Association of Plasma Cortisol Levels with Gestational Age and Anthropometric Values at Birth in Preterm Infants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligible Infants
2.2. Blood Sample Collection and Plasma Cortisol Measurement
2.3. Nutrition Policy from Birth to One-Month of Age
2.4. Clinical Characteristics and Disease Definitions
2.5. Statistical Analysis
3. Results
3.1. Study 1
3.1.1. Clinical Characteristics
3.1.2. Plasma Cortisol Levels at Birth and at 1 Month of Age
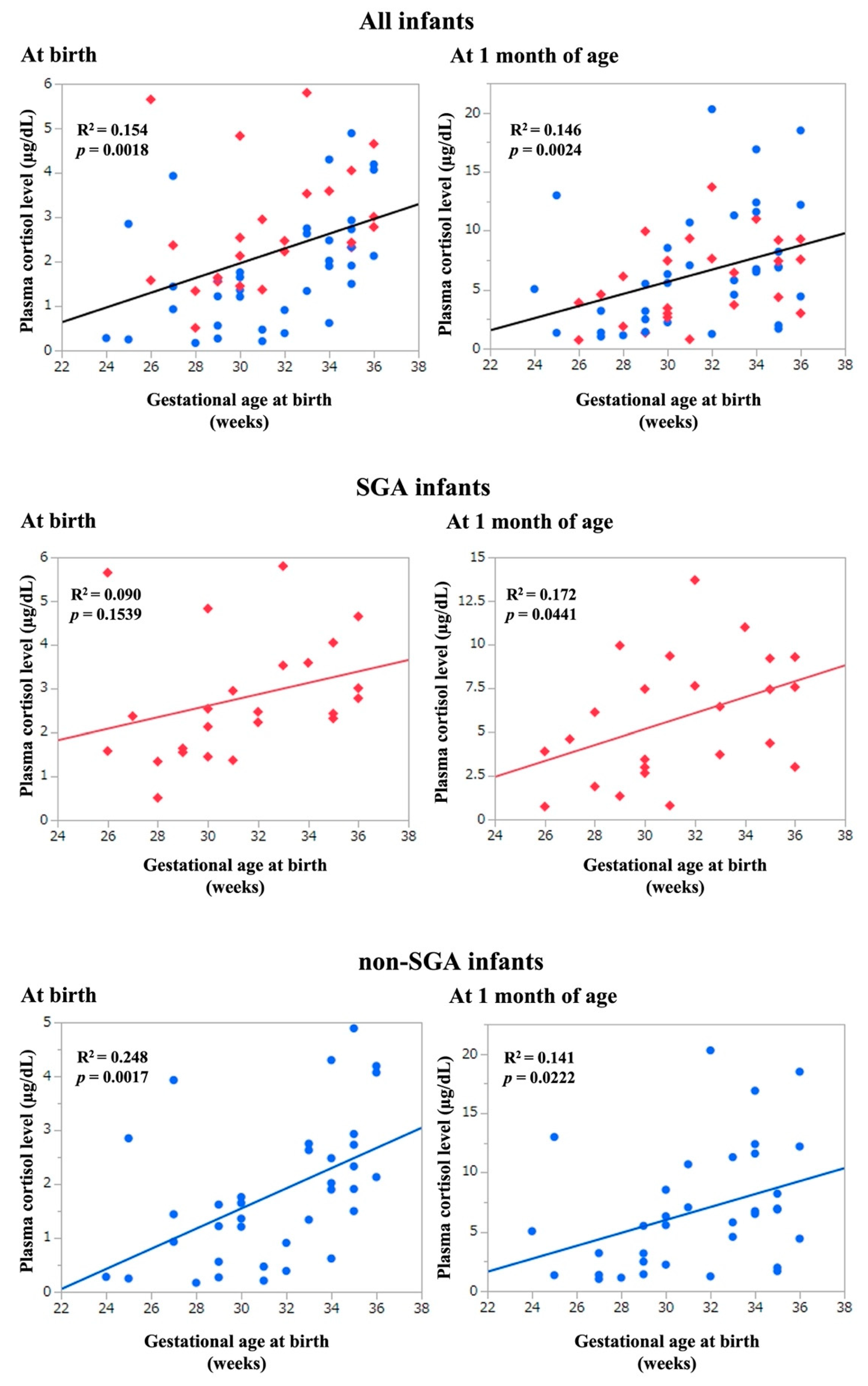
3.1.3. Association between Plasma Cortisol Levels and GA, and Anthropometric Values at Birth
3.2. Study 2
3.2.1. Clinical Characteristics
3.2.2. Plasma Cortisol Levels
4. Discussion
4.1. Maternal Steroid Administration and Fetal Cortisol Levels
4.2. Factors Affecting Plasma Cortisol Levels and Physique
4.3. Plasma Cortisol Levels in Preterm SGA Infants
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McTernan, C.L.; Draper, N.; Nicholson, H.; Chalder, S.M.; Driver, P.; Hewison, M.; Kilby, M.D.; Stewart, P.M. Reduced placental 11beta-hydroxysteroid dehydrogenase type 2 mRNA levels in human pregnancies complicated by intrauterine growth restriction: An analysis of possible mechanisms. J. Clin. Endocrinol. Metab. 2001, 86, 4979–4983. [Google Scholar] [PubMed]
- Mesiano, S.; Jaffe, R.B. Developmental and functional biology of the primate fetal adrenal cortex. Endocr. Rev. 1997, 18, 378–403. [Google Scholar] [PubMed]
- Vaughan, O.R.; De Blasio, M.J.; Fowden, A.L. Ovine uteroplacental and fetal metabolism during and after fetal cortisol overexposure in late gestation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R791–R801. [Google Scholar] [CrossRef]
- Long, N.M.; Ford, S.P.; Nathanielsz, P.W. Multigenerational effects of fetal dexamethasone exposure on the hypothalamic-pituitary-adrenal axis of first- and second-generation female offspring. Am. J. Obstet. Gynecol. 2013, 208, 217.e1–217.e8. [Google Scholar] [CrossRef] [PubMed]
- Papageorghiou, A.T.; Ohuma, E.O.; Altman, D.G.; Todros, T.; Cheikh Ismail, L.; Lambert, A.; Jaffer, Y.A.; Bertino, E.; Gravett, M.G.; Purwar, M.; et al. International standards for fetal growth based on serial ultrasound measurements: The fetal growth longitudinal study of the INTERGROWTH-21st project. Lancet 2014, 384, 869–879. [Google Scholar] [CrossRef]
- Iwata, S.; Kinoshita, M.; Okamura, H.; Tsuda, K.; Saikusa, M.; Harada, E.; Saitoh, S.; Iwata, O. Intrauterine growth and the maturation process of adrenal function. PeerJ 2019, 7, e6368. [Google Scholar] [CrossRef]
- Grunau, R.E.; Haley, D.W.; Whitfield, M.F.; Weinberg, J.; Yu, W.; Thiessen, P. Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. J. Pediatr. 2007, 150, 151–156. [Google Scholar] [CrossRef]
- Banks, B.A.; Stouffer, N.; Cnaan, A.; Ning, Y.; Merrill, J.D.; Ballard, R.A.; Ballard, P.L. Association of plasma cortisol and chronic lung disease in preterm infants. Pediatrics 2001, 107, 494–498. [Google Scholar] [CrossRef]
- Ng, P.C.; Lee, C.H.; Lam, C.W.; Ma, K.C.; Chan, I.H.; Wong, E.; Fok, T.F. Early pituitary-adrenal response and respiratory outcomes in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2004, 89, F127–F130. [Google Scholar] [CrossRef]
- Srivastava, T.; Merchant, R.H.; Ambadekar, M.C. Cord blood cortisol levels and respiratory distress syndrome. Indian Pediatr. 1994, 31, 923–928. [Google Scholar]
- Sybulski, S.; Maughan, G.B. Relationship between cortisol levels in umbilical cord plasma and development of the respiratory distress syndrome in premature newborn infants. Am. J. Obstet. Gynecol. 1976, 125, 239–243. [Google Scholar] [CrossRef]
- Masumoto, K.; Kusuda, S.; Aoyagi, H.; Tamura, Y.; Obonai, T.; Yamasaki, C.; Sakuma, I.; Uchiyama, A.; Nishida, H.; Oda, S.; et al. Comparison of serum cortisol concentrations in preterm infants with or without late-onset circulatory collapse due to adrenal insufficiency of prematurity. Pediatr. Res. 2008, 63, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Itabashi, K.; Miura, F.; Uehara, R.; Nakamura, Y. New Japanese neonatal anthropometric charts for gestational age at birth. Pediatr. Int. 2014, 56, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Noel, M.M.; Fraissinet, F.; Lefebvre, H.; Benichou, J.; Brunel, V.; Ziegler, F. Novel threshold value of midnight serum cortisol for diagnosis of hypercortisolism using the Roche Cortisol II assay. Clin. Biochem. 2022, 101, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Matsubara, K.; Nakamoto, O.; Ushijima, J.; Ohkuchi, A.; Koide, K.; Makino, S.; Mimura, K.; Morikawa, M.; Naruse, K.; et al. Outline of the new definition and classification of “Hypertensive Disorders of Pregnancy (HDP)”; A revised JSSHP statement of 2005. Hypertens. Res. Pregnancy 2018, 6, 33–37. [Google Scholar] [CrossRef]
- Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; de Leiva, A.; Hod, M.; Kitzmiler, J.L.; et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef]
- Nishikawa, T.; Ono, K.; Hashimoto, S.; Kinoshita, H.; Watanabe, T.; Araki, H.; Otsu, K.; Sakamoto, W.; Harada, M.; Toyonaga, T.; et al. One-hour oral glucose tolerance test plasma glucose at gestational diabetes diagnosis is a common predictor of the need for insulin therapy in pregnancy and postpartum impaired glucose tolerance. J. Diabetes Investig. 2018, 9, 1370–1377. [Google Scholar] [CrossRef]
- Kim, C.J.; Romero, R.; Chaemsaithong, P.; Chaiyasit, N.; Yoon, B.H.; Kim, Y.M. Acute chorioamnionitis and funisitis: Definition, pathologic features, and clinical significance. Am. J. Obs. Gynecol. 2015, 213, S29–S52. [Google Scholar] [CrossRef]
- Liszewski, M.C.; Stanescu, A.L.; Phillips, G.S.; Lee, E.Y. Respiratory distress in neonates: Underlying causes and current imaging assessment. Radiol. Clin. North Am. 2017, 55, 629–644. [Google Scholar] [CrossRef]
- Ogawa, Y. Chronic lung disease of the very low birth weight infant—Is it preventable? Turk. J. Pediatr. 1998, 40, 35–44. [Google Scholar]
- Kato, T.; Mandai, T.; Iwatani, S.; Koda, T.; Nagasaka, M.; Fujita, K.; Kurokawa, D.; Yamana, K.; Nishida, K.; Taniguchi-Ikeda, M.; et al. Extremely preterm infants small for gestational age are at risk for motor impairment at 3 years corrected age. Brain Dev. 2016, 38, 188–195. [Google Scholar] [CrossRef]
- Seckl, J.R. Glucocorticoids, feto-placental 11 beta-hydroxysteroid dehydrogenase type 2, and the early life origins of adult disease. Steroids 1997, 62, 89–94. [Google Scholar] [CrossRef]
- Kapoor, A.; Dunn, E.; Kostaki, A.; Andrews, M.H.; Matthews, S.G. Fetal programming of hypothalamo-pituitary-adrenal function: Prenatal stress and glucocorticoids. J. Physiol. 2006, 572, 31–44. [Google Scholar] [CrossRef]
- Stoye, D.Q.; Sullivan, G.; Galdi, P.; Kirschbaum, C.; Lamb, G.J.; Black, G.S.; Evans, M.J.; Boardman, J.P.; Reynolds, R.M. Perinatal determinants of neonatal hair glucocorticoid concentrations. Psychoneuroendocrinology 2021, 128, 105223. [Google Scholar] [CrossRef] [PubMed]
- Elhassan, M.E.; Miller, A.L.; Vazquez, D.M.; Lumeng, J.C. Associations of prenatal and perinatal factors with cortisol diurnal pattern and reactivity to stress at preschool age among children living in poverty. J. Clin. Res. Pediatr. Endocrinol. 2015, 7, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Buske-Kirschbaum, A.; Krieger, S.; Wilkes, C.; Rauh, W.; Weiss, S.; Hellhammer, D.H. Hypothalamic-pituitary-adrenal axis function and the cellular immune response in former preterm children. J. Clin. Endocrinol. Metab. 2007, 92, 3429–3435. [Google Scholar] [CrossRef]
- Kinoshita, M.; Iwata, S.; Okamura, H.; Tsuda, K.; Saikusa, M.; Harada, E.; Yamashita, Y.; Saitoh, S.; Iwata, O. Feeding-induced cortisol response in newborn infants. J. Clin. Endocrinol. Metab. 2018, 103, 4450–4455. [Google Scholar] [CrossRef]
- Polglase, G.R.; Ong, T.; Hillman, N.H. Cardiovascular alterations and multiorgan dysfunction after birth asphyxia. Clin. Perinatol. 2016, 4, 469–483. [Google Scholar] [CrossRef]
- Arends, N.J.; Boonstra, V.H.; Hokken-Koelega, A.C. Head circumference and body proportions before and during growth hormone treatment in short children who were born small for gestational age. Pediatrics 2004, 114, 683–690. [Google Scholar] [CrossRef]
- Ester, W.; Bannink, E.; van Dijk, M.; Willemsen, R.; van der Kaay, D.; de Ridder, M.; Hokken-Koelega, A. Subclassification of small for gestational age children with persistent short stature: Growth patterns and response to GH treatment. Horm. Res. 2008, 69, 89–98. [Google Scholar] [CrossRef]
- Hardy, D.B.; Yang, K. The expression of 11 beta-hydroxysteroid dehydrogenase type 2 is induced during trophoblast differentiation: Effects of hypoxia. J. Clin. Endocrinol. Metab. 2002, 87, 3696–3701. [Google Scholar] [PubMed]
- Kajantie, E.; Dunkel, L.; Turpeinen, U.; Stenman, U.H.; Wood, P.J.; Nuutila, M.; Andersson, S. Placental 11 beta-hydroxysteroid dehydrogenase-2 and fetal cortisol/cortisone shuttle in small preterm infants. J. Clin. Endocrinol. Metab. 2003, 88, 493–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, L.W.; Ehrenkranz, R.A.; Halliday, H.L. Early (<8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst. Rev. 2014, 5, CD001146. [Google Scholar]
- Słabuszewska-Jóżwiak, A.; Włodarczyk, M.; Kilian, K.; Rogulski, Z.; Ciebiera, M.; Szymańska-Majchrzak, J.; Zaręba, K.; Szymański, J.K.; Raczkiewicz, D.; Nowicka, G.; et al. Does the caesarean section impact on 11β HSD2 and fetal cortisol? Int. J. Environ. Res. Public Health 2020, 17, 5566. [Google Scholar] [CrossRef] [PubMed]
| Mothers | N = 53 |
| Age at delivery, years | 31 (24–36) |
| Pre-pregnancy BMI, kg/m2 | 21.5 (15.6–33.8) |
| Weight gain during pregnancy, kg | 5.9 (0.0–24.4) |
| Height, cm | 156.9 (144.6–172.0) |
| Placenta weight, g | 348 (136–804) |
| Di- and tri-chorionic births, n (%) | 6 (11.3) |
| Hypertensive disorders of pregnancy, n (%) | 16 (30.2) |
| Gestational diabetes mellitus, n (%) | 1 (1.9) |
| Chorioamnionitis, n (%) | 28 (52.8) |
| Prepartum use of betamethasone, n (%) | 37 (69.8) |
| Last use of betamethasone, days before delivery, n = 37 | 6 (0–35) |
| Infants | N = 61 |
| Male sex, n (%) | 22 (36) |
| GA, weeks | 31 (24–36) |
| BW, g | 1424 (464–2834) |
| BW SDS | −0.91(−4.23–1.69) |
| BL, cm | 39.0 (28.0–47.0) |
| BL SDS | −0.9 (−4.23–1.69) |
| Birth head circumstance, cm | 28.8 (20.0–34.5) |
| Birth head circumstance SDS | −0.16 (−2.48–1.92) |
| Respiratory distress syndrome, n (%) | 26 (42.6) |
| Chronic lung disease, n (%) | 14 (23.0) |
| Late-onset circulatory collapse, n (%) | 5 (8.2) |
| Patent ductus arteriosus, n (%) | 0 (0) |
| Sepsis, n (%) | 1 (1.6) |
| Retinopathy of prematurity, n (%) | 2 (3.3) |
| Necrotizing enterocolitis, n (%) | 0 (0) |
| Meconium disease, n (%) | 2 (3.3) |
| Intraventricular hemorrhage, n (%) | 0 (0) |
| Periventricular leukomalacia, n (%) | 2 (3.3) |
| A. Cortisol Level at Birth | ||
| Coefficient of Determination | p-Value | |
| GA, weeks | 0.154 | 0.002 |
| BW SDS | 0.057 | 0.063 |
| BL SDS | 0.063 | 0.051 |
| Birth head circumference SDS | 0.015 | 0.344 |
| B. Cortisol Level at 1 Month of Age | ||
| Coefficient of Determination | p-Value | |
| GA, weeks | 0.146 | 0.002 |
| BW SDS | 0.014 | 0.370 |
| BL SDS | 0.010 | 0.453 |
| Birth head circumference SDS | 0.058 | 0.062 |
| C. Δ Cortisol | ||
| Coefficient of Determination | p-Value | |
| GA, weeks | 0.067 | 0.044 |
| BW SDS | 0.037 | 0.137 |
| BL SDS | 0.031 | 0.172 |
| Birth head circumference SDS | 0.078 | 0.029 |
| A. Cortisol Level at Birth | ||
| Partial Correlation Coefficient (95% CI) | p-Value | |
| GA, weeks | 0.191 (0.092–0.290) | <0.001 |
| BW SDS | −0.459 (−0.934–0.015) | 0.058 |
| BL SDS | −0.127 (−0.581–0.328) | 0.580 |
| Birth head circumference SDS | 0.495 (−0.125–1.114) | 0.115 |
| B. Cortisol Level at 1 Month of Age | ||
| Partial Correlation Coefficient (95% CI) | p-Value | |
| GA, weeks | 0.558 (0.236–0.881) | 0.001 |
| BW SDS | −1.151 (−2.700–0.398) | 0.142 |
| BL SDS | 0.095 (−1.389–1.578) | 0.899 |
| Birth head circumference SDS | 2.533 (0.514–4.552) | 0.015 |
| C. Δ Cortisol | ||
| Partial Correlation Coefficient (95% CI) | p-Value | |
| GA, weeks | 0.367 (0.028–0.707) | 0.034 |
| BW SDS | −0.692 (−2.323–0.939) | 0.399 |
| BL SDS | 0.221 (−1.341–1.783) | 0.778 |
| Birth head circumference SDS | 2.039 (−0.876–4.165) | 0.060 |
| Mothers N = 53 | SGA N = 23 | Non-SGA N = 30 | p-Value |
| Age at delivery, years | 31 (26–36) | 32 (24–36) | 0.212 |
| Pre-pregnancy BMI, kg/m2 | 22.2 (16.7–33.8) | 20.9 (15.6–26.6) | 0.049 |
| Weight gain during pregnancy, kg | 7.1 (1.0–24.4) | 5.8 (0.0–14.8) | 0.543 |
| Height, cm | 157.6 (149.0–172.0) | 156.2 (144.6–168.0) | 0.287 |
| Placenta weight, g | 311 (136–480) | 420 (242–804) | <0.001 |
| Di- and tri-chorionic births, n (%) | 3 (13.0) | 3 (10.0) | 1.000 |
| Hypertensive disorders of pregnancy, n (%) | 12 (52.2) | 4 (13.3) | 0.006 |
| Gestational diabetes mellitus, n (%) | 1 (4.3) | 0 (0.0) | 0.434 |
| Chorioamnionitis, n (%) | 10 (43.5) | 18 (60.0) | 0.276 |
| Prepartum use of betamethasone, n (%) | 16 (69.6) | 21 (70.0) | 1.000 |
| Last use of betamethasone, days before delivery, n = 37 | 14 (0–35), n = 16 | 4 (0–28), n = 21 | 0.113 |
| Infants N = 61 | SGA N = 24 | Non-SGA N = 37 | |
| Male sex, n (%) | 11 (45.8) | 11 (29.7) | 0.068 |
| GA, weeks | 31 (26–36) | 32 (24–36) | 0.796 |
| BW, g | 1051 (464–1900) | 1575 (578–2834) | <0.001 |
| BW SDS | −2.5 (–4.23–−1.29) | −0.23 (−1.27–1.69) | <0.001 |
| BL, cm | 36.4 (28.0–46.5) | 40.5 (30.0–47.0) | 0.001 |
| BL SDS | −2.2 (−4.17–0.33) | −0.31 (−1.89–1.71) | <0.001 |
| Birth head circumstance, cm | 26.7 (20.7–31.0) | 29.2 (20.0–34.5) | 0.013 |
| Birth head circumstance SDS | −1.0 (−2.48–0.50) | 0.14 (−1.07–1.92) | <0.001 |
| Respiratory distress syndrome, n (%) | 11 (45.8) | 15 (40.5) | 0.793 |
| Chronic lung disease, n (%) | 7 (29.2) | 7 (18.9) | 0.370 |
| Late-onset circulatory collapse, n (%) | 3 (12.5) | 2 (5.4) | 0.373 |
| Sepsis, n (%) | 0 (0) | 1 (2.7) | 1.000 |
| Retinopathy of prematurity, n (%) | 1 (4.2) | 1 (2.7) | 1.000 |
| Meconium disease, n (%) | 1 (4.2) | 1 (2.7) | 1.000 |
| Periventricular leukomalacia, n (%) | 1 (4.2) | 1 (2.7) | 1.000 |
| All Infants N = 61 | SGA N = 24 | Non-SGA N = 37 | p-Value | |
|---|---|---|---|---|
| At birth, μg/dL | 2.02 (0.17–5.80) | 2.45 (0.51–5.80) | 1.62 (0.17–4.89) | 0.010 |
| 1 month of age, μg/dL | 5.80 (0.74–20.3) | 5.37 (0.74–13.7) | 5.80 (1.03–20.3) | 0.816 |
| Δ cortisol, μg/dL | 4.21 (−4.91–19.39) | 2.28 (−4.91–11.47) | 4.61 (−2.55–19.39) | 0.181 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aoki, M.; Urakami, T.; Nagano, N.; Aoki, R.; Morioka, I. Association of Plasma Cortisol Levels with Gestational Age and Anthropometric Values at Birth in Preterm Infants. Int. J. Environ. Res. Public Health 2022, 19, 11448. https://doi.org/10.3390/ijerph191811448
Aoki M, Urakami T, Nagano N, Aoki R, Morioka I. Association of Plasma Cortisol Levels with Gestational Age and Anthropometric Values at Birth in Preterm Infants. International Journal of Environmental Research and Public Health. 2022; 19(18):11448. https://doi.org/10.3390/ijerph191811448
Chicago/Turabian StyleAoki, Masako, Tatsuhiko Urakami, Nobuhiko Nagano, Ryoji Aoki, and Ichiro Morioka. 2022. "Association of Plasma Cortisol Levels with Gestational Age and Anthropometric Values at Birth in Preterm Infants" International Journal of Environmental Research and Public Health 19, no. 18: 11448. https://doi.org/10.3390/ijerph191811448





