Psychophysiological Responses of Cut Flower Fragrances as an Olfactory Stimulation by Measurement of Electroencephalogram in Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
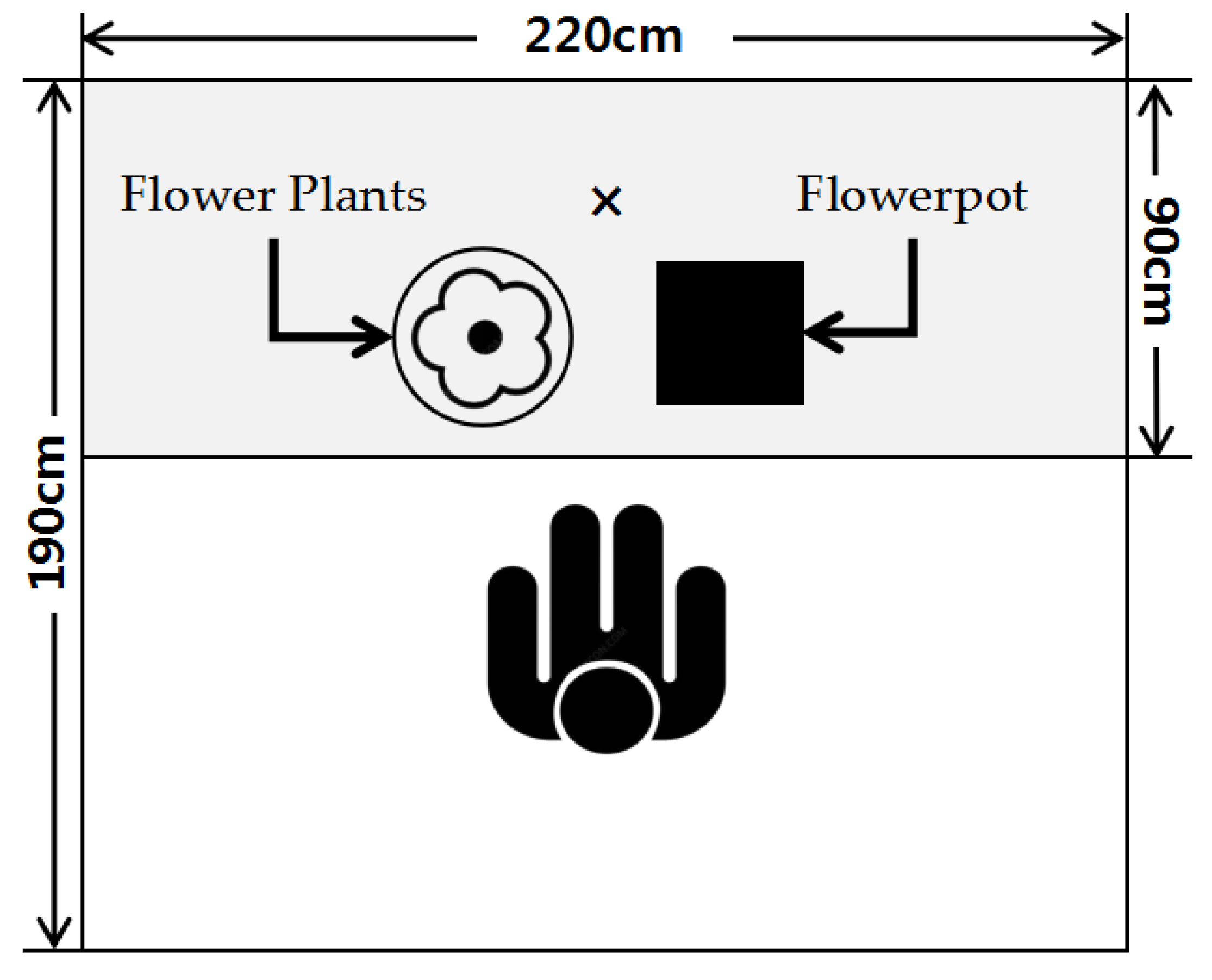
2.2. Experimental Environment
2.3. Experimental Protocol
2.4. Measurements
2.4.1. Electroencephalogram (EEG)
2.4.2. Subjective Evaluation of the Emotional States
2.4.3. Scent Strength of Flowers
2.5. Data Analysis
3. Results
3.1. Demographic Information
3.2. Electroencephalogram (EEG)
3.3. Subjective Evaluation of the Emotional States
3.4. Scent Strength
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohly, H.; White, M.P.; Wheeler, B.W.; Bethel, A.; Ukoumunne, O.C.; Nikolaou, V.; Garside, R. Attention Restoration Theory: A systematic review of the attention restoration potential of exposure to natural environments. J. Toxicol. Environ. Health Part B 2016, 19, 305–343. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, S.; Kaplan, R. The Experience of Nature: A Psychological Perspective; Cambridge University Press: Cambridge, UK, 1989; p. 340. [Google Scholar]
- Ulrich, R.S. Natural versus Urban Scenes: Some Psychophysiological Effects. Environ. Behav. 1981, 13, 523–556. [Google Scholar] [CrossRef]
- Gloster, A.T.; Lamnisos, D.; Lubenko, J.; Presti, G.; Squatrito, V.; Constantinou, M.; Nicolaou, C.; Papacostas, S.; Aydın, G.; Chong, Y.Y.; et al. Impact of COVID-19 pandemic on mental health: An international study. PLoS ONE 2020, 15, e0244809. [Google Scholar] [CrossRef]
- Anderson, K.A.; Chapin, K.P.; Reimer, Z.; Siffri, G. On fertile ground: An initial evaluation of green care farms in the United States. Home Health Care Serv. Q. 2017, 36, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Kim, S.O.; Gim, G.M.; Kim, D.S.; Park, S. Care farming program for family health: A pilot study with mothers and children. Int. J. Environ. Res. Public Health 2020, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- Sempik, J. Green care and mental health: Gardening and farming as health and social care. Ment. Health Soc. Incl. 2010, 14, 15–22. [Google Scholar] [CrossRef]
- Relf, P.D. Historical perspective on theoretical models for research and program development in horticultural therapy. Acta Hortic. 2008, 775, 79–91. [Google Scholar] [CrossRef]
- Son, K.C.; Jung, S.J.; Lee, A.Y.; Park, S.A. The theoretical model and universal definition of horticultural therapy. Acta Hortic. 2016, 1121, 79–88. [Google Scholar] [CrossRef]
- Park, S.; Lee, A.; Lee, G.J.; Kim, D.S.; Kim, W.S.; Shoemaker, C.A.; Son, K.C. Horticultural activity interventions and outcomes: A review. Hortic. Sci. Technol. 2016, 34, 513–527. [Google Scholar] [CrossRef]
- Chen, H.M.; Tu, H.M.; Ho, C.I. Understanding biophilia leisure as facilitating well-being and the environment: An examination of participants’ attitudes toward horticultural activity. Leis. Sci. 2013, 35, 301–319. [Google Scholar] [CrossRef]
- Oh, D.M. A Study on an Application of Horticultural Therapy to Social Welfare Policy. Ph.D. Thesis, Jeju University, Jeju, Korea, 2004. [Google Scholar]
- Esfandiary, E.; Karimipour, M.; Mardani, M.; Alaei, H.; Ghannadian, M.; Kazemi, M.; Mohammadnejad, D.; Hosseini, N.; Esmaeili, A. Novel effects of Rosa damascena extract on memory and neurogenesis in a rat model of Alzheimer’s disease. J. Neurosci. Res. 2014, 92, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Haviland-Jones, J.; Rosario, H.H.; Wilson, P.; McGuire, T.R. An environmental approach to positive emotion: Flowers. Evol. Psychol. 2005, 3, 104–132. [Google Scholar] [CrossRef]
- Kim, M.J.; Han, C.W.; Min, K.Y.; Cho, C.Y.; Lee, C.W.; Ogawa, Y.; Mori, E.; Kohzuki, M. Physical exercise with multicomponent cognitive intervention for older adults with Alzheimer’s disease: A 6-month randomized controlled trial. Dement. Geriatr. Cogn. Disord. Extra 2016, 6, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Lavin, J.; Lavin, C.; Bai, X.; Mastropaolo, S.; Feldman, D. Determining the effect of group flower arranging sessions on caregiver self-efficacy and stress levels in an in-patient hospice. OMEGA-J. Death Dying 2021, 84, 491–511. [Google Scholar] [CrossRef]
- Han, I.J. Effect of Horticultual Therapy Program on Mental Symptomatic Relief and Rehabilitation of Schizophrenics. Ph.D. Thesis, Catholic University of Daegu, Gyeongsan, Korea, 2005. [Google Scholar]
- Kang, S.J. Evaluation of the effects of horticultural therapy on physical ability in elderly people. Master’s Thesis, Yonsei University, Seoul, Korea, 2002. Unpublished. [Google Scholar]
- Lee, S.; Um, S.; Song, J.; Son, K. Effect of horticultural therapy using the floral decoration training on the improvement of occupational performance ability and vocational rehabilitation in mentally retarded. Hortic. Sci. Technol. 2007, 25, 474–484. [Google Scholar]
- Lee, S.S.; Park, S.; Kwon, O.Y.; Song, J.E.; Son, K.C. Measuring range of motion and muscle activation of flower arrangement tasks and application for improving upper limb function. Hortic. Sci. Technol. 2012, 30, 449–462. [Google Scholar] [CrossRef]
- Yang, J.W.; Lee, M.S.; Joung, D.; Park, B.J. Effects of using Natural and Artificial Flowers in Flower Arrangement on Psychological and Physiological Relaxation. J. People Plants Environ. 2022, 25, 39–48. [Google Scholar] [CrossRef]
- Du, J.; Yin, J.; Chen, X.; Hassan, A.; Fu, E.; Li, X. Electroencephalography (EEG)-Based Neural Emotional Response to Flower Arrangements (FAs) on Normal Elderly (NE) and Cognitively Impaired Elderly (CIE). Int. J. Environ. Res. Public Health 2022, 19, 3971. [Google Scholar] [CrossRef]
- Tao, J.; Hassan, A.; Qibing, C.; Yinggao, L.; Li, G.; Jiang, M.; Li, D.; Nian, L.; Bing-Yang, L.; Ziqin, Z. Psychological and physiological relaxation induced by nature-working with ornamental plants. Discret. Dyn. Nat. Soc. 2020, 2020, 6784512. [Google Scholar] [CrossRef]
- Morita, Y.; Ebara, F.; Morita, Y.; Horikawa, E. Increased activity in the right prefrontal cortex measured using near-infrared spectroscopy during a flower arrangement task. Int. J. Psychiatry Clin. Pract. 2018, 22, 34–39. [Google Scholar] [CrossRef]
- Mita, S.; Hosokawa, M.; Hayashi, T. The effect of reproducing two-dimensional photographs of flower arrangements in three dimensions on prefrontal blood flow in elderly patients with dementia. Acta Hortic. 2020, 1330, 139–146. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, H.S.; Yun, S.Y.; Choi, B.J.; Jang, E.J. Effects of horticultural activities and flower tea drinking based on reminiscent storytelling on demented elders’ cognitive and emotional functions. J. People Plants Environ. 2017, 20, 351–360. [Google Scholar] [CrossRef]
- Tarkka, I.M.; Hallett, M. Cortical topography of premotor and motor potentials preceding self-paced, voluntary movement of dominant and non-dominant hands. Electroencephalogr. Clin. Neurophysiol. 1990, 75, 36–43. [Google Scholar] [CrossRef]
- Moulton, E.; Galléa, C.; Kemlin, C.; Valabregue, R.; Maier, M.A.; Lindberg, P.; Rosso, C. Cerebello-cortical differences in effective connectivity of the dominant and non-dominant hand during a visuomotor paradigm of grip force control. Front. Hum. Neurosci. 2017, 11, 511. [Google Scholar] [CrossRef]
- Igarashi, M.; Song, C.; Ikei, H.; Ohira, T.; Miyazaki, Y. Effect of olfactory stimulation by fresh rose flowers on autonomic nervous activity. J. Altern. Complement. Med. 2014, 20, 727–731. [Google Scholar] [CrossRef]
- Jasper, H.H. The ten-twenty electrode system of the International Federation. Electroencephalogr. Clin. Neurophysiol. 1958, 10, 370–375. [Google Scholar]
- Rolls, E.T. The orbitofrontal cortex. Philos. Trans. R. Soc. B Biol. Sci. 1996, 351, 1433–1443. [Google Scholar] [CrossRef]
- McNair, D.M.; Heuchert, J.P.; Shilony, E. Profile of Mood States: Bibliography 1964–2002; Multi-Health Systems: Toronto, ON, Canada, 2003. [Google Scholar]
- Kim, E.J.; Lee, S.I.; Jeong, D.U.; Shin, M.S.; Yoon, I.Y. Standardization and reliability and validity of the Korean edition of Profile of Mood States (K-POMS). Sleep Med. Psychophysiol. 2003, 10, 39–51. [Google Scholar]
- McNair, D.M. Profile of Mood States; Educational and Industrial Testing Service: San Diego, CA, USA, 1992. [Google Scholar]
- Osgood, C.E.; Suci, G.J.; Tannenbaum, P.H. The Measurement of Meaning; University of Illinois Press: Champaign, IL, USA, 1957; p. 47. [Google Scholar]
- Green, B.G.; Dalton, P.; Cowart, B.; Shaffer, G.; Rankin, K.; Higgins, J. Evaluating the ‘Labeled Magnitude Scale’for measuring sensations of taste and smell. Chem. Senses 1996, 21, 323–334. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Cho, H.; Yu, B.; Kim, S. Effect of olfactory stimulation of isomeric aroma compounds,(+)-limonene and terpinolene on human electroencephalographic activity. Eur. J. Integr. Med. 2015, 7, 561–566. [Google Scholar] [CrossRef]
- Cho, S.H. Effects of horseback riding exercise on the relative alpha power spectrum in the elderly. Arch. Gerontol. Geriatr. 2017, 70, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Kober, S.E.; Witte, M.; Stangl, M.; Väljamäe, A.; Neuper, C.; Wood, G. Shutting down sensorimotor interference unblocks the networks for stimulus processing: An SMR neurofeedback training study. Clin. Neurophysiol. 2015, 126, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.C.; Yoo, Y.S.; Kim, E.S.; Kim, S.C.; Park, S.H.; Kim, J.K.; Kang, S.H. Development of KVSS test (Korean version of Sniffin’Sticks Test). Korean J. Otorhinolaryngol.-Head Neck Surg. 1999, 42, 855–860. [Google Scholar]
- Yang, Y.; Choi, H.R.; Cho, J.H.; Hong, S.C.; Kim, J.K. Clinical Feasibility of Scent Survey for Screening Test for Olfactory Function. J. Rhinol. 2018, 25, 14–20. [Google Scholar] [CrossRef]
- Stevenson, R.J.; Wilson, D.A. Learning to Smell: Olfactory Perception from Neurobiology to Behavior; JHU Press: Baltimore, MD, USA, 2006. [Google Scholar]
- Huang, L.C. An Evaluation of Flower Visual and Olfactory Perceptions Influencing Consumer Attitudes within Socio-Economic Groups. Ph.D. Thesis, Kansas State University, Manhattan, KS, USA, 1997. [Google Scholar]
- Çelikel, F.G.; Reid, M.S. Postharvest handling of stock (Matthiola incana). HortScience 2002, 37, 144–147. [Google Scholar] [CrossRef]
- Bahmanzadegan, A.; Rowshan, V. Static headspace analysis and polyphenol content of Tagetes erecta, Matthiola incana, Erysimum cheiri, Gaillardia grandiflora and Dahlia pinnata in Iran. Anal. Chem. Lett. 2018, 8, 794–802. [Google Scholar] [CrossRef]
- Hussain, S.; Rahman, R.; Mushtaq, A.; Belaskri, A.E. Clove: A review of a precious species with multiple uses. Int. J. Chem. Biochem. Sci. 2017, 11, 129–133. [Google Scholar]
- Kadohisa, M. Effects of odor on emotion, with implications. Front. Syst. Neurosci. 2013, 7, 66. [Google Scholar] [CrossRef]
- Weber, S.T.; Heuberger, E. The impact of natural odors on affective states in humans. Chem. Senses 2008, 33, 441–447. [Google Scholar] [CrossRef]
- Campenni, C.E.; Crawley, E.J.; Meier, M.E. Role of suggestion in odor-induced mood change. Psychol. Rep. 2004, 94, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.-T.; Li, X.; Jin, Z.-L.; Sun, M.; Wang, J.; Yang, W.-R.; Kong, Y.; Zhang, Q.-X. Major aroma ingredients of oriental lily’Siberia’and their effect on humans. Acta Hortic. 2011, 925, 307–313. [Google Scholar] [CrossRef]
- Kong, Y.; Sun, M.; Pan, H.T.; Zhang, Q.X. Composition and emission rhythm of floral scent volatiles from eight lily cut flowers. J. Am. Soc. Hortic. Sci. 2012, 137, 376–382. [Google Scholar] [CrossRef]
- Lee, Y.; Jeoung, B. The relationship between the behavior problems and motor skills of students with intellectual disability. J. Exerc. Rehabil. 2016, 12, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Ehrlichman, H.; Bastone, L. Olfaction and emotion. In Science of Olfaction; Springer: New York, NY, USA, 1992; pp. 410–438. [Google Scholar] [CrossRef]
- Betts, K.A.; Sikirica, V.; Hodgkins, P.; Zhou, Z.; Xie, J.; DeLeon, A.; Erder, M.H.; Wu, E.Q. Period prevalence of concomitant psychotropic medication usage among children and adolescents with attention-deficit/hyperactivity disorder during 2009. J. Child Adolesc. Psychopharmacol. 2014, 24, 260–268. [Google Scholar] [CrossRef]
- Di Carmine, F.; Berto, R. Contact with nature can help ADHD children to cope with their symptoms. The state of the evidence and future directions for research. Vis. Sustain. 2020, 14, 24–33. [Google Scholar] [CrossRef]
- Ressler, K.J.; Nemeroff, C.B. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress. Anxiety 2000, 12, 2–19. [Google Scholar] [CrossRef]






| Analysis Indicator | The Full Name of the EEG Power Spectrum Indicator | Indicator Estimate (Ratio) | State |
|---|---|---|---|
| RFA | Relative fast alpha | Higher alpha (11–13 Hz)/Total frequency (4–50 Hz) | Attention and concentration in a relaxed state [38,39]. |
| RSA | Relative slow alpha | Lower alpha (11–13 Hz)/Total frequency (4–50 Hz) | Stability and relax [38,39]. |
| SMR/theta | Sensory Motor Rhythm to theta | Lower beta (12–15 Hz)/theta (4–8 Hz) | Attention related to cognitive function [40]. |
| Variable | Male (n = 7) | Female (n = 23) | Total (N = 30) | |
|---|---|---|---|---|
| Mean ± SD | ||||
| Age (years) | 32.57 ± 12.46 | 35.57 ± 13.41 | 34.87 ± 13.04 | |
| Body Height (cm) | 175.94 ± 3.36 | 162.16 ± 4.33 | 165.61 ± 7.30 | |
| Body Weight (kg) | 70.94 ± 13.86 | 57.56 ± 9.80 | 60.91 ± 12.19 | |
| Olfactory Function | SSS 1 | 85.71 ± 7.89 | 83.7 ± 13.66 | 83.77 ± 12.47 |
| VAS 2 | 8.57 ± 0.98 | 7.78 ± 1.62 | 7.97 ± 1.51 | |
| Flower Arrangement Activity | RFA 1 | SMR 2 | RSA 3 | ||||
|---|---|---|---|---|---|---|---|
| Fp1 6 | Fp2 6 | Fp1 | Fp2 | Fp1 | Fp2 | ||
| Mean ± SD | |||||||
| Total (N = 30) | Chrysanthemum | 0.048 ± 0.006 ab5 | 0.049 ± 0.008 | 0.060 ± 0.007 | 0.064 ± 0.012 | 0.121 ± 0.016 | 0.122 ± 0.017 |
| Lily | 0.044 ± 0.004 b | 0.049 ± 0.009 | 0.057 ± 0.009 | 0.062 ± 0.013 | 0.113 ± 0.022 | 0.116 ± 0.020 | |
| Stock | 0.052 ± 0.013 a | 0.051 ± 0.007 | 0.065 ± 0.015 | 0.066 ± 0.013 | 0.118 ± 0.021 | 0.118 ± 0.015 | |
| Rose | 0.048 ± 0.007 ab | 0.049 ± 0.009 | 0.060 ± 0.009 | 0.062 ± 0.011 | 0.118 ± 0.024 | 0.116 ± 0.016 | |
| Carnation | 0.050 ± 0.011 a | 0.049 ± 0.008 | 0.063 ± 0.018 | 0.061 ± 0.010 | 0.121 ± 0.027 | 0.119 ± 0.014 | |
| P 4 | 0.024 * | 0.886 NS | 0.157 NS | 0.518 NS | 0.508 NS | 0.676 NS | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-T.; Lee, A.-Y.; Choi, N.-Y.; Park, S.-A. Psychophysiological Responses of Cut Flower Fragrances as an Olfactory Stimulation by Measurement of Electroencephalogram in Adults. Int. J. Environ. Res. Public Health 2022, 19, 11639. https://doi.org/10.3390/ijerph191811639
Wu Y-T, Lee A-Y, Choi N-Y, Park S-A. Psychophysiological Responses of Cut Flower Fragrances as an Olfactory Stimulation by Measurement of Electroencephalogram in Adults. International Journal of Environmental Research and Public Health. 2022; 19(18):11639. https://doi.org/10.3390/ijerph191811639
Chicago/Turabian StyleWu, Yu-Tong, A-Young Lee, Na-Yoon Choi, and Sin-Ae Park. 2022. "Psychophysiological Responses of Cut Flower Fragrances as an Olfactory Stimulation by Measurement of Electroencephalogram in Adults" International Journal of Environmental Research and Public Health 19, no. 18: 11639. https://doi.org/10.3390/ijerph191811639







