Controlling AMR in the Pig Industry: Is It Enough to Restrict Heavy Metals?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Sampling
2.2. Measurements of Heavy Metals in Original Manure and Soil
2.3. DNA Extraction from Soils
2.4. Metagenomic Sequencing and Bioinformatic Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Concentrations of Heavy Metals in Soil
3.2. Bacterial α Diversity in Soil
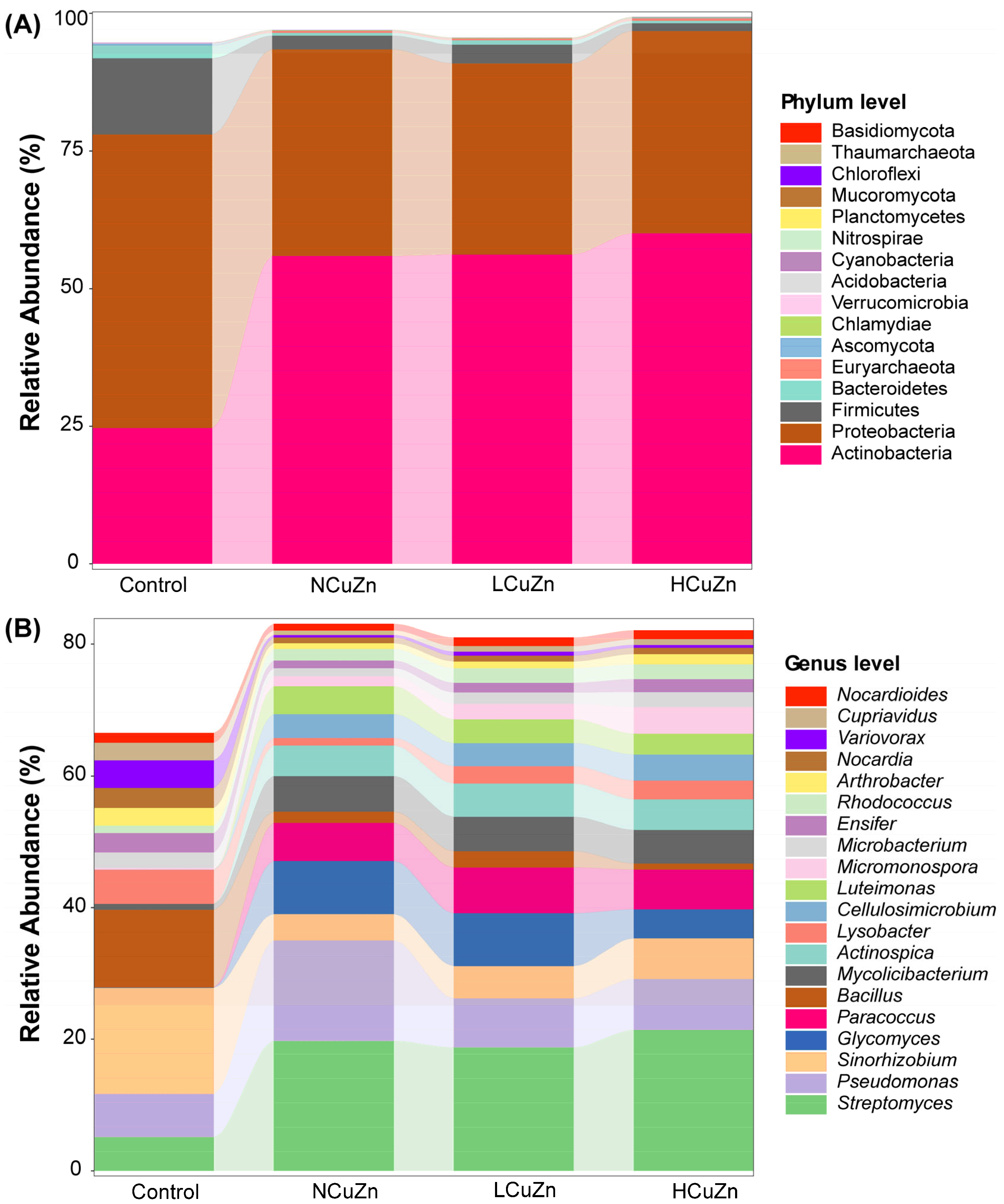
3.3. Bacterial Community Composition in Soils
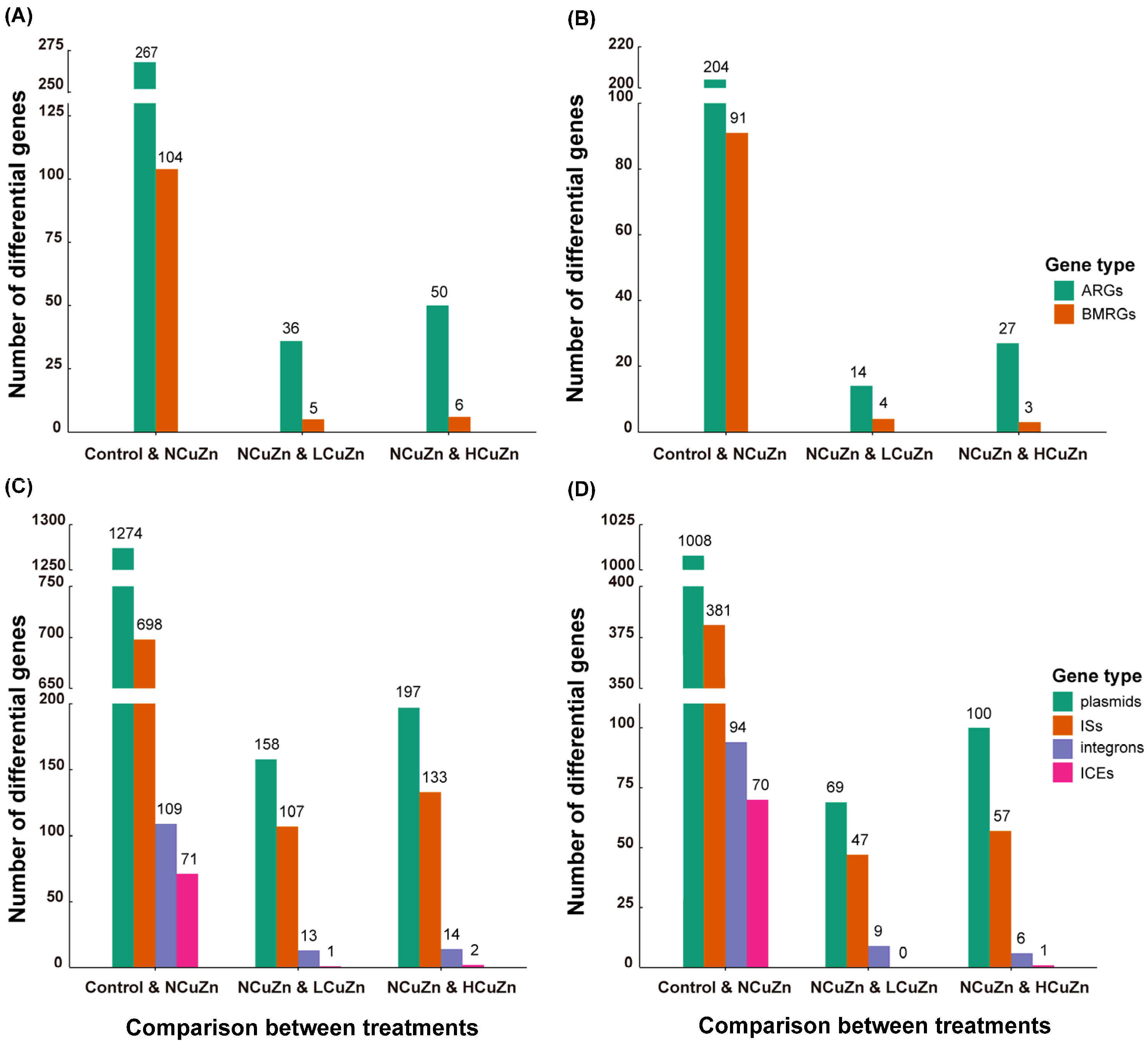
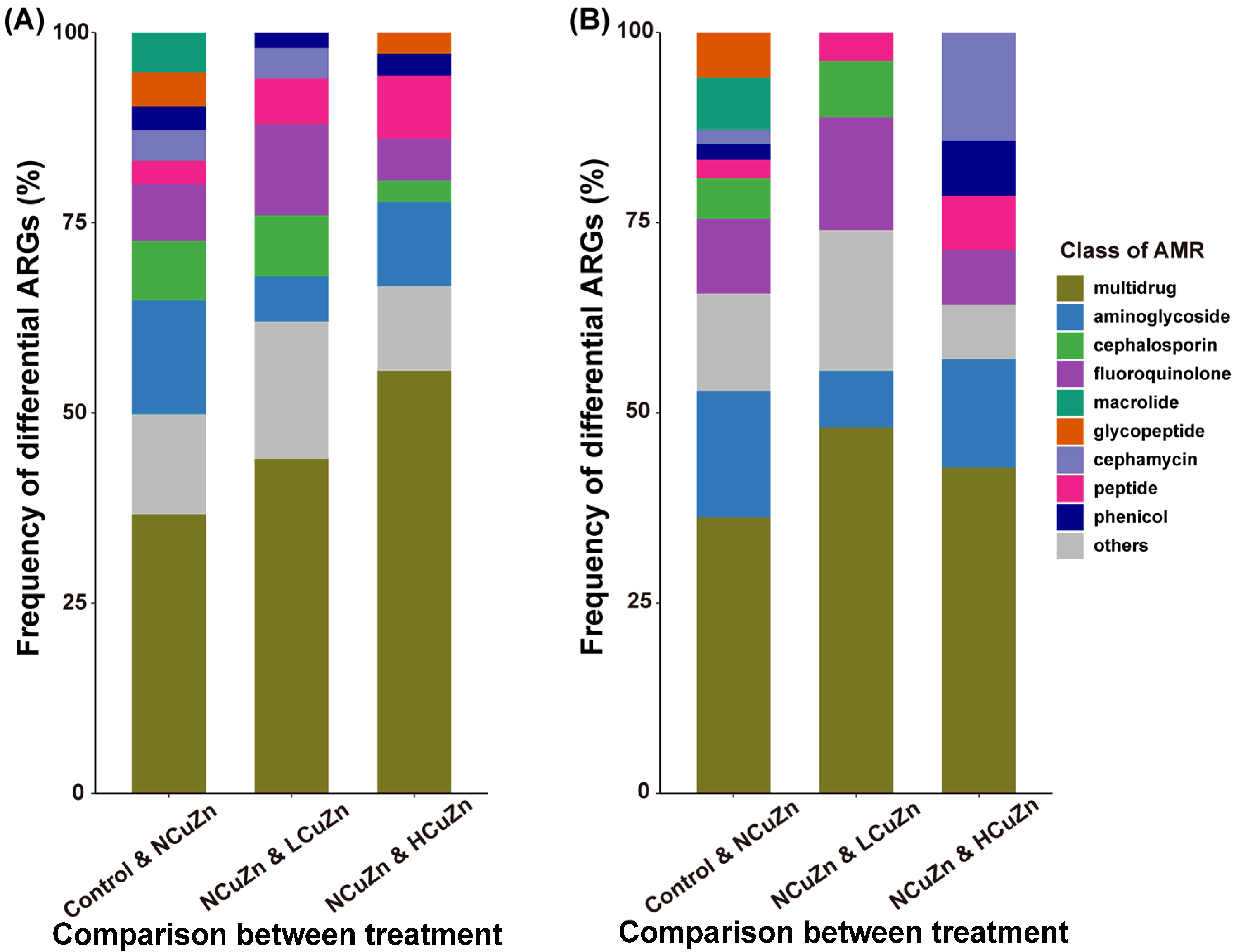
3.4. Comparison of Differential Resistance Genes between Soil Groups
3.5. Mobile Genetic Elements in Soil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yazdankhah, S.; Rudi, K.; Bernhoft, A. Zinc and copper in animal feed–development of resistance and co-resistance to antimicrobial agents in bacteria of animal origin. Microb. Ecol. Health Dis. 2014, 25, 25862. [Google Scholar] [CrossRef]
- Jensen, J.; Larsen, M.M.; Bak, J. National monitoring study in Denmark finds increased and critical levels of copper and zinc in arable soils fertilized with pig slurry. Environ. Pollut. 2016, 214, 334–340. [Google Scholar] [CrossRef]
- Hejna, M.; Gottardo, D.; Baldi, A.; Dell’Orto, V.; Cheli, F.; Zaninelli, M.; Rossi, L. Review: Nutritional ecology of heavy metals. Animal 2018, 12, 2156–2170. [Google Scholar] [CrossRef]
- Espinosa, C.D.; Stein, H.H. Digestibility and metabolism of copper in diets for pigs and influence of dietary copper on growth performance, intestinal health, and overall immune status: A review. J. Anim. Sci. Biotechnol. 2021, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Yanxia, L.; Wei, L.; Chunye, L.; Wei, H.; Ming, Y. Copper content in animal manures and potential risk of soil copper pollution with animal manure use in agriculture. Resour. Conserv. Recycl. 2010, 54, 985–990. [Google Scholar] [CrossRef]
- Choudhary, S.; Sar, P. Real-time PCR based analysis of metal resistance genes in metal resistant Pseudomonas aeruginosa strain J007. J. Basic Microbiol. 2016, 56, 688–697. [Google Scholar] [CrossRef]
- Liu, K.; Sun, M.; Ye, M.; Chao, H.; Zhao, Y.; Xia, B.; Jiao, W.; Feng, Y.; Zheng, X.; Liu, M.; et al. Coexistence and association between heavy metals, tetracycline and corresponding resistance genes in vermicomposts originating from different substrates. Environ. Pollut. 2018, 244, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.; Thorsen, M.K.; Holm, P.E.; Jensen, J.; Nybroe, O.; Brandt, K.K. Cu Exposure under Field Conditions Coselects for Antibiotic Resistance as Determined by a Novel Cultivation-Independent Bacterial Community Tolerance Assay. Environ. Sci. Technol. 2010, 44, 8724–8728. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Jiang, L.; Li, B.; Dong, Y.; He, Y.; Qiu, Y. Screening and evaluation of heavy metals facilitating antibiotic resistance gene transfer in a sludge bacterial community. Sci. Total Environ. 2019, 695, 133862. [Google Scholar] [CrossRef]
- Thomas, J.C.; Oladeinde, A.; Kieran, T.J.; Finger, J.W.; Bayona-Vásquez, N.J.; Cartee, J.C.; Beasley, J.C.; Seaman, J.C.; McArthur, J.V.; Rhodes, O.E.; et al. Co-occurrence of antibiotic, biocide, and heavy metal resistance genes in bacteria from metal and radionuclide contaminated soils at the Savannah River Site. Microb. Biotechnol. 2020, 13, 1179–1200. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Wright, M.S.; Stepanauskas, R.; McArthur, J. Co-selection of antibiotic and metal resistance. Trends Microbiol. 2006, 14, 176–182. [Google Scholar] [CrossRef]
- FEEDAP. Revision of the currently authorised maximum copper content in complete feed. EFSA J. 2016, 14, 4563. [Google Scholar] [CrossRef]
- Mazili, S.R. 5 Key Facts Pig Producers Need to Know about the EU’s ZnO ban the Pig Site. 2020. Available online: https://www.pigprogress.net/health-nutrition/5-key-facts-about-the-eus-zno-ban/ (accessed on 27 February 2021).
- Liu, W.-R.; Zeng, D.; She, L.; Su, W.-X.; He, D.-C.; Wu, G.-Y.; Ma, X.-R.; Jiang, S.; Jiang, C.-H.; Ying, G.-G. Comparisons of pollution characteristics, emission situations, and mass loads for heavy metals in the manures of different livestock and poultry in China. Sci. Total Environ. 2020, 734, 139023. [Google Scholar] [CrossRef]
- Blau, K.; Samuel, J.; Sørensen, S.J.; Su, J.; Zhu, Y.; Smalla, K.; Jechalke, S. Manure and doxycycline affect the bacterial community and its resistome in lettuce rhizosphere and bulk soil. Front. Microbiol. 2019, 10, 725. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhu, C.; Liu, C.; Zhang, X.; Ding, J.; Zandi, P.; Li, H. The persistence of antimicrobial resistance and related environmental factors in abandoned and working swine feedlots. Environ. Pollut. 2019, 255, 113116. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.E.; Lu, J.; Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef]
- Li, N.; Chen, J.; Liu, C.; Yang, J.; Zhu, C.; Li, H. Cu and Zn exert a greater influence on antibiotic resistance and its transfer than doxycycline in agricultural soils. J. Hazard. Mater. 2021, 423, 127042. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R 4.0.0; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Zhang, B.; Yuan, Q.; Wang, M.M.; Sun, R.; Liu, H.; Wang, P. Insights into the effects of Zn exposure on the fate of tylosin resistance genes and dynamics of microbial community during co-composting with tylosin fermentation dregs and swine manure. Sci. Pollut. Res. Int. 2021, 28, 14423–14433. [Google Scholar] [CrossRef]
- He, H.; Liu, H.; Shen, T.; Wei, S.; Dai, J.; Wang, R. Influence of Cu application on ammonia oxidizers in fluvo-aquic soil. Geoderma 2018, 321, 141–150. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Lin, Q.; Chen, X.; Chen, Y. Heavy metal availability and impact on activity of soil microorganisms along a Cu/Zn contamination gradient. J. Environ. Sci. 2007, 19, 848–853. [Google Scholar] [CrossRef]
- Alvarez, A.; Saez, J.M.; Costa, J.S.D.; Colin, V.L.; Fuentes, M.S.; Cuozzo, S.A.; Benimeli, C.S.; Polti, M.A.; Amoroso, M.J. Actinobacteria: Current research and perspectives for bioremediation of pesticides and heavy metals. Chemosphere 2017, 166, 41–62. [Google Scholar] [CrossRef]
- Majzlik, P.; Strasky, A.; Adam, V.; Nemec, M.; Trnkova, L.; Zehnalek, J.; Hubalek, J.; Provaznik, I.; Kizek, R. Influence of Zinc(II) and Copper(II) Ions on Streptomyces Bacteria Revealed by Electrochemistry. Int. J. Electrochem. Sci. 2011, 6, 2171–2191. [Google Scholar]
- Hamdan, A.M.; Abd-El-Mageed, H.; Ghanem, N. Biological treatment of hazardous heavy metals by Streptomyces rochei ANH for sustainable water management in agriculture. Sci. Rep. 2021, 11, 9314. [Google Scholar] [CrossRef]
- Lu, M.; Jiao, S.; Gao, E.; Song, X.; Li, Z.; Hao, X.; Rensing, C.; Wei, G. Transcriptome response to heavy metals in Sinorhizo-bium meliloti CCNWSX0020 reveals new metal resistance determinants that also promote bioremediation by medicago lupulina in metal-contaminated soil. Appl. Environ. Microbiol. 2017, 83, e01244-17. [Google Scholar] [CrossRef]
- Alotaibi, B.S.; Khan, M.; Shamim, S. Unraveling the Underlying Heavy Metal Detoxification Mechanisms of Bacillus Species. Microorganisms 2021, 9, 1628. [Google Scholar] [CrossRef]
- Puopolo, G.; Tomada, S.; Sonego, P.; Moretto, M.; Engelen, K.; Perazzolli, M.; Pertot, I. The Lysobacter capsici AZ78 Genome Has a Gene Pool Enabling it to Interact Successfully with Phytopathogenic Microorganisms and Environmental Factors. Front. Microbiol. 2016, 7, 96. [Google Scholar] [CrossRef]
- El-Gendy, M.M.A.A.; El-Bondkly, A.M.A. Evaluation and enhancement of heavy metals bioremediation in aqueous solutions by Nocardiopsis sp. MORSY1948, and Nocardia sp. MORSY2014. Braz. J. Microbiol. 2016, 47, 571–586. [Google Scholar] [CrossRef]
- Jalilvand, N.; Akhgar, A.; Alikhani, H.A.; Rahmani, H.A.; Rejali, F. Removal of Heavy Metals Zinc, Lead, and Cadmium by Biomineralization of Urease-Producing Bacteria Isolated from Iranian Mine Calcareous Soils. J. Soil Sci. Plant Nutr. 2019, 20, 206–219. [Google Scholar] [CrossRef]
- von Rozycki, T.; Nies, D.H. Cupriavidus metallidurans: Evolution of a metal-resistant bacterium. Antonie Van Leeuwenhoek 2008, 96, 115–139. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Lou, C.; Wang, S.; Lu, Y.; Liu, M.; Hashmi, M.Z.; Liang, X.; Li, Z.; Liao, Y.; Qin, W.; et al. Effects of long-term manure applications on the occurrence of antibiotics and antibiotic resistance genes (ARGs) in paddy soils: Evidence from four field experiments in south of China. Soil Biol. Biochem. 2015, 90, 179–187. [Google Scholar] [CrossRef]
- Martínez, J.L. Antibiotics and Antibiotic Resistance Genes in Natural Environments. Science 2008, 321, 365–367. [Google Scholar] [CrossRef]
- D’Costa, V.M.; King, C.E.; Kalan, L.; Morar, M.; Sung, W.W.L.; Schwarz, C.; Froese, D.; Zazula, G.; Calmels, F.; Debruyne, R.; et al. Antibiotic resistance is ancient. Nature 2011, 477, 457–461. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, G.; Hou, S.; Zhang, T.; Li, Z.; Liang, F. Distribution of ARGs and MGEs among glacial soil, permafrost, and sediment using metagenomic analysis. Environ. Pollut. 2018, 234, 339–346. [Google Scholar] [CrossRef]
- van Hoek, A.H.A.M.; Mevius, D.; Guerra, B.; Mullany, P.; Roberts, A.P.; Aarts, H.J.M. Acquired Antibiotic Resistance Genes: An Overview. Front. Microbiol. 2011, 2, 203. [Google Scholar] [CrossRef]
- Sánchez, M.B.; Hernández, A.; Rodríguez-Martínez, J.M.; Martínez-Martínez, L.; Martinez, J.L. Predictive analysis of transmissible quinolone resistance indicates Stenotrophomonas maltophilia as a potential source of a novel family of Qnr determinants. BMC Microbiol. 2008, 8, 148. [Google Scholar] [CrossRef]
- Fan, X.-T.; Li, H.; Chen, Q.; Zhang, Y.-S.; Ye, J.; Zhu, Y.-G.; Su, J.-Q. Fate of Antibiotic Resistant Pseudomonas putida and Broad Host Range Plasmid in Natural Soil Microcosms. Front. Microbiol. 2019, 10, 194. [Google Scholar] [CrossRef]
- Lerminiaux, N.A.; Cameron, A.D.S. Horizontal transfer of antibiotic resistance genes in clinical environments. Can. J. Microbiol. 2019, 65, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Mahillon, J.; Chandler, M. Insertion sequences. Microbiol. Mol. Biol. Rev. 1998, 62, 725–774. [Google Scholar] [CrossRef]
- Callanan, M.J.; Ross, R.P.; Beresford, T.P. Insertion sequence elements as mediators of strain diversity in Lactobacillus helveticus. Int. J. Food Microbiol. 2007, 120, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Marathe, N.P.; Gillings, M.R.; Flach, C.-F.; Kristiansson, E.; Joakim Larsson, D.G. Discovery of the fourth mobile sulfonamide resistance gene. Microbiome 2017, 5, 160. [Google Scholar] [CrossRef] [PubMed]
- Cury, J.; Jové, T.; Touchon, M.; Néron, B.; Rocha, E.P. Identification and analysis of integrons and cassette arrays in bacterial genomes. Nucleic Acids Res. 2016, 44, 4539–4550. [Google Scholar] [CrossRef] [Green Version]
- Wozniak, R.A.F.; Waldor, M.K. Integrative and conjugative elements: Mosaic mobile genetic elements enabling dynamic lateral gene flow. Nat. Rev. Microbiol. 2010, 8, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Santoro, F.; Romeo, A.; Pozzi, G.; Iannelli, F. Excision and Circularization of Integrative Conjugative Element Tn5253 of Streptococcus pneumoniae. Front. Microbiol. 2018, 9, 1779. [Google Scholar] [CrossRef]
- Johnson, C.M.; Grossman, A.D. Integrative and Conjugative Elements (ICEs): What They Do and How They Work. Annu. Rev. Genet. 2015, 49, 577–601. [Google Scholar] [CrossRef]
- Delavat, F.; Miyazaki, R.; Carraro, N.; Pradervand, N.; van der Meer, J.R. The hidden life of integrative and conjugative elements. FEMS Microbiol. Rev. 2017, 41, 512–537. [Google Scholar] [CrossRef]
- Zhou, Z.C.; Zheng, J.; Wei, Y.Y.; Chen, T.; Dahlgren, R.A.; Shang, X.; Chen, H. Antibiotic resistance genes in an urban river as impacted by bacterial community and physicochemical parameters. Environ. Sci. Pollut. Res. 2017, 24, 23753–23762. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wen, Q.; Chen, Z. Impacts of Cu and Zn on the performance, microbial community dynamics and resistance genes variations during mesophilic and thermophilic anaerobic digestion of swine manure. Bioresour. Technol. 2020, 312, 123554. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Peng, F.; Gao, X.; Xiao, P.; Yang, J. Decoupling the Dynamics of Bacterial Taxonomy and Antibiotic Resistance Function in a Subtropical Urban Reservoir as Revealed by High-Frequency Sampling. Front. Microbiol. 2019, 10, 1448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, A.Z.; Cen, T.; Li, X.; He, M.; Li, D.; Chen, J. Sub-inhibitory concentrations of heavy metals facilitate the horizontal transfer of plasmid-mediated antibiotic resistance genes in water environment. Environ. Pollut. 2018, 237, 74–82. [Google Scholar] [CrossRef]
- Xu, G.; Yang, L.; Li, Y.; Fu, J.; Xia, J.; Wu, K.; Wu, J. Study on heavy metal content and its correlation on large-scale pig farms in Jiangxi Province. Anim. Husband. Vet. Med. 2020, 52, 44–49. (In Chinese) [Google Scholar]
- Legros, S.; Chaurand, P.; Rose, J.; Masion, A.; Briois, V.; Ferrasse, J.-H.; Macary, H.S.; Bottero, J.-Y.; Doelsch, E. Investigation of Copper Speciation in Pig Slurry by a Multitechnique Approach. Environ. Sci. Technol. 2010, 44, 6926–6932. [Google Scholar] [CrossRef]

| Metal | Cu | Zn | Cd | As | Pb | Ni |
|---|---|---|---|---|---|---|
| Soil * | 22.16 ± 0.29 | 49.60 ± 0.44 | 0.16 ± 0.02 | ND | ND | ND |
| Pig manure | 27.48 ± 1.29 | 93.14 ± 6.04 | 0.92 ± 0.04 | 0.87 ± 0.07 | 7.07 ± 0.35 | 7.44 ± 0.54 |
| Index | ACE | ACE Significance | Shannon | Shannon Significance |
|---|---|---|---|---|
| Control | 202.33 ± 6.03 1 | C 2 | 5.54 ± 0.13 | C 2 |
| NCuZn | 620.67 ± 7.02 | b | 6.04 ± 0.064 | B |
| LCuZn | 656.67 ± 44.46 | b | 6.21 ± 0.064 | B |
| HCuZn | 757.67 ± 35.53 | a | 6.47 ± 0.036 | A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Li, H.; Zhu, C.; Liu, C.; Su, G.; Chen, J. Controlling AMR in the Pig Industry: Is It Enough to Restrict Heavy Metals? Int. J. Environ. Res. Public Health 2022, 19, 11265. https://doi.org/10.3390/ijerph191811265
Li N, Li H, Zhu C, Liu C, Su G, Chen J. Controlling AMR in the Pig Industry: Is It Enough to Restrict Heavy Metals? International Journal of Environmental Research and Public Health. 2022; 19(18):11265. https://doi.org/10.3390/ijerph191811265
Chicago/Turabian StyleLi, Na, Hongna Li, Changxiong Zhu, Chong Liu, Guofeng Su, and Jianguo Chen. 2022. "Controlling AMR in the Pig Industry: Is It Enough to Restrict Heavy Metals?" International Journal of Environmental Research and Public Health 19, no. 18: 11265. https://doi.org/10.3390/ijerph191811265
APA StyleLi, N., Li, H., Zhu, C., Liu, C., Su, G., & Chen, J. (2022). Controlling AMR in the Pig Industry: Is It Enough to Restrict Heavy Metals? International Journal of Environmental Research and Public Health, 19(18), 11265. https://doi.org/10.3390/ijerph191811265








