Functional Therapeutic Strategies Used in Different Stages of Alzheimer’s Disease—A Systematic Review
Abstract
:1. Introduction
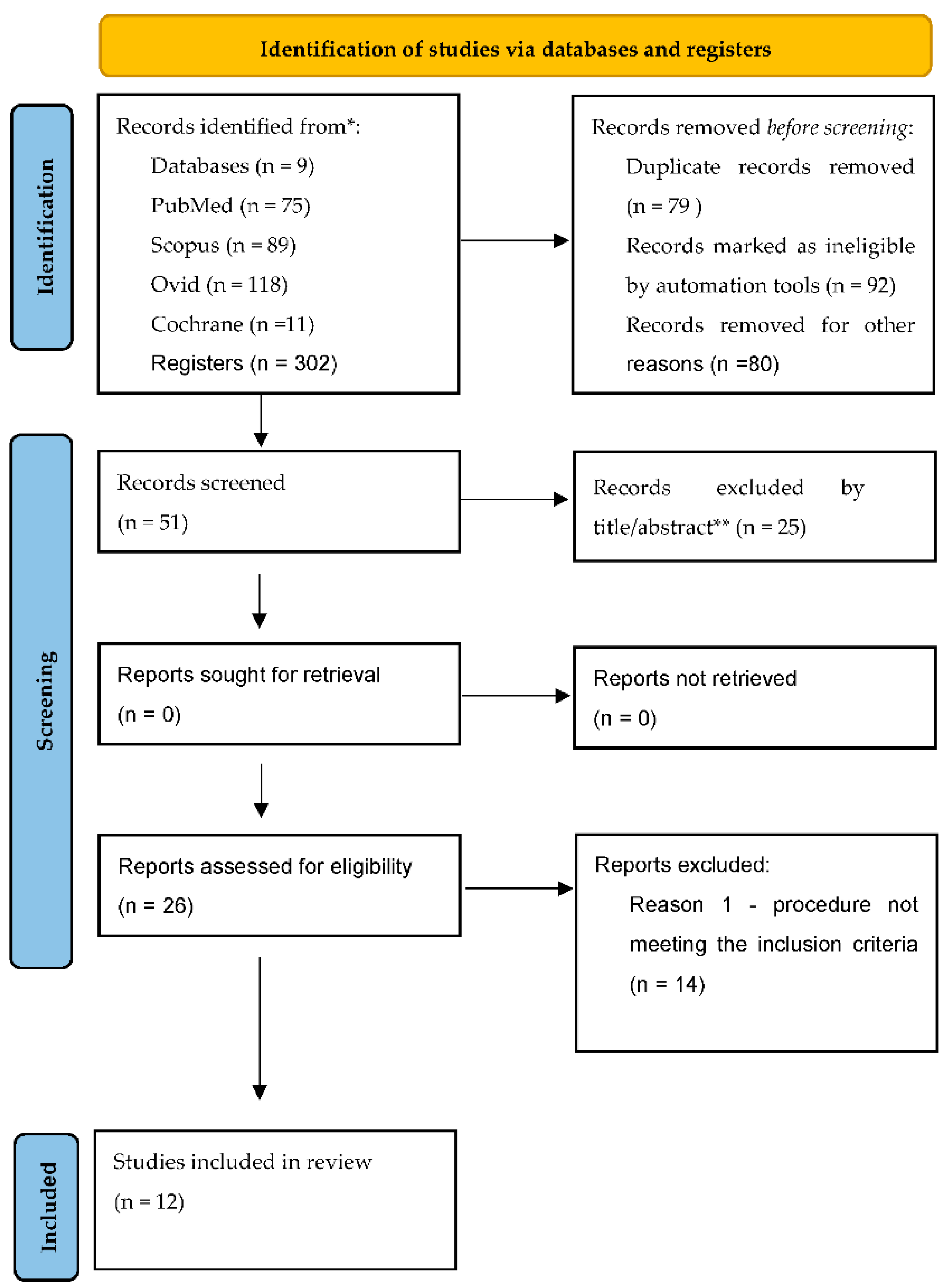
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection and Data Extraction
3. Results
3.1. Characteristics of the Studies
3.2. Evaluation of the Methodological Quality of the Included Research
4. Discussion
4.1. Research Value
4.2. Study Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Epperly, T.; Dunay, M.A.; Boice, J.L. Alzheimer Disease: Pharmacologic and Nonpharmacologic Therapies for Cognitive and Functional Symptoms. Am. Fam. Physician 2017, 95, 771–778. [Google Scholar] [PubMed]
- Abreu, A.; Armas, B.; Fuentes, C. Anti-ageing therapies in Alzheimer’s disease. Rev. Española Geriatría Gerontol. 2018, 53, 45–53. [Google Scholar] [CrossRef]
- Derakhshankhah, H.; Sajadimajd, S.; Jafari, S.; Izadi, Z.; Sarvari, S.; Sharifi, M.; Falahati, M.; Moakedi, F.; Muganda, W.C.A.; Müller, M.; et al. Novel therapeutic strategies for Alzheimer’s disease: Implications from cell-based therapy and nanotherapy. Nanomedicine 2020, 24, 102149. [Google Scholar] [CrossRef]
- Gao, L.B.; Yu, X.F.; Chen, Q.; Zhou, D. Alzheimer’s Disease therapeutics: Current and future therapies. Minerva Med. 2016, 107, 108–113. [Google Scholar]
- Alzheimer’s Association Report. Alzheimer’s Disease Facts and Figures. Alzheimer's Association Report 2021, 17, 327–406. [Google Scholar] [CrossRef]
- Citron, M. Alzheimer’s disease. treatments in discovery and development. Nat. Neurosci. 2002, 5, 1055–1057. [Google Scholar] [CrossRef] [PubMed]
- Neugroschl, J.; Wang, S. Alzheimer’s disease: Diagnosis and treatment across the spectrum of disease severity. Mt. Sinai J. Med. 2011, 78, 596–612. [Google Scholar] [CrossRef]
- Coccaro, E.F.; Siever, L.J. Pathophysiology and treatment of aggression. In Neuropsychopharmacology. The Fifth Generation of Progress; Davis, K.L., Charney, D., Coyle, J.T., Nemeroff, C., Eds.; Lippincott, Wiliams & Wilkins: Philadelphia, PA, USA, 2002; pp. 1709–1723. [Google Scholar]
- Raskind, M.A. Evaluation and management of aggressive behavior in the elderly demented patient. J. Clin. Psychiatry 1999, 60, 45–49. [Google Scholar]
- Cummings, J.L.; Frank, J.C.; Cherry, D.; Kohatsu, N.D.; Kemp, B.; Hewett, L.; Mittman, B. Guidelines for managing Alzheimer’s disease. Part II. Treatment. Am. Fam. Physician 2002, 65, 2525–2534. [Google Scholar] [PubMed]
- Alberca, G. Cognitive intervention therapy as treatment for behaviour disorders in Alzheimer disease: Evidence on efficacy and neurobiological correlations. Neurologia 2015, 30, 8–15. [Google Scholar] [CrossRef]
- Tromeur, E. Music therapy and Alzheimer disease. Soins Gerontol. 2014, 107, 16–18. [Google Scholar] [CrossRef]
- Yang, H.; Luo, Y.; Hu, Q.; Tian, X.; Wen, H. Benefits in Alzheimer’s Disease of Sensory and Multisensory Stimulation. J Alzheimers Dis. 2021, 82, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Morris, J.K.; Vidoni, E.D.; Johnson, D.K.; Van Sciver, A.; Mahnken, J.D.; Honea, R.A.; Wilkins, H.M.; Brooks, W.M.; Billinger, S.A.; Swerdlow, R.H.; et al. Aerobic exercise for Alzheimer’s disease: A randomized controlled pilot trial. PLoS ONE 2017, 12, e0170547. [Google Scholar] [CrossRef] [PubMed]
- Sobola, N.A.; Dalla, C.H.; Høghc, P.; Hoffmannd, K.; Frederiksend, K.S.; Vogeld, A.; Siersmae, V.; Waldemard, G.; Steen GHasselbalchd, S.G.; Beyera, N. Change in Fitness and the Relation to Change in Cognition and Neuropsychiatric Symptoms After Aerobic Exercise in Patients with Mild Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 65, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Vidoni, E.D.; Perales, J.; Alshehri, M.; Giles, A.-M.; Siengsukon, C.F.; Burns, J.M. Aerobic Exercise Sustains Performance of Instrumental Activities of Daily Living in Early-Stage Alzheimer Disease. J. Geriatr. Phys. Ther. 2019, 42, E129–E134. [Google Scholar] [CrossRef] [PubMed]
- Lam, L.C.W.; Chan, W.M.; Kwok, T.C.Y.; Chiu, H.F.K. Effectiveness of Tai Chi in maintenance of cognitive and functional abilities in mild cognitive impairment: A randomised controlled trial. Hong Kong Med. J. 2014, 20 (Suppl. S3), S20–S23. [Google Scholar]
- Law, L.L.F.; Barnett, F.; Yau, M.K.; Gray, M.A. Effects of functional tasks exercise on older adults with cognitive impairment at risk of Alzheimer’s disease: A randomised controlled trial. Age Ageing 2014, 43, 813–820. [Google Scholar] [CrossRef]
- Kim, D.J. The Effects of a Recollection-Based Occupational Therapy Program of Alzheimer’s Disease: A Randomized Controlled Trial. Hindawi Occup. Ther. Int. 2020, 2020, 6305727. [Google Scholar] [CrossRef]
- Parra, E.V.; Hernández Garre, J.M.; Echevarría Pérez, P. Benefits of Dog-Assisted Therapy in Patients with Dementia Residing in Aged Care Centers in Spain. Int. J. Environ. Res. Public Health 2021, 18, 1471. [Google Scholar] [CrossRef]
- Yu, F.; Swartwood, R.M. Swartwood, Feasibility and Perception of the Impact From Aerobic Exercise in Older Adults with Alzheimer’s Disease. Am. J. Alzheimer’s Dis. Other Dement. 2021, 27, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Cao, Y.; Gao, J. The impacts of a GO-game (Chinese chess) intervention on Alzheimer disease in a Northeast Chinese population. Front. Aging Neurosci. 2015, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Simmons-Stern, N.R.; Deason, R.G.; Brandler, B.J.; Frustace, B.S.; O’Connor, M.K.; Ally, B.A.; Budson, A.E. Music-Based Memory Enhancement in Alzheimer’s Disease: Promise and Limitations. Neuropsychologia 2012, 50, 3295–3303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eftychios, A.; Nektarios, S.; Nikoleta, G. Alzheimer Disease and Music-Therapy: An Interesting Therapeutic Challenge and Proposal. Adv. Alzheimer’s Dis. 2021, 10, 1–18. [Google Scholar] [CrossRef]
- Zhang, A.; Yang, Y.; Xu, M. Clinical Observation of Computer Vision Technology Combined with Music Therapy in the Treatment of Alzheimer’s Disease. Emerg. Med. Int. 2022, 2022, 2567340. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.H.; Wieland, L.S.; Lee, H.; Sim, H.; Lee, M.S.; Shin, B.C. Acupuncture and Related Interventions for the Treatment of Symptoms Associated with Carpal Tunnel Syndrome. Cochrane Database Syst. Rev. 2018, 12, CD011215. [Google Scholar] [CrossRef]
- Rankin, I.A.; Sargeant, H.; Rehman, H.; Gurusamy, K.S. Low-Level Laser Therapy for Carpal Tunnel Syndrome. Cochrane Database Syst Rev. 2017, 2017, CD012765. [Google Scholar] [CrossRef]
- Rosenberga, A.; Ngandub, T.; Rusanenb, M.; Antikainene, R.; B€ackmanh, L.; Havulinnai, S.; H€anninenj, T.; Laatikainenb, T.; Lehtisalob, J.; Lev€alahtib, E.; et al. Multidomain lifestyle intervention benefits a large elderly population at risk for cognitive decline and dementia regardless of baseline characteristics: The FINGER trial. Alzheimer’s Dement. 2018, 14, 263–270. [Google Scholar] [CrossRef]
- Beaunieux, H.; Eustache, F.; Busson, P.; de la Sayette, V.; Viader, F.; Desgranges, B. Cognitive procedural learning in early Alzheimer’s disease: Impaired processes and compensatory mechanisms. J. Neuropsychol. 2012, 6, 31–42. [Google Scholar] [CrossRef]
- Nestor, P.J.; Fryer, T.D.; Hodges, J.R. Declarative memory impairments in Alzheimer’s disease and semantic dementia. NeuroImage 2006, 30, 1010–1020. [Google Scholar] [CrossRef]
- Arroyo-Anlló, E.M.; Chamorro-Sánchez, J.; Díaz-Marta, J.P.; Gil, R. Proceeding memory in Alzheimer’s disease. Rev. Médica Inst. Mex. Seguro Soc. 2013, 51, 402–413. [Google Scholar]
- van Halteren-van Tilborg, I.A.; Scherder, E.J.; Hulstijn, W. Motor-skill learning in Alzheimer’s disease: A review with an eye to the clinical practice. Neuropsychol. Rev. 2007, 17, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Elvsåshagen, T.; Malt, U.F. Structural plasticity in the adult central nervous system. Tidsskr Nor Laegeforen. 2008, 128, 298–302. [Google Scholar] [PubMed]
- Duffau, H. Brain plasticity: From pathophysiological mechanisms to therapeutic applications. J. Clin. Neurosci. 2006, 9, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Annunciato, N.F. Plasticity of the nervous system. Int. J. Orofac. Myol. 1995, 21, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.D.; Zhu, L.L.; Fan, W.H. Role of semaphorins in neural development and synaptic plasticity. Sheng Li Ke Xue Jin Zhan 2007, 38, 68–70. [Google Scholar]
- Tohill, M.; Terenghi, G. Stem-cell plasticity and therapy for injuries of the peripheral nervous system. Biotechnol. Appl. Biochem. 2004, 40, 17–24. [Google Scholar] [CrossRef]
- Hirono, N.; Mori, E.; Ikejiri, Y.; Imamura, T.; Shimomura, T.; Ikeda, M.; Yamashita, H.; Takatsuki, Y.; Tokimasa, A.; Yamadori, A. Procedural memory in patients with mild Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 1997, 8, 210–216. [Google Scholar] [CrossRef]
| Author | Sample (N) | Intervention | Duration, Frequency, and Exercise Intensity |
|---|---|---|---|
| Morris et al. [15] | AEx = 39, ST = 37 older adults over 55 early stage of AD | AEx: aerobic exercise ST: non-aerobic (stretching and toning control program) | 26 weeks 150 min per week/3–5 sessions HR from 40–55% to 60–75% |
| Sobol et al. [16] | IG = 29, CG = 26 age 52–83 years mild stage of AD | IG/CG: aerobic exercises (rgometer bicycle, cross trainer, treadmill), Non-aerobic (strength training of the lower extremity) | IG/CG: 16 weeks 1 h 3 times weekly moderate-to-high intensity The following 12 weeks comprised 10 min of warm up followed by 3 times 10 min of moderate-to high intensity with small breaks of 2–5 min |
| Vidoni et al. [17] | AEx = 33, ST = 32 adults over 55 early stage of AD | AEx: aerobic exercise; ST: non-aerobic (stretching and toning control program, core strengthening, resistance bands, modified Tai Chi, modified yoga) | AEx: 26 weeks from 60 min a week, increasing the weekly exercise time by about 21 min a week, reached 150 min a week, over 3–5 sessions. ST: keep HR below 100 beats per min. |
| Lam et al. [18] | IG = 171, CG = 218 65 years old mild cognitive impairment | IG: 24-style Tai Chi CG: stretching and relaxation exercises | 12 months ≥30 min per day ≥3 days per week intensity not specified |
| Law et al. [19] | IG = 43, AC = 40 older adults over 60 mild cognitive impairment | IG—FcTSim (program facilitated by an occupational therapist) AC—Computer-based cognitive training (visual searching, forwardbackward digit recall and calculation) Cognitive strategy training | IG: 10 weeks 13 sessions till 40 to 50 min AC: over 10 weeks, 6 sessions Total 60 min intensity not specified |
| Kim [20] | EG = 18, CG = 17 older adults mild stage of AD | EG: cognitive stimulus program based on recollection (physical, horticultural, musical, art, IADL activity) CG: activities at centers (physical activity, recreation, watching TV) | EG: 24 sessions 5 times per week 60 min per session Easy, medium to hard CG—regular activities intensity not specified |
| Parra et al. [21] | EG = 171, CG = 163 adults over 65 did not report stage | EG/CG—DAT Occupational therapy Physical therapy Cognitive therapy (psychology, socio-cultural animation, and complementary therapies) | EG: 8 months every day, 1 or 2 a week weekly sessions of 45 min intensity not specified |
| Yu et al. [22] | AD = 10, older adults over 65, mild-to-moderate stage AD | AEx: aerobic exercise (cycling), focus group, | EG: 6 months, 3 times a week, session of 45 min |
| Lin et al. [23] | AD = 147 (CG = 49, SGGI = 49 LGGI = 49) Older adults, did not report stage | Playing Go-game | SGGI—1 h daily for 6 months LGGI—2 h dayly for 6 months CG—non playing |
| Simmons-Sterna et al. [24] | AD/EG = 12, CG = 12 Older adults, did not report stage | EG/CG Musicaltherapy, simple songs and spoken recorgings | EG: 1.5 h, one session intensity not specified |
| Eftychios et al. [25] | AD/EG = 31, Older adults, Mild, moderate, severe stage of AD | Musicaltherapy, playing on instruments, singing, litening | EG: 30 months 1 h, three times a week, |
| Zhang et al. [26] | AD/EG = 9, Older adults, Mild and moderate stage of AD | Musicaltherapy, music listenig | EG: 45 min of 16 sessions, Twice a week |
| Author | Type of Intervention | Instruments | Outcomes | Effect of Exercise |
|---|---|---|---|---|
| Morris et al. [15] | Aerobic exercise Non-aerobic exercise | DAD, CSDD, CPX, MRI | Memory, executive function composite scores, functional ability, depressive symptoms, peak VO2, brain volumes | Positive |
| Sobol et al. [16] | Aerobic exercises Non-aerobic exercise | CEPT, MMSE, SDMT, NPI | VO2 peak, Mental speed Attention, Neuropsychiatric symptoms, | Positive |
| Vidoni et al. [17] | Aerobic exercise Non-aerobic exercise | DAD, RUD-Lite | Functional dependence, Informal caregiver time required, Cognition | Positive |
| Lam et al. [18] | Tai Chi Stretching and relaxation exercises | CDR, DAD, CSDD, NPI, BBS | Cognitive functions, Neuropsychiatric assessments, Depression, body balance | Positive |
| Law et al. [19] | Program facilitated by an occupational therapist Computer-based cognitive training Cognitive strategy training | NCSE, CVVLT, CVFT, TMT-A, TMT-B | Cognitive functions, Lawton IADLC-PEDL | Positive |
| Kim [20] | Cognitive stimulus program based on recollection Regular activities at centers | FIM, K-MMSE, SMCQ, SGDS-K, GQOL-D | Functional independence, Mental speed, Memory, Depression level, Quality of Life | Positive |
| Parra et al. [21] | DAT Occupational therapy Physical therapy Cognitive therapy | MEC, Mdified Barthel Index, CSDD, NPI | Mental speed, Motor skills, Depression level, Neuropsychiatric assessments, | Positive |
| Yu et al. [22] | Aerobic exercise (cycling) Focus group | MMSE, CDR, Borg Rating of Perceived Exertion Scale, Heart Rate Reserve | Mental speed, Memory, Overall functioning, Stress reduction, Improvement of attitude | Positive |
| Lin et al. [23] | Playing Go-game | MADRS, HADS, GAF, RAND-36, KICA-dep, TAS-20, CDR, MMSE, BDNF | Quality of life, Depression level, Mental speed, Memory, Protein Levels of BDNF | Positive |
| Simmons-Sterna et al. [24] | Listening to recorded songs, and spoken recordings | MMSE, MOCA | Mental speed, Memory enhancement | Positive |
| Eftychios et al. [25] | Music listening, playing on instruments | MMSE | Mental speed, Memory, Increase the life quality of the participants | Positive |
| Zhang et al. [26] | Music listening | AMT, GDS-15 | Memory enhancement | Positive |
| Sample Randomization | Random Sequence Generation | Blinding of Evaluators | Similar Groups in the Initial Assessment | Inclusion Criteria for Participants | Description of the Experimental Protocol | Statistical Comparison among Groups | Description of Sample Losses | Results Description | |
|---|---|---|---|---|---|---|---|---|---|
| Morris et al. [15] | YES | YES | YES | YES | YES | YES | YES | YES | YES |
| Sobol et al. [16] | YES | YES | YES | YES | YES | YES | YES | YES | YES |
| Vidoni et al. [17] | YES | NO | YES | YES | YES | YES | YES | NO | YES |
| Lam et al. [18] | YES | NO | YES | YES | YES | YES | YES | NO | YES |
| Law et al. [19] | YES | YES | YES | YES | YES | YES | YES | YES | YES |
| Kim et al. [20] | YES | NO | NO | YES | YES | NO | YES | NO | YES |
| Parra et al. [21] | YES | NO | NO | YES | YES | YES | YES | YES | YES |
| Yu et al. [22] | YES | NO | NO | YES | YES | YES | NO | NO | YES |
| Lin et al. [23] | YES | NO | YES | YES | YES | YES | YES | NO | YES |
| Simmons-Sterna et al. [24] | YES | NO | YES | YES | YES | YES | YES | YES | YES |
| Eftychios et al. [25] | YES | NO | NO | YES | YES | YES | YES | YES | YES |
| Zhang et al. [26] | YES | NO | NO | YES | YES | YES | YES | NO | YES |
| Statistical analysis according to assessment item n/N(%) | 12/12 (100%) | 3/12 (25%) | 7/12 (58%) | 12/12 (100%) | 12/12 (100%) | 11/12 (92%) | 11/12 (92%) | 6/12 (50%) | 12/12 (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olczak, A.; Truszczyńska-Baszak, A.; Stępień, A.; Górecki, K. Functional Therapeutic Strategies Used in Different Stages of Alzheimer’s Disease—A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 11769. https://doi.org/10.3390/ijerph191811769
Olczak A, Truszczyńska-Baszak A, Stępień A, Górecki K. Functional Therapeutic Strategies Used in Different Stages of Alzheimer’s Disease—A Systematic Review. International Journal of Environmental Research and Public Health. 2022; 19(18):11769. https://doi.org/10.3390/ijerph191811769
Chicago/Turabian StyleOlczak, Anna, Aleksandra Truszczyńska-Baszak, Adam Stępień, and Krzysztof Górecki. 2022. "Functional Therapeutic Strategies Used in Different Stages of Alzheimer’s Disease—A Systematic Review" International Journal of Environmental Research and Public Health 19, no. 18: 11769. https://doi.org/10.3390/ijerph191811769





