratesticular, 10 mg, at time of surgery) | nd | 2a |
| Esposito et al. [20], 2019 | Varicocele (n = 30); Non-functioning kidney (n = 3); Duplex kidney (n = 2) | Laparoscopic Palomo varicocelectomy; Laparoscopic radical nephrectomy; Laparoscopic partial nephrectomy | ICG (intratesticular, 6.25 mg, at the time of thelaparoscopic Palomo varicocelectomy; iv, 0.5 mg/kg, at the time of the laparoscopic radical nephrectomy; iv, 0.3 mg/kg, at the time of the laparoscopic partial nephrectomy) | IMAGE1 S system (KARL STORZ SE & Co., KG, Tuttlingen, Germany) | 2a |
| Esposito et al. [21], 2020 | Varicocele (n = 41); Non-functioning kidney (n = 3); Duplex kidney (n = 9); Renal cysts (n = 4) | Laparoscopic and robotic Palomo varicocelectomy; Laparoscopic radical nephrectomy; Laparoscopic partial nephrectomy; Robotic renal cyst deroofing | ICG (intratesticular, 6.25 mg, at the time of thelaparoscopic Palomo varicocelectomy; iv, 0.3 mg/kg, at the time of the laparoscopic radical nephrectomy; iv, 0.3 mg/kg, at the time of the laparoscopic partial nephrectomy; iv, 0.3 mg/kg, at the time of the laparoscopic radical nephrectomy; iv, 0.3 mg/kg, at the time of the robotic deroofing of simple renal cysts) | Storz D-light (KARL STORZ SE & Co., KG, Tuttlingen, Germany) and FireflyTM system, da Vinci Xi robotic platform (Intuitive Surgical Inc., Sunnyvale, CA, USA) | 3 |
| Esposito et al. [22], 2021 | Duplex kidney (n = 12) | Laparoscopic partial nephrectomy | ICG, 1st step: 25 mg into the ureteral catheter just before surgery to identify the ureter; 2nd step: 0.3 mg/kg, iv, to identify the hilar vessel and the vasculature of the non-functioning pole during surgery; 3rd step: 0.3 mg/kg, iv, to identify the boundary plane between the avascular and the perfused pole after ligation of the vessel supplying the non- functioning moiety | nd (KARL STORZ SE & Co., KG, Tuttlingen, Germany) and ICG-NIRF RUBINATM system (KARL STORZ SE & Co., KG, Tuttlingen, Germany). | 3 |
| Carty et al. [23], 2021 | Congenital ureteral stricture (n = 1); Mid-ureteral polyp disease (n = 1); Distal ureteral polyp disease (n = 1) | Robotic Heineke–Mikulicz strictureplasty (n = 1); Robotic ureteroureterostomy (n = 2); Robotic right lower pole ureterocalicostomy (n = 1) | ICG (iv, 0.086 mg/kg at the time of the robotic Heineke–Mikulicz strictureplasty; iv, 0.039 mg/kg at the time of the robotic ureteroureterostomy in the 2nd patient; iv, 0.067 mg/kg at the time of the robotic ureteroureterostomy in the 3rd patient; 0.046 mg/kg, at the time of the robotic right lower pole ureterocalicostomy in the 3rd patient) | FireflyTM system, da Vinci Xi robotic platform (Intuitive Surgical Inc., Sunnyvale, CA, USA) | 1 |
| Abdelhafeez et al. [24], 2021 | Wilms tumor (n = 7); Epithelioid angiomyolipoma (n = 1) | Bilateral nephron-sparing surgery (n = 3); Unilateral radical nephrectomy and concurrent contralateral nephron-sparing surgery (n = 1); Unilateral nephron-sparing surgery (n = 4) | ICG (iv, 1.5 mg/kg, 24 h before surgery) | Iridium system (Visionsense Corp, Philadelphia, PA) | 2a |
| Author, Year | Type of Study | 3D Technology Developed | Aim of the 3D Technology Developed | Authors’ Conclusions |
|---|---|---|---|---|
| Baughman et al. [28], 2007 | Prospective study comparing standard contrast genitography, endoscopy, and 3D MRI genitography for the preoperative surgical planning of four female infants with cloacal malformations | 3D MRI genitography | Preoperative surgical planning | The 3D MRI genitography provided excellent anatomical details of the complex genitourinary anomalies, augmented the information obtained by standard MRI, and added complementary information to that of endoscopy. |
| Patel et al. [29], 2012 | Review article describing the use of 3D rotational fluoroscopy for surgical planning and prognosis determination in children with complex cloacal malformations | 3D rotational fluoroscopy | Preoperative surgical planning and prognosis determination | The 3D rotational fluoroscopy provided surgeons with detailed anatomical findings, enabling them to more accurately plan for surgical repair and predict functional prognosis. |
| Jarboe et al. [30], 2012 | Case series on the use of 3D rotational fluoroscopy combined with high-resolution 3D pelvic MRI for the delineation of the pelvic anatomy of two female patients with complex genitourinary anomalies | 3D rotational fluoroscopy combined with high-resolution 3D pelvic MRI | Preoperative surgical planning | The 3D rotational fluoroscopy combined with high-resolution 3D pelvic MRI provided excellent delineation of the pelvic anatomy to aid in operative planning. |
| Ahn et al. [31], 2017 | Retrospective review comparing 3D reconstruction cloacagrams and endoscopic and intraoperative findings of four cloaca patients and reporting the use of 3D printing technology for preoperative planning and education | 3D reconstruction cloacagrams and 3D-printed models of cloacal malformations | Parent counseling, surgical education for trainees, and preoperative surgical planning | The 3D reconstruction cloacagrams yielded accurate measurements of urethral length and level of cloaca common channel, consistent with the endoscopic findings. The 3D-printed models were useful for surgical planning and education. |
| Gasior et al. [32], 2019 | Prospective study comparing a 2D contrast study cloacagram, a 3D model rotatable CT scan reconstruction, a software-enhanced 3D video animation, and a printed physical 3D model for preoperative planning of a cloaca malformation | 3D model rotatable CT scan reconstruction; software-enhanced 3D video animation; 3D-printed physical cloaca model | Improve learning and understanding of cloaca malformations for preoperative surgical planning | The 3D reconstruction and printed models enabled surgeons to make significant strides in the comprehension of intricate cloacal anatomy and achieve a higher level of preparedness for surgery. |
| Krois et al. [33], 2021 | Prospective study investigating the quality and the feasibility of a real-size rubber-like 3D model of an infant pelvis with a cloacal malformation for cysto-vaginoscopy | 3D-printed cloaca model | Education, procedural simulation, and preoperative surgical planning | The 3D-printed cloaca model was useful for preoperative training to enhance the understating of the patient-specific pelvic anatomy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paraboschi, I.; Mantica, G.; Minoli, D.G.; De Marco, E.A.; Gnech, M.; Bebi, C.; Manzoni, G.; Berrettini, A. Fluorescence-Guided Surgery and Novel Innovative Technologies for Improved Visualization in Pediatric Urology. Int. J. Environ. Res. Public Health 2022, 19, 11194. https://doi.org/10.3390/ijerph191811194
Paraboschi I, Mantica G, Minoli DG, De Marco EA, Gnech M, Bebi C, Manzoni G, Berrettini A. Fluorescence-Guided Surgery and Novel Innovative Technologies for Improved Visualization in Pediatric Urology. International Journal of Environmental Research and Public Health. 2022; 19(18):11194. https://doi.org/10.3390/ijerph191811194
Chicago/Turabian StyleParaboschi, Irene, Guglielmo Mantica, Dario Guido Minoli, Erika Adalgisa De Marco, Michele Gnech, Carolina Bebi, Gianantonio Manzoni, and Alfredo Berrettini. 2022. "Fluorescence-Guided Surgery and Novel Innovative Technologies for Improved Visualization in Pediatric Urology" International Journal of Environmental Research and Public Health 19, no. 18: 11194. https://doi.org/10.3390/ijerph191811194





