Electroneurographic Evaluation of Neural Impulse Transmission in Patients after Ischemic Stroke Following Functional Electrical Stimulation of Antagonistic Muscles at Wrist and Ankle in Two-Month Follow-Up
Abstract
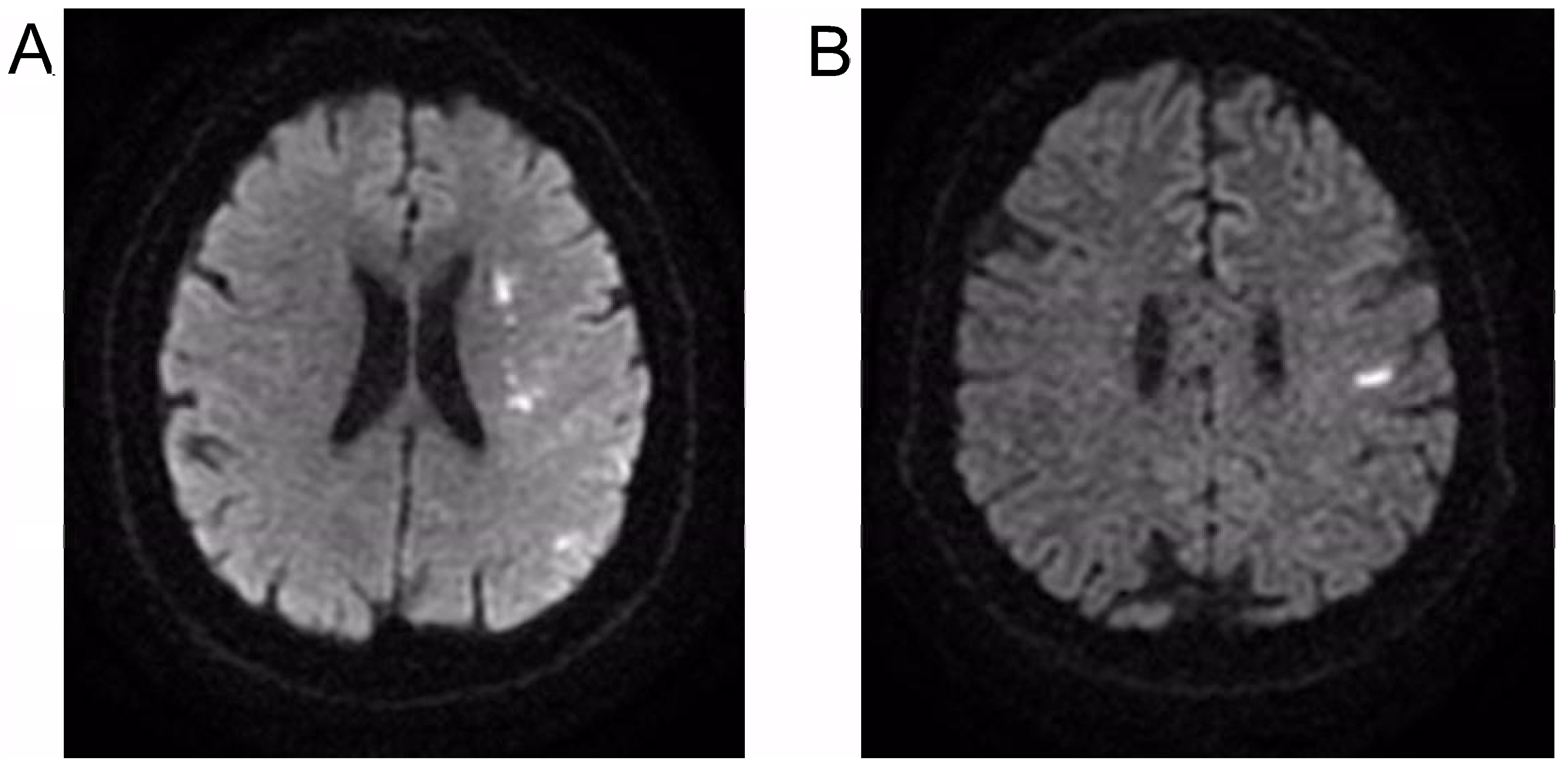
:1. Introduction
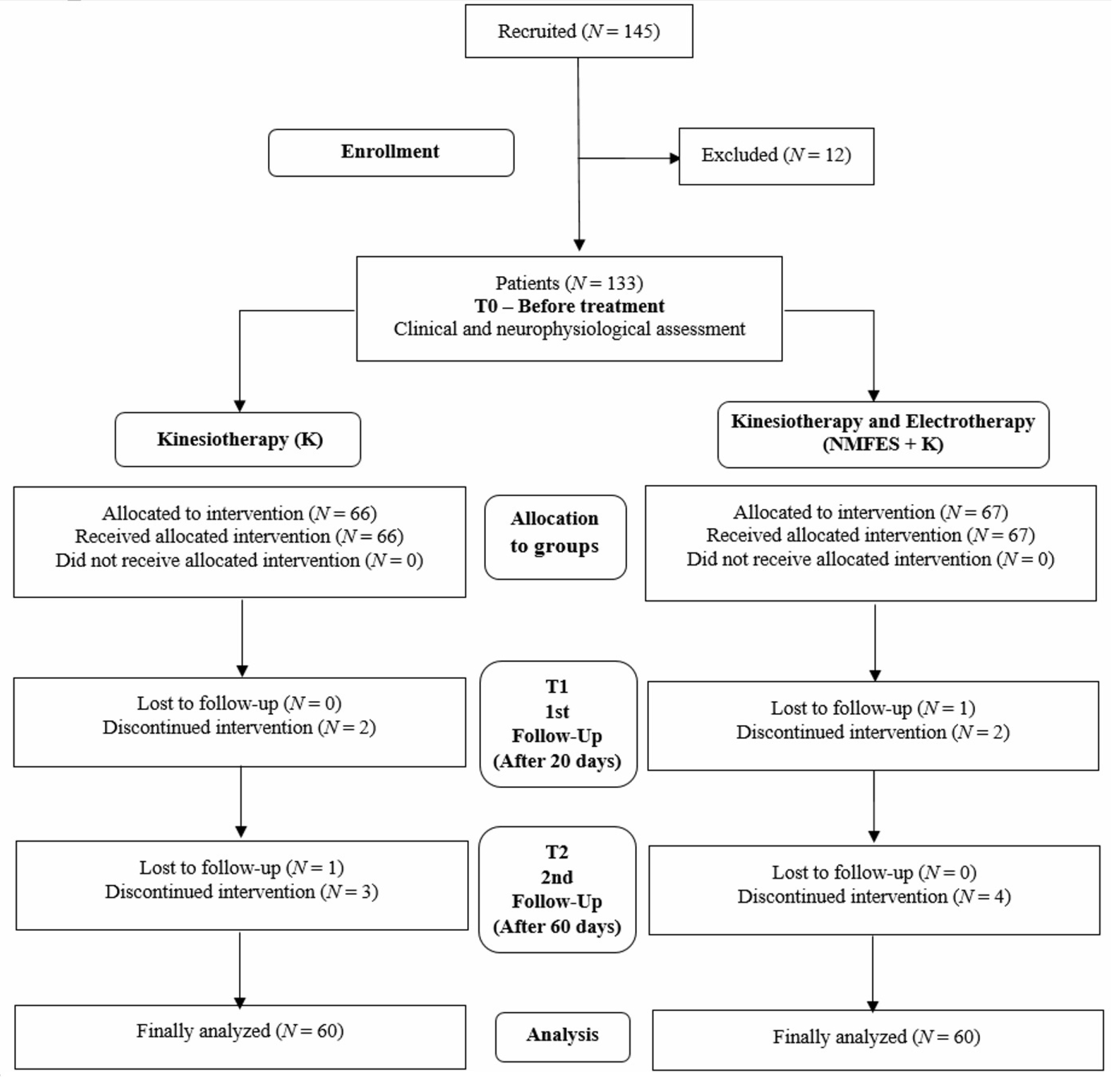
2. Materials and Methods
2.1. Subjects and Study Design
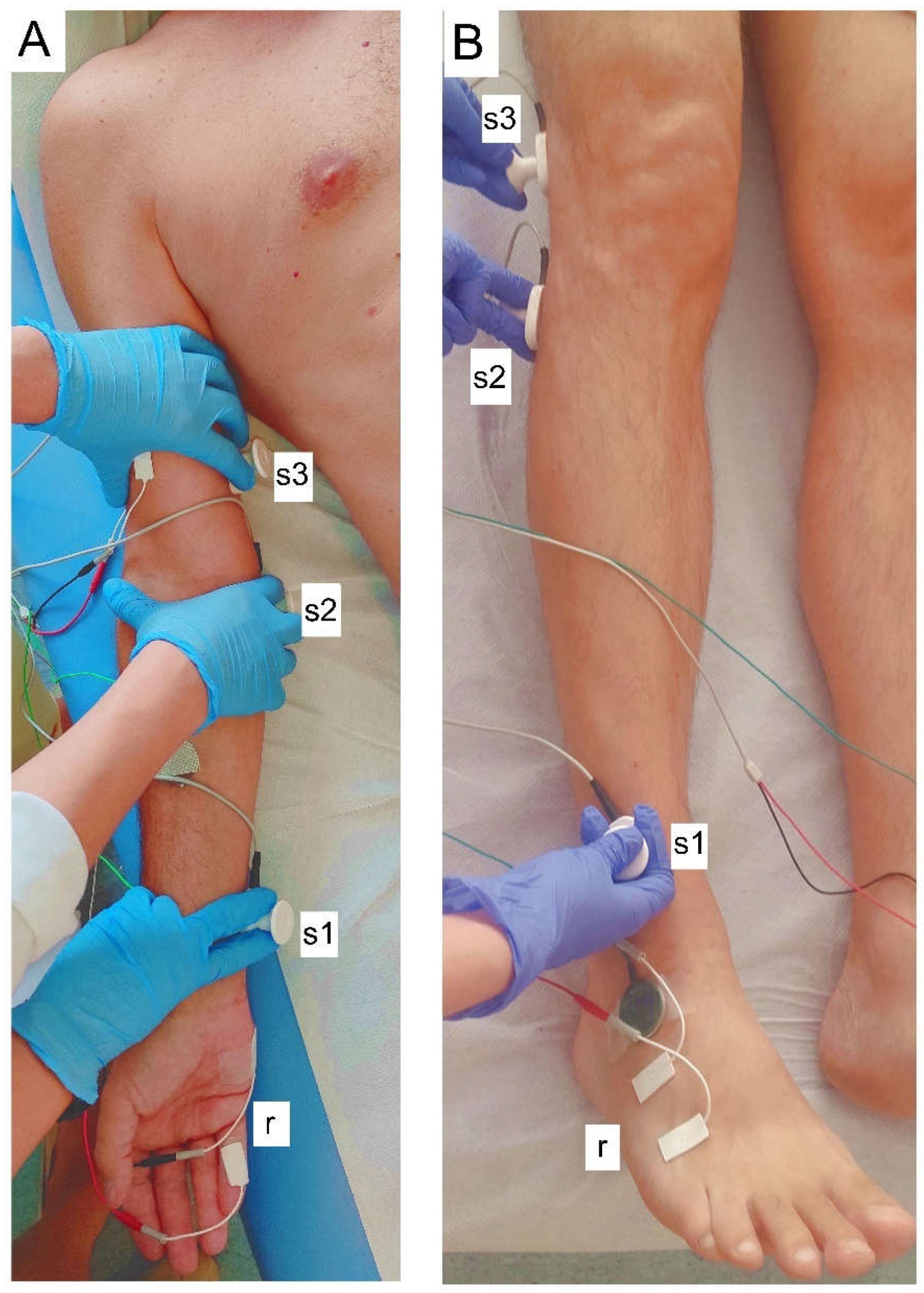
2.2. Neurophysiological Evaluation
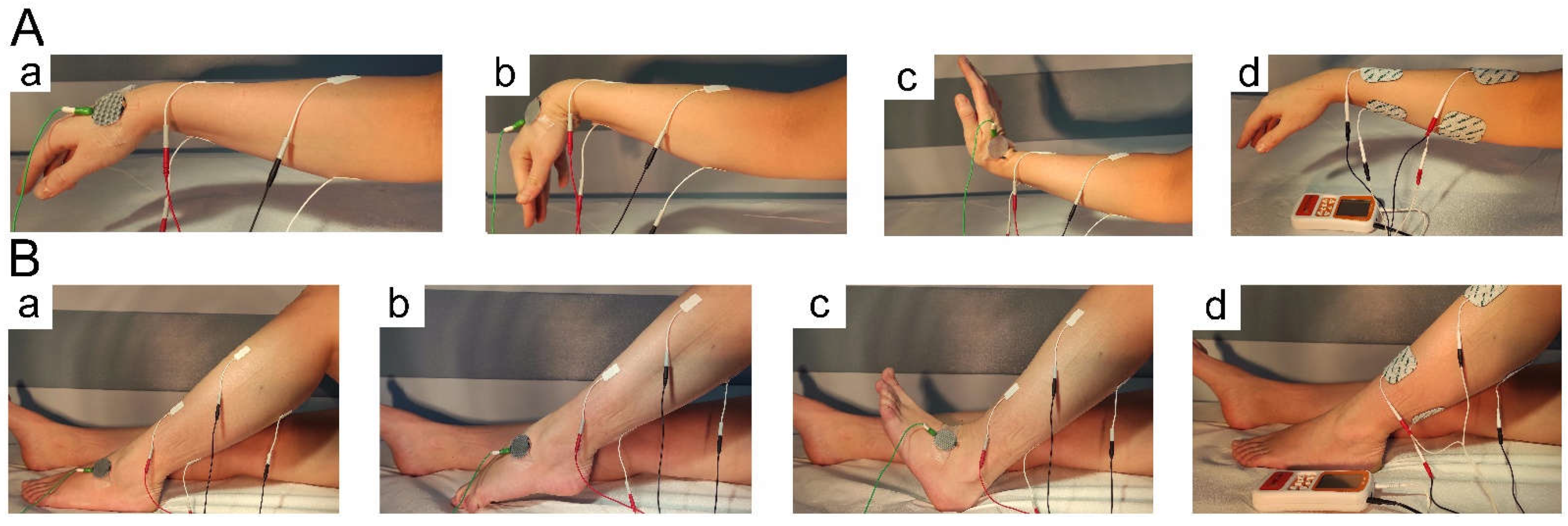
2.3. Treatment with NMFES
2.4. Kinesiotherapy
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berge, E.; Whiteley, W.; Audebert, H.; De Marchis, G.M.; Fonseca, A.C.; Padiglioni, C.; de la Ossa, N.P.; Strbian, D.; Tsivgoulis, G.; Turc, G. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur. Stroke J. 2021, 6, 1–62. [Google Scholar] [CrossRef] [PubMed]
- Westwater-Wood, S.; Adams, N.; Kerry, R. The use of proprioceptive neuromuscular facilitation in physiotherapy practice. Phys. Ther. Rev. 2010, 15, 23–28. [Google Scholar] [CrossRef]
- Hindle, K.B.; Whitcomb, T.J.; Briggs, W.O.; Hong, J. Proprioceptive neuromuscular facilitation (PNF): Its mechanisms and effects on range of motion and muscular function. J. Hum. Kinet. 2013, 31, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Adler, S.S.; Beckers, D.; Buck, M. PNF in Practice: An Illustrated Guide, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Knutson, J.S.; Fu, M.J.; Sheffler, L.R.; Chae, J. Neuromuscular electrical stimulation for motor restoration in hemiplegia. Phys. Med. Rehabil. Clin. N. Am. 2015, 26, 729–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisinski, P.; Huber, J.; Samborski, W.; Witkowska, A. Neurophysiological assessment of the electrostimulation procedures used in stroke patients during rehabilitation. Int. J. Artif. Organs 2008, 31, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Kraft, G.H.; Fitts, S.S.; Hammond, M.C. Techniques to improve function of the arm and hand in chronic hemiplegia. Arch. Phys. Med. Rehabil. 1992, 73, 220–227. [Google Scholar] [PubMed]
- Paoloni, M.; Volpe, B.; Mangone, M.; Ioppolo, F.; Santilli, V. Peripheral nerve conduction abnormalities in nonparetic side of ischemic stroke patients. J. Clin. Neurophysiol. 2010, 27, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Picelli, A.; Tamburin, S.; Berto, G.; Chemello, E.; Gandolfi, M.; Saltuari, L.; Waldner, A.; Smania, N. Electrodiagnostic and nerve ultrasonographic features in upper limb spasticity: An observational study. Funct. Neurol. 2017, 32, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Huber, J.; Lisiński, P.; Polowczyk, A. Reinvestigation of the dysfunction in neck and shoulder girdle muscles as The reason of cervicogenic headache among office workers. Disabil. Rehabil. 2013, 35, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Lisiński, P.; Huber, J. Evolution of muscles dysfunction From myofascial pain syndrome through cervical disc-root conflict to degenerative spine disease. Spine 2017, 42, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Huber, J.; Leszczyńska, K.; Wincek, A.; Szymankiewicz-Szukała, A.; Fortuna, W.; Okurowski, S.; Tabakow, P. The role of peripheral nerve electrotherapy in functional recovery of muscle motor units in patients after incomplete spinal cord injury. Appl. Sci. 2021, 11, 9764. [Google Scholar] [CrossRef]
- Sentandreu-Mañó, T.; Tomás, J.M.; Ricardo Salom Terrádez, J. A randomised clinical trial comparing 35 Hz versus 50 Hz frequency stimulation effects on hand motor recovery in older adults after stroke. Sci. Rep. 2021, 11, 9131. [Google Scholar] [CrossRef] [PubMed]
- Sabut, S.K.; Sikdar, C.; Kumar, R.; Mahadevappa, M. Functional electrical stimulation of dorsiflexor muscle: Effects on dorsiflexor strength, plantarflexor spasticity, and motor recovery in stroke patients. NeuroRehabilitation 2011, 29, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Eraifej, J.; Clark, W.; France, B.; Desando, S.; Moore, D. Effectiveness of upper limb functional electrical stimulation after stroke for the improvement of activities of daily living and motor function: A systematic review and meta-analysis. Syst. Rev. 2017, 6, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anas, R.; Alashram, A.A.; Alghwiri, E.P.; Giuseppe, A. Efficacy of proprioceptive neuromuscular facilitation on spasticity in patients with stroke: A systematic review. Phys. Ther. Rev. 2021, 26, 168–176. [Google Scholar]
- Guiu-Tula, F.X.; Cabanas-Valdés, R.; Sitjà-Rabert, M.; Urrútia, G.; Gómara-Toldrà, N. The Efficacy of the proprioceptive neuromuscular facilitation (PNF) approach in stroke rehabilitation to improve basic activities of daily living and quality of life: A systematic review and meta-analysis protocol. BMJ Open 2017, 7, e016739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahin, N.; Ugurlu, H.; Albayrak, I. The efficacy of electrical stimulation in reducing the post-stroke spasticity: A randomized controlled study. Disabil. Rehabil. 2012, 34, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.X.; Fan, B.Y.; Mao, Y.Y. Effectiveness of neuromuscular electrical stimulation for wrist rehabilitation after acute ischemic stroke. Medicine 2018, 97, 12299. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, E. Interneuronal relay in spinal pathways from proprioceptors. Prog. Neurobiol. 1992, 38, 335–378. [Google Scholar] [CrossRef]
- Sharman, M.; Cresswell, A.; Riek, S. Proprioceptive neuromuscular facilitation stretching: Mechanisms and clinical implications. Sport Med. 2006, 36, 929–939. [Google Scholar] [CrossRef] [PubMed]
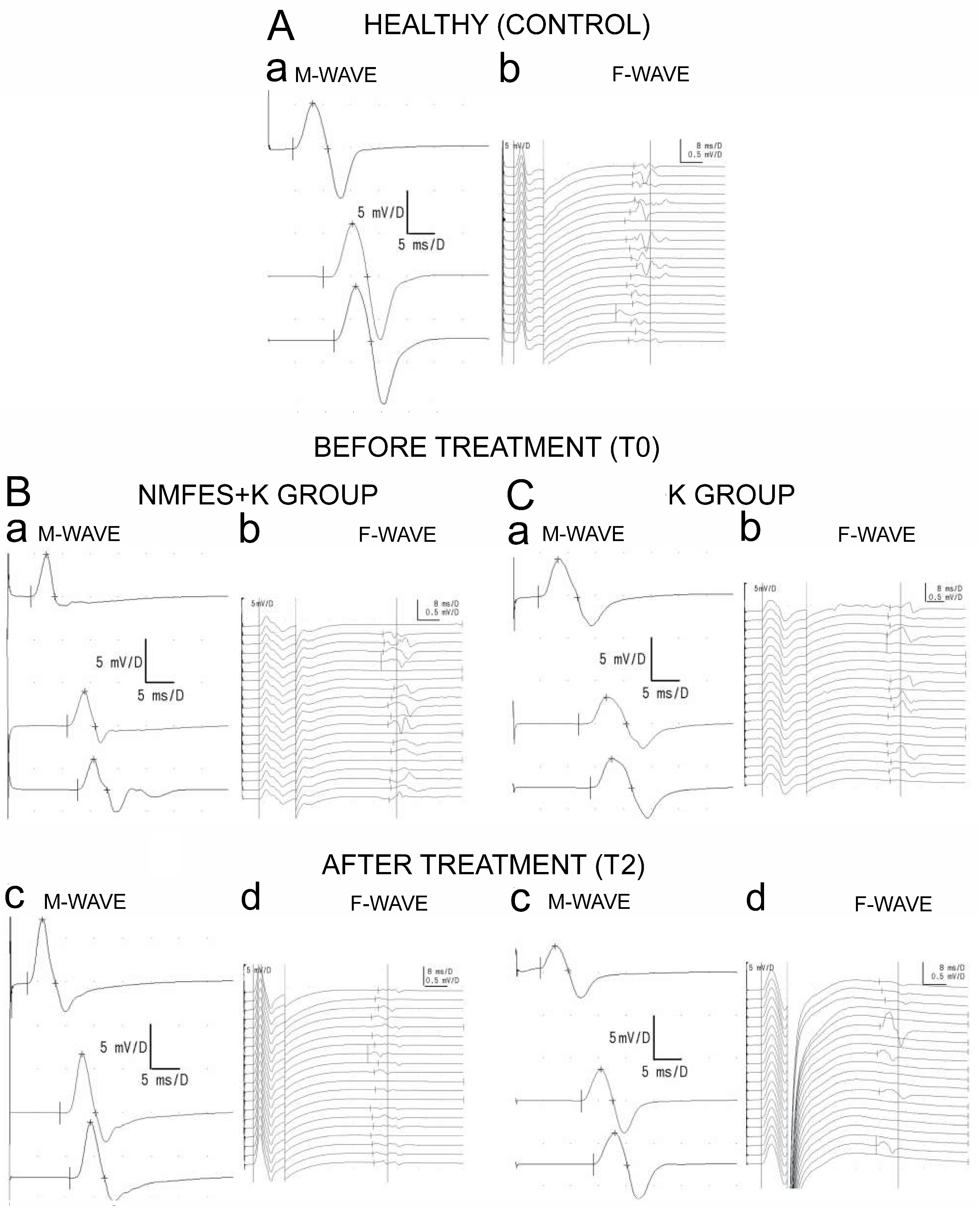
| Study Group Variable | Healthy Volunteers (Control) N = 60 41♀, 19♂ | Patients NMFES+K Group N = 60 44♀, 16♂ | Patients K Group N = 60 45♀, 15♂ | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Min–Max | Mean ± SD | Min–Max | Mean ± SD | Min–Max | |
| Age | 48.6 ± 4.3 | 30–52 | 62 ± 6.1 | 47–70 | 65 ± 5.2 | 56–70 |
| Height (cm) | 166.0 ± 4.8 | 161–180 | 16 3± 10.3 | 148–178 | 167 ± 7.2 | 157–180 |
| Weight (kg) | 75.3 ± 9.5 | 52–81 | 72 ± 11.1 | 55–95 | 74 ± 11.4 | 52–98 |
| Observation time T0–T2 (days) | NA | NA | 62 ± 6 | 50–72 | 63 ± 6 | 50–72 |
| Study Group Variable | Healthy Volunteers (Control) N = 60 41♀, 19♂ | Patients NMFES+K Group N = 60 44♀, 16♂ | Patients K Group N = 60 45♀, 15♂ | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Min-Max | Mean ± SD | Min-Max | Mean ± SD | Min-Max | |
| Expected stimulation (hours) | NA | NA | 19.2 ± 2.1 | 15–23 | NA | NA |
| Detected stimulation (hours) | NA | NA | 18.4 ± 4.3 | 16–24 | NA | NA |
| Train stimulation frequency (Hz) | NA | NA | 48.6 ± 6.1 | 35–70 | NA | NA |
| Single stimulus duration (ms) | NA | NA | 14.1 ± 15.2 | 12.5–17.5 | NA | NA |
| Train duration (sec) | NA | NA | 4.1 ± 1.7 | 3–6 | NA | NA |
| Interval between trains (sec) | NA | NA | 4.3 ± 1.2 | 2–5 | NA | NA |
| Session duration (mins) | NA | NA | 19.1 ± 2.2 | 15–20 | NA | NA |
| Applied stimulus strength (mA) Upper extremity muscles -flexors -extensors | NA | NA | 25.9 ± 3.1 26.2 ± 3.0 | 27–33 21–35 | NA | NA |
| Applied stimulus strength (mA) Lower extremity muscles -flexors -extensors | NA | NA | 25.4 ± 3.1 28.2 ± 3.3 | 21–37 23–32 | NA | NA |
| Test or Parameter | Healthy Volunteers N = 60 | T0 Acute Phase (up to 7 Days after the Incident) | T1 Subacute phase (after 2–3 Weeks of Treatment) | T2 (after 2 Months of Rehabilitation Centre Treatment) | p Patients T0 vs. T2 before- -after | p Healthy vs. Patients T0 before | p Healthy vs. Patients T2 after | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Group NMFES+K Patients N = 60 | Group K Patients N = 60 | Group NMFES+K Patients N = 60 | Group K Patients N = 60 | Group NMFES+K Patients N = 60 | Group K Patients N = 60 | |||||
| ENG (M-waves parameters) | ||||||||||
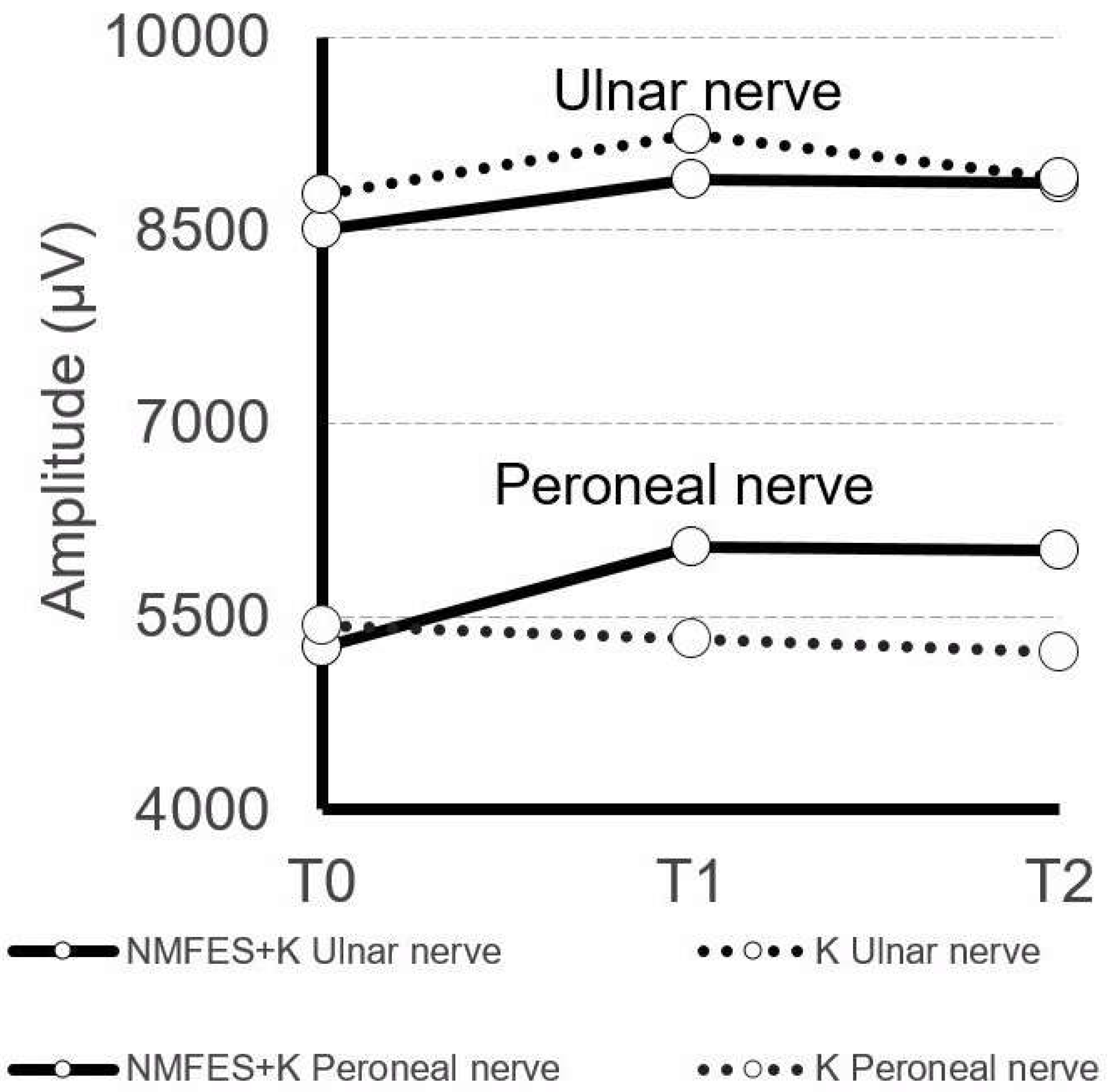
| Ulnar nerve Amplitude (µV) | 9533 ± 2009 | 8580 ± 2227 | 8783 ± 2127 | 8809 ± 2122 | 9240 ± 2324 | 8872 ± 1175 | 8905 ± 2126 | NMFES+K NS K NS | NMFES+K p = 0.05 K NS | NMFES+K NS K NS |
| Latency (ms) | 3.5 ± 0.4 | 3.1 ± 0.8 | 3.0 ± 0.9 | 3.1 ± 0.7 | 3.2 ± 0.9 | 3.2 ± 0.8 | 3.2 ± 0.9 | NMFES+K NS K NS | NMFES+K NS K NS | NMFES+K NS K NS |
| Peroneal nerve Amplitude (µV) | 8677 ± 1122 | 5268 ± 1211 | 5432 ± 1125 | 6035 ± 1225 | 5321 ± 1028 | 6009 ± 928 | 5224 ± 1005 | NMFES+K p = 0.04 K NS | NMFES+K p = 0.04 K p = 0.04 | NMFES+K p = 0.04 K p = 0.04 |
| Latency (ms) | 4.5 ± 0.8 | 4.6 ± 0.9 | 4.7 ± 0.7 | 4.6 ± 0.7 | 4.5 ± 0.9 | 4.6 ± 0.9 | 4.6 ± 0.7 | NMFES+K NS K NS | NMFES+K NS K NS | NMFES+K NS K NS |
| ENG (F-waves frequencies) | ||||||||||
| Ulnar nerve Frequency | 18 ± 1 | 17 ± 3 | 18 ± 2 | 18 ± 2 | 18 ± 2 | 19 ± 1 | 18 ± 2 | NMFES+K NS K NS | NMFES+K NS K NS | NMFES+K NS K NS |
| Peroneal nerve Frequency | 19 ± 1 | 17 ± 4 | 16 ± 3 | 16 ± 4 | 15 ± 5 | 16 ± 4 | 15 ± 5 | NMFES+K NS K p = 0.05 | NMFES+K p = 0.05 K p = 0.05 | NMFES+K p = 0.05 K p = 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczmarek, K.; Huber, J.; Leszczyńska, K.; Daroszewski, P. Electroneurographic Evaluation of Neural Impulse Transmission in Patients after Ischemic Stroke Following Functional Electrical Stimulation of Antagonistic Muscles at Wrist and Ankle in Two-Month Follow-Up. Int. J. Environ. Res. Public Health 2022, 19, 713. https://doi.org/10.3390/ijerph19020713
Kaczmarek K, Huber J, Leszczyńska K, Daroszewski P. Electroneurographic Evaluation of Neural Impulse Transmission in Patients after Ischemic Stroke Following Functional Electrical Stimulation of Antagonistic Muscles at Wrist and Ankle in Two-Month Follow-Up. International Journal of Environmental Research and Public Health. 2022; 19(2):713. https://doi.org/10.3390/ijerph19020713
Chicago/Turabian StyleKaczmarek, Katarzyna, Juliusz Huber, Katarzyna Leszczyńska, and Przemysław Daroszewski. 2022. "Electroneurographic Evaluation of Neural Impulse Transmission in Patients after Ischemic Stroke Following Functional Electrical Stimulation of Antagonistic Muscles at Wrist and Ankle in Two-Month Follow-Up" International Journal of Environmental Research and Public Health 19, no. 2: 713. https://doi.org/10.3390/ijerph19020713






