Highly Efficient Degradation of Tetracycline Hydrochloride in Water by Oxygenation of Carboxymethyl Cellulose-Stabilized FeS Nanofluids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of CMC-FeS Nanofluids
2.3. TC Removal in Oxic FeS Suspensions or CMC-FeS Nanofluids
2.4. Analyses
3. Results and Discussion
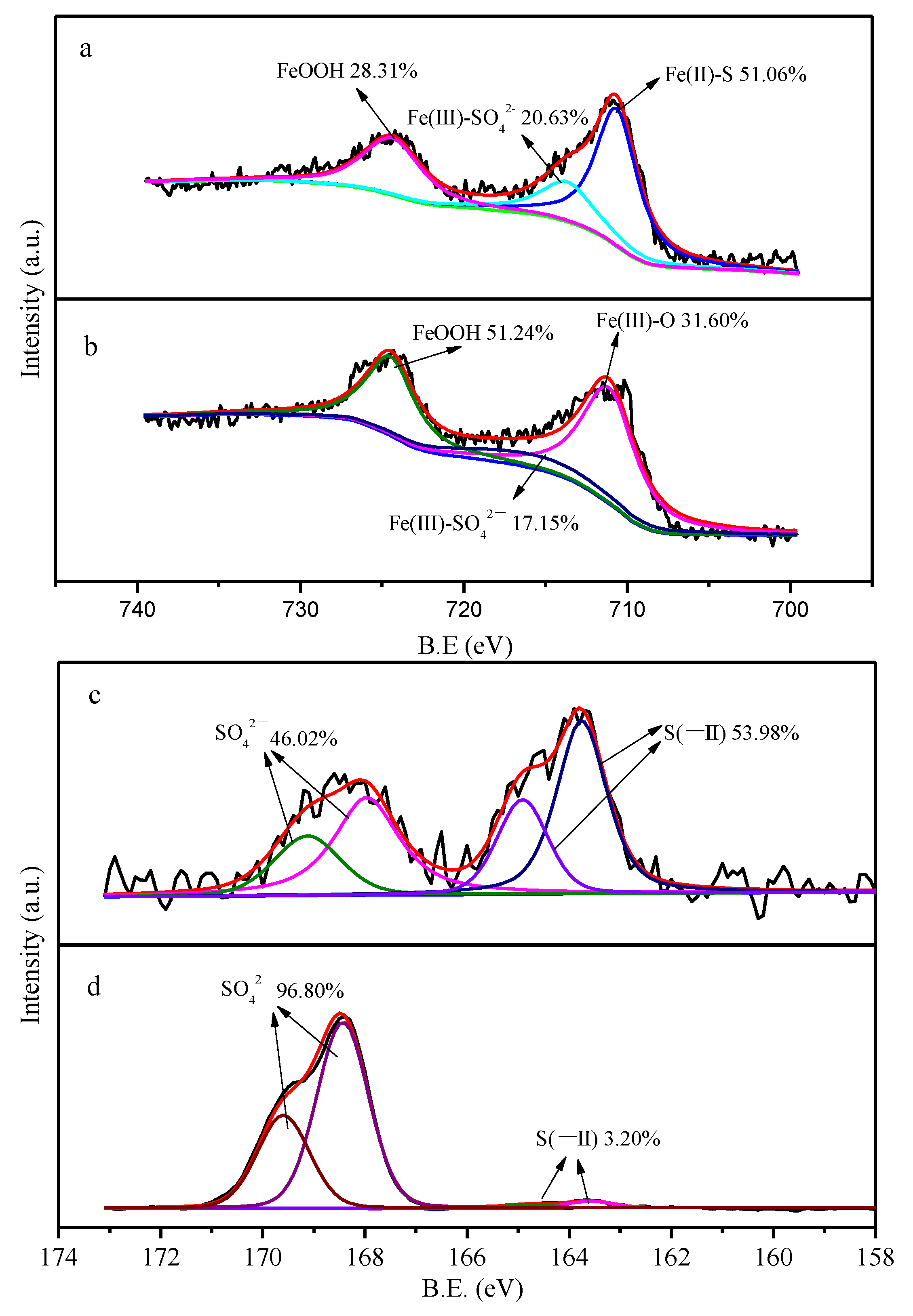
3.1. Characterizations
3.2. TC Removal at Different CMC-FeS Dosages
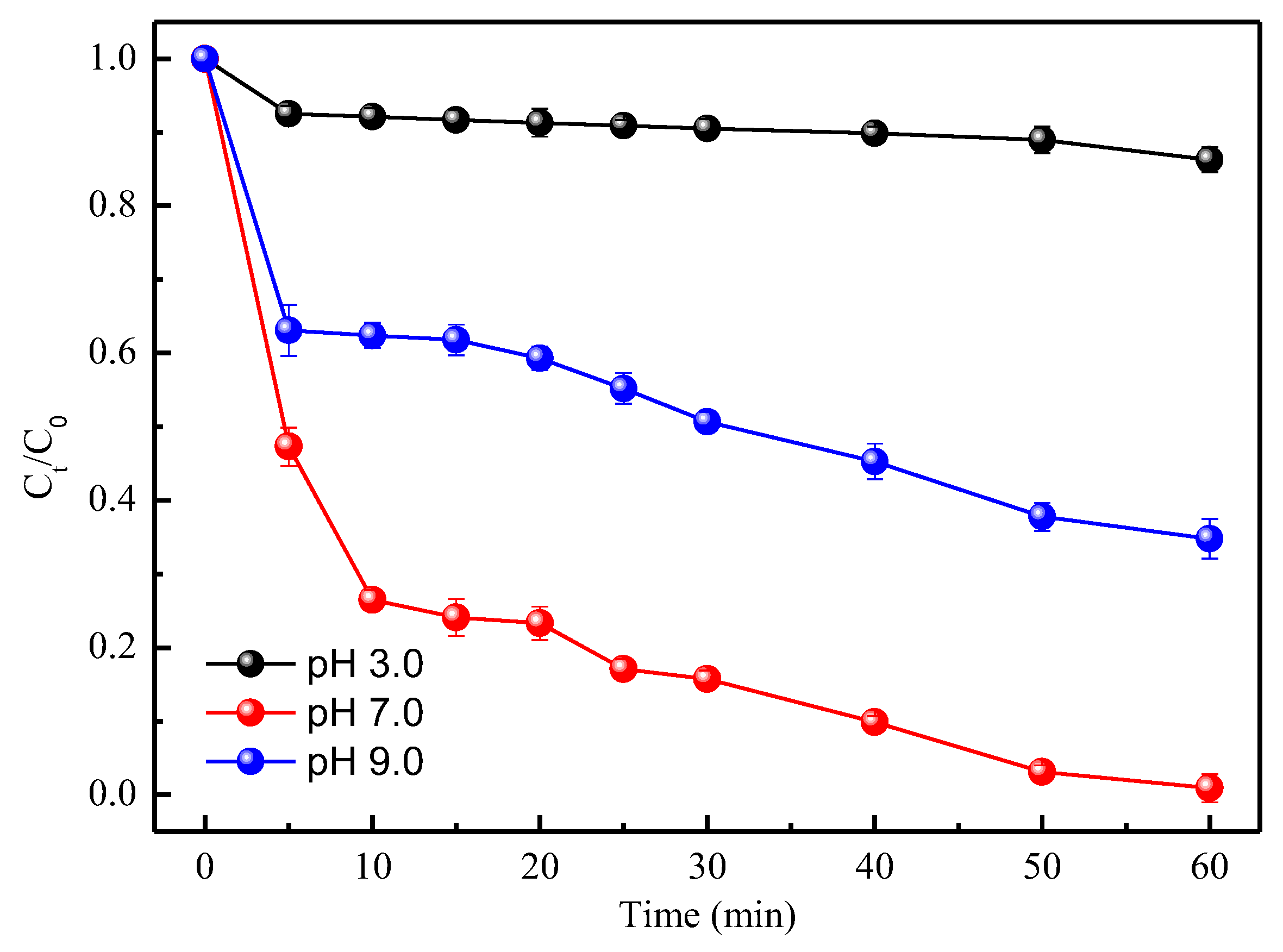
3.3. TC Removal at Different Initial pH Values
3.4. Mechanisms of TC Removal
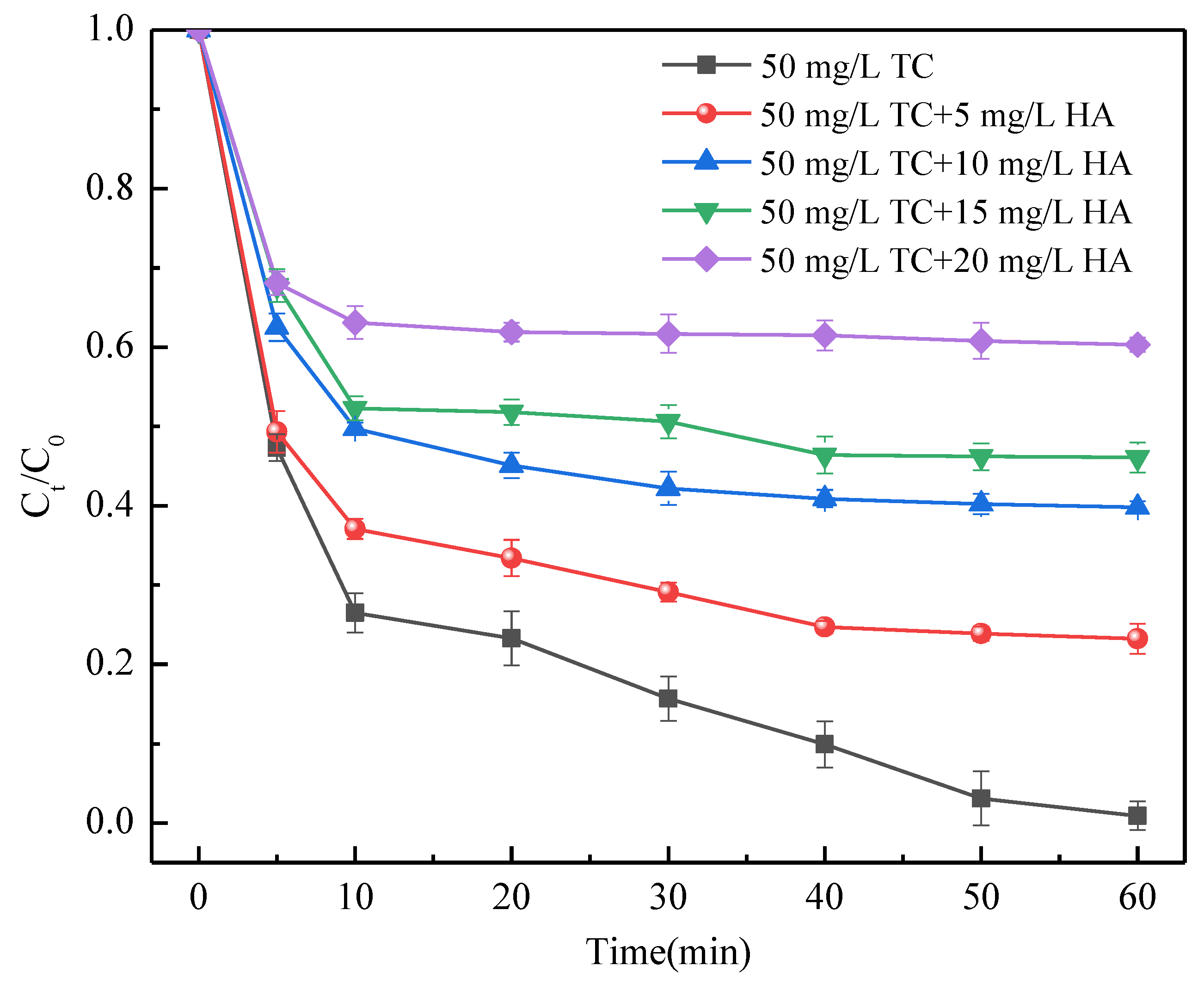
3.5. TC Removal in the Presence of Co-Existing Organic Impurities
3.6. Environmental Implications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pan, X.; Cheng, S.; Zhang, C.; Jiao, Y.; Lin, X.; Dong, W.; Qi, X. Mussel-inspired magnetic pullulan hydrogels for enhancing catalytic degradation of antibiotics from biomedical wastewater. Chem. Eng. J. 2021, 409, 128203. [Google Scholar] [CrossRef]
- Chang, P.H.; Li, Z.; Jean, J.S.; Jiang, W.T.; Wu, Q.; Kuo, C.Y.; Kraus, J. Desorption of tetracycline from montmorillonite by aluminum, calcium, and sodium: An indication of intercalation stability. Int. J. Environ. Sci. Technol. 2014, 11, 633–644. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; de Pedro, C.; Paxeus, N. Effluent from drug manufactures contains extremely high levels of pharmaceuticals. J. Hazard. Mater. 2007, 148, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, J.; Wang, J.; Fan, Y.; Zhang, S.; Dai, W. A novel multifunctional p-type semiconductor@MOFs nanoporous platform for simultaneous sensing and photodegradation of tetracycline. ACS Appl. Mater. Interfaces 2020, 12, 11036–11044. [Google Scholar] [CrossRef]
- Malik, A.H.; Iyer, P.K. Conjugated polyelectrolyte based sensitive detection and removal of antibiotics tetracycline from water. ACS Appl. Mater. Interfaces 2017, 9, 4433–4439. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wang, X.; Liu, Q.; Shi, B. N-doped FeOOH/RGO hydrogels with a dual-reaction-center for enhanced catalytic removal of organic pollutants. Chem. Eng. J. 2020, 379, 122310. [Google Scholar] [CrossRef]
- Gopal, G.; Alex, S.A.; Chandrasekaran, N.; Mukherjee, A. A review on tetracycline removal from aqueous systems by advanced treatment techniques. Rsc Adv. 2020, 10, 27081–27095. [Google Scholar] [CrossRef]
- Dey, P.; Parai, D.; Banerjee, M.; Hossain, S.T.; Mukherjee, S.K. Naringin sensitizes the antibiofilm effect of ciprofloxacin and tetracycline against Pseudomonas aeruginosa biofilm. Int. J. Med. Microbiol. 2020, 310, 151410. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, F.; Guo, H.; Peng, L.E.; Ma, X.-H.; Song, X.-X.; Wang, Z.; Tang, C.Y. Mechanistic insights into the role of polydopamine interlayer toward improved separation performance of polyamide nanofiltration membranes. Environ. Sci. Technol. 2020, 54, 11611–11621. [Google Scholar] [CrossRef]
- Jiao, Y.; Cheng, S.; Wu, F.; Pan, X.; Xie, A.; Zhu, X.; Dong, W. MOF-Guest complex derived Cu/C nanocomposites with multiple heterogeneous interfaces for excellent electromagnetic waves absorption. Compos. Part B-Eng. 2021, 211, 108643. [Google Scholar] [CrossRef]
- Wang, Z.; Muhammad, Y.; Tang, R.; Lu, C.; Yu, S.; Song, R.; Tong, Z.; Han, B.; Zhang, H. Dually organic modified bentonite with enhanced adsorption and desorption of tetracycline and ciprofloxacine. Sep. Purif. Technol. 2021, 274, 119059. [Google Scholar] [CrossRef]
- Gong, Y.; Tang, J.; Zhao, D. Application of iron sulfide particles for groundwater and soil remediation: A review. Water Res. 2016, 89, 309–320. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, W.; Li, Y.; Wu, Y.; Chen, Y.; Xiao, W.; Zhao, L.; Zhang, J.; Li, H. Modification, application and reaction mechanisms of nano-sized iron sulfide particles for pollutant removal from soil and water: A review. Chem. Eng. J. 2019, 362, 144–159. [Google Scholar] [CrossRef]
- Cheng, D.; Neumann, A.; Yuan, S.; Liao, W.; Qian, A. Oxidative degradation of organic contaminants by FeS in the presence of O2. Environ. Sci. Technol. 2020, 54, 4091–4101. [Google Scholar] [CrossRef]
- He, J.; Miller, C.J.; Collins, R.; Wang, D.; Waite, T.D. Production of a surface-localized oxidant during oxygenation of mackinawite (FeS). Environ. Sci. Technol. 2020, 54, 1167–1176. [Google Scholar] [CrossRef]
- Cheng, D.; Yuan, S.; Liao, P.; Zhang, P. Oxidizing impact induced by mackinawite (FeS) nanoparticles at oxic conditions due to production of hydroxyl radicals. Environ. Sci. Technol. 2016, 50, 11646–11653. [Google Scholar] [CrossRef]
- Wang, H.; Yao, H.; Sun, P.; Li, D.; Huang, C.-H. Transformation of tetracycline antibiotics and Fe(II) and Fe(III) species induced by their complexation. Environ. Sci. Technol. 2016, 50, 145–153. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, Y.; Xiong, Z.; Zhao, D. Immobilization of mercury by carboxymethyl cellulose stabilized iron sulfide nanoparticles: Reaction mechanisms and effects of stabilizer and water chemistry. Environ. Sci. Technol. 2014, 48, 3986–3994. [Google Scholar] [CrossRef]
- He, F.; Zhao, D.; Paul, C. Field assessment of carboxymethyl cellulose stabilized iron nanoparticles for in situ destruction of chlorinated solvents in source zones. Water Res. 2010, 44, 2360–2370. [Google Scholar] [CrossRef]
- Liu, J.; Valsaraj, K.T.; Devai, I.; DeLaune, R.D. Immobilization of aqueous Hg(II) by mackinawite (FeS). J. Hazard. Mater. 2008, 157, 432–440. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, Y.; Xiong, Z.; Kaback, D.; Zhao, D. Immobilization of mercury in field soil and sediment using carboxymethyl cellulose stabilized iron sulfide nanoparticles. Nanotechnology 2012, 23, 294007. [Google Scholar] [CrossRef]
- Xiao, H.; Peng, H.; Deng, S.; Yang, X.; Zhang, Y.; Li, Y. Preparation of activated carbon from edible fungi residue by microwave assisted K2CO3 activation-Application in reactive black 5 adsorption from aqueous solution. Bioresour. Technol. 2012, 111, 127–133. [Google Scholar] [CrossRef]
- Solis, R.R.; Dinc, O.; Fang, G.; Nadagouda, M.N.; Dionysiou, D.D. Activation of inorganic peroxides with magnetic graphene for the removal of antibiotics from wastewater. Environ. Sci. Nano 2021, 8, 960–977. [Google Scholar] [CrossRef]
- Solis, R.R.; Gomez-Aviles, A.; Belver, C.; Rodriguez, J.J.; Bedia, J. Microwave-assisted synthesis of NH2-MIL-125(Ti) for the solar photocatalytic degradation of aqueous emerging pollutants in batch and continuous tests. J. Environ. Chem. Eng. 2021, 9, 106230. [Google Scholar] [CrossRef]
- Leandri, V.; Gardner, J.M.; Jonsson, M. Coumarin as a quantitative probe for hydroxyl radical formation in heterogeneous photocatalysis. J. Phys. Chem. C 2019, 123, 6667–6674. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Z.; Zhang, Y. Anaerobic ammonium removal pathway driven by the Fe(II)/Fe(III) cycle through intermittent aeration. Environ. Sci. Technol. 2021, 55, 7615–7623. [Google Scholar] [CrossRef]
- Kong, H.; Yang, J.; Zhang, Y.; Fang, Y.; Nishinari, K.; Phillips, G.O. Synthesis and antioxidant properties of gum arabic-stabilized selenium nanoparticles. Int. J. Biol. Macromol. 2014, 65, 155–162. [Google Scholar] [CrossRef]
- Tian, S.; Gong, Y.; Ji, H.; Duan, J.; Zhao, D. Efficient removal and long-term sequestration of cadmium from aqueous solution using ferrous sulfide nanoparticles: Performance, mechanisms, and long-term stability. Sci. Total Environ. 2020, 704, 135402. [Google Scholar] [CrossRef]
- Tso, C.-P.; Shih, Y.-H. The influence of carboxymethylcellulose (CMC) on the reactivity of Fe NPs toward decabrominated diphenyl ether: The Ni doping, temperature, pH, and anion effects. J. Hazard. Mater. 2017, 322, 145–151. [Google Scholar] [CrossRef]
- Yanzhi, R.; Ken-ichi, I.; Teiji, K. Structure of barium stearate films at the air/water interface investigated by polarization modulation infrared spectroscopy and πA isotherms. Langmuir 2001, 17, 2688–2693. [Google Scholar]
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Wu, N.; Fu, L.; Su, M.; Aslam, M.; Wong, K.C.; Dravid, V.P. Interaction of Fatty Acid Monolayers with Cobalt Nanoparticles. Nano Lett. 2015, 4, 383–386. [Google Scholar] [CrossRef]
- He, F.; Zhao, D.; Liu, J.; Roberts, C.B. Stabilization of Fe-Pd nanoparticles with sodium carboxymethyl cellulose for enhanced transport and dechlorination of trichloroethylene in soil and groundwater. Ind. Eng. Chem. Res. 2007, 46, 29–34. [Google Scholar] [CrossRef]
- Sylvestre, J.P.; Kabashin, A.V.; Sacher, E.; Meunier, M.; Luong, J.H.T. Stabilization and Size Control of Gold Nanoparticles during Laser Ablation in Aqueous Cyclodextrins. J. Am. Chem. Soc. 2004, 126, 7176–7177. [Google Scholar] [CrossRef]
- Yao, Y.; Mi, N.; He, C.; He, H.; Zhang, Y.; Zhang, Y.; Yin, L.; Li, J.; Yang, S.; Li, S.; et al. Humic acid modified nano-ferrous sulfide enhances the removal efficiency of Cr(VI). Sep. Purif. Technol. 2020, 240, 116623. [Google Scholar] [CrossRef]
- Yao, Y.; Mi, N.; He, C.; Zhang, Y.; Yin, L.; Li, J.; Wang, W.; Yang, S.; He, H.; Li, S.; et al. A novel colloid composited with polyacrylate and nano ferrous sulfide and its efficiency and mechanism of removal of Cr(VI) from Water. J. Hazard. Mater. 2020, 399, 123082. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.B.; Zeng, R.J. Reactivity enhancement of iron sulfide nanoparticles stabilized by sodium alginate: Taking Cr (VI) removal as an example. J. Hazard. Mater. 2017, 333, 275–284. [Google Scholar] [CrossRef]
- Duan, J.; Ji, H.; Zhao, X.; Tian, S.; Liu, X.; Liu, W.; Zhao, D. Immobilization of U(VI) by stabilized iron sulfide nanoparticles: Water chemistry effects, mechanisms, and long-term stability. Chem. Eng. J. 2020, 393, 124692. [Google Scholar] [CrossRef]
- Han, Y.-S.; Seong, H.J.; Chon, C.-M.; Park, J.H.; Nam, I.-H.; Yoo, K.; Ahn, J.S. Interaction of Sb(III) with iron sulfide under anoxic conditions: Similarities and differences compared to As(III) interactions. Chemosphere 2018, 195, 762–770. [Google Scholar] [CrossRef]
- Chen, W.-R.; Huang, C.-H. Transformation of tetracyclines mediated by Mn(II) and Cu(II) ions in the presence of oxygen. Environ. Sci. Technol. 2009, 43, 401–407. [Google Scholar] [CrossRef]
- Ohyama, T.; Cowan, J.A. Calorimetric studies of metal binding to tetracycline. Role of solvent structure in defining the selectivity of metal ion-drug interactions. Inorg. Chem. 1995, 34, 3083–3086. [Google Scholar] [CrossRef]
- Oladipo, A.A.; Ifebajo, A.O. Highly efficient magnetic chicken bone biochar for removal of tetracycline and fluorescent dye from wastewater: Two-stage adsorber analysis. J. Environ. Manag. 2018, 209, 9–16. [Google Scholar] [CrossRef]
- Xie, Y.; Gu, L.; Mao, S.; Wu, D.; Fan, J. The role of structural elements and its oxidative products on the surface of ferrous sulfide in reducing the electron-withdrawing groups of tetracycline. Chem. Eng. J. 2019, 378. [Google Scholar] [CrossRef]
- Zhao, Y.; Tong, F.; Gu, X.; Gu, C.; Wang, X.; Zhang, Y. Insights into tetracycline adsorption onto goethite: Experiments and modeling. Sci. Total Environ. 2014, 470–471, 19–25. [Google Scholar] [CrossRef]
- Cao, J.; Xiong, Z.; Lai, B. Effect of initial pH on the tetracycline (TC) removal by zero-valent iron: Adsorption, oxidation and reduction. Chem. Eng. J. 2018, 343, 492–499. [Google Scholar] [CrossRef]
- Davis, C.C.; Chen, H.W.; Edwards, M. Modeling silica sorption to iron hydroxide. Environ. Sci. Technol. 2002, 36, 582–587. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Z.; Yang, Z.; Yang, Q.; Li, B.; Bai, Z. Heterogeneous fenton-like catalytic degradation of 2,4-dichlorophenoxyacetic acid in water with FeS. Chem. Eng. J. 2015, 273, 481–489. [Google Scholar] [CrossRef]
- Cao, J.; Lai, L.; Lai, B.; Yao, G.; Chen, X.; Song, L. Degradation of tetracycline by peroxymonosulfate activated with zero-valent iron: Performance, intermediates, toxicity and mechanism. Chem. Eng. J. 2019, 364, 45–56. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, H.; Zhang, G.; Yang, F.; Yuan, G.-E. Natural bornite as an efficient and cost-effective persulfate activator for degradation of tetracycline: Performance and mechanism. Chem. Eng. J. 2020, 381, 122717. [Google Scholar] [CrossRef]
- Keenan, C.R.; Sedlak, D.L. Factors affecting the yield of oxidants from the reaction of nanoparticulate zero-valent iron and oxygen. Environ. Sci. Technol. 2008, 42, 1262–1267. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J. Magnetic nanoscaled Fe3O4/CeO2 composite as an efficient Fenton-like heterogeneous catalyst for degradation of 4-chlorophenol. Environ. Sci. Technol. 2012, 46, 10145–10153. [Google Scholar] [CrossRef]
- Maier, A.C.; Iglebaek, E.H.; Jonsson, M. Confirming the formation of hydroxyl radicals in the catalytic decomposition of H2O2 on metal oxides using coumarin as a probe. ChemCatChem 2019, 11, 5435–5438. [Google Scholar] [CrossRef]
- Zhang, P.; Yuan, S.; Liao, P. Mechanisms of hydroxyl radical production from abiotic oxidation of pyrite under acidic conditions. Geochim. Et Cosmochim. Acta 2016, 172, 444–457. [Google Scholar] [CrossRef]
- Fan, J.; Gu, L.; Wu, D.; Liu, Z. Mackinawite (FeS) activation of persulfate for the degradation of p-chloroaniline: Surface reaction mechanism and sulfur-mediated cycling of iron species. Chem. Eng. J. 2018, 333, 657–664. [Google Scholar] [CrossRef]
- Jeong, J.; Song, W.; Cooper, W.J.; Jung, J.; Greaves, J. Degradation of tetracycline antibiotics: Mechanisms and kinetic studies for advanced oxidation/reduction processes. Chemosphere 2010, 78, 533–540. [Google Scholar] [CrossRef]
- Rose, A.L.; Waite, T.D. Kinetic model for Fe(II) oxidation in seawater in the absence and presence of natural organic matter. Environ. Sci. Technol. 2002, 36, 433–444. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, H.; Wang, Y.; Peng, H.; Zhu, Y.; Fang, D.; Wu, G.; Li, L.; Zeng, Z. Highly Efficient Degradation of Tetracycline Hydrochloride in Water by Oxygenation of Carboxymethyl Cellulose-Stabilized FeS Nanofluids. Int. J. Environ. Res. Public Health 2022, 19, 11447. https://doi.org/10.3390/ijerph191811447
Xiao H, Wang Y, Peng H, Zhu Y, Fang D, Wu G, Li L, Zeng Z. Highly Efficient Degradation of Tetracycline Hydrochloride in Water by Oxygenation of Carboxymethyl Cellulose-Stabilized FeS Nanofluids. International Journal of Environmental Research and Public Health. 2022; 19(18):11447. https://doi.org/10.3390/ijerph191811447
Chicago/Turabian StyleXiao, Hong, Yingjun Wang, Hong Peng, Ying Zhu, Dexin Fang, Ganxue Wu, Li Li, and Zhenxing Zeng. 2022. "Highly Efficient Degradation of Tetracycline Hydrochloride in Water by Oxygenation of Carboxymethyl Cellulose-Stabilized FeS Nanofluids" International Journal of Environmental Research and Public Health 19, no. 18: 11447. https://doi.org/10.3390/ijerph191811447






