Preparation and Characterization of a Novel Amidoxime-Modified Polyacrylonitrile/Fly Ash Composite Adsorbent and Its Application to Metal Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polyacrylonitrile/Fly Ash Composite
2.3. Preparation of Amidoxime-Modified Polyacrylonitrile/Fly Ash Composite
2.4. Characterization
2.5. Adsorption of Zn2+ in Aqueous Solution
2.5.1. Effect of pH
2.5.2. Dynamic Test
2.5.3. Effect of Temperature
3. Results and Discussions
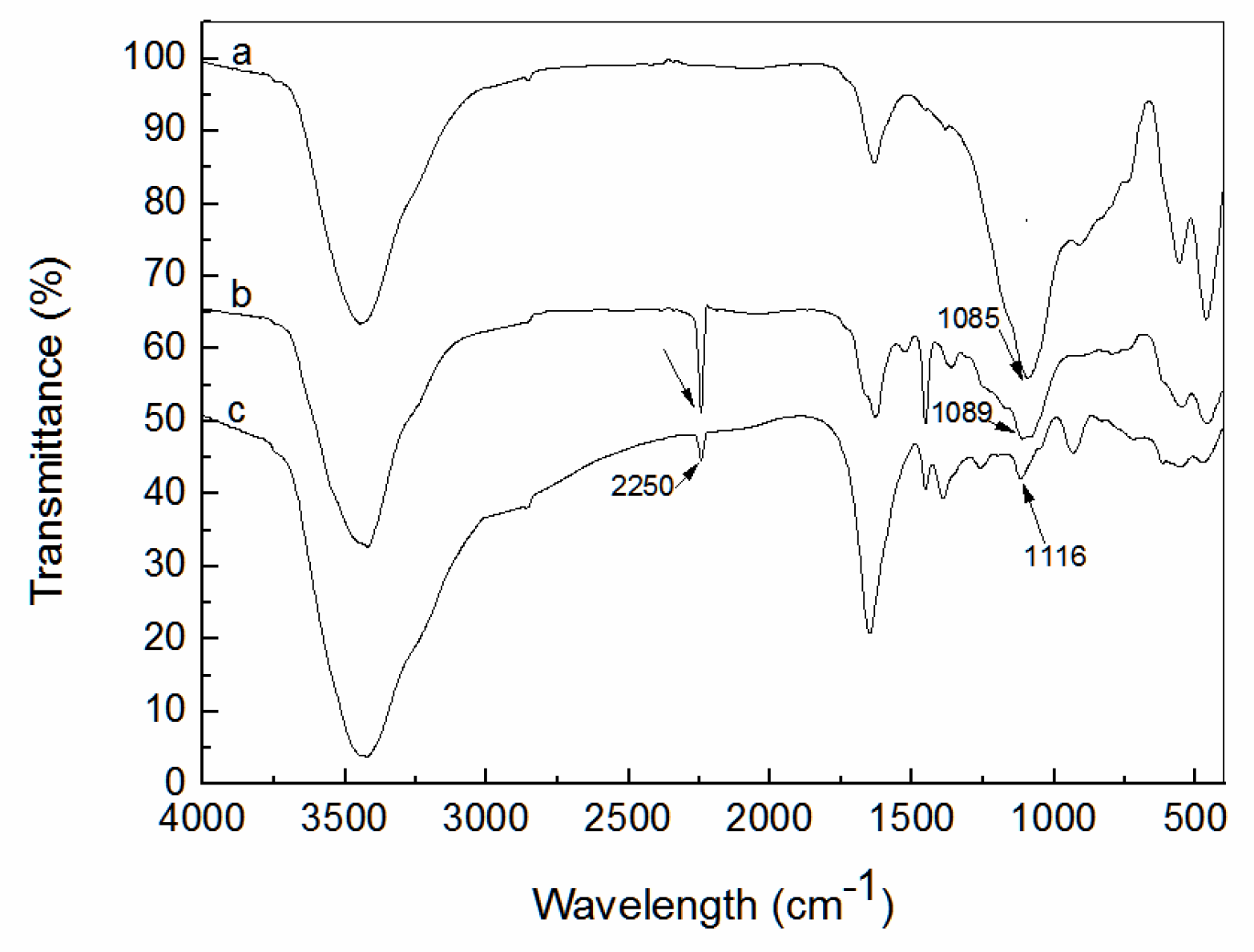
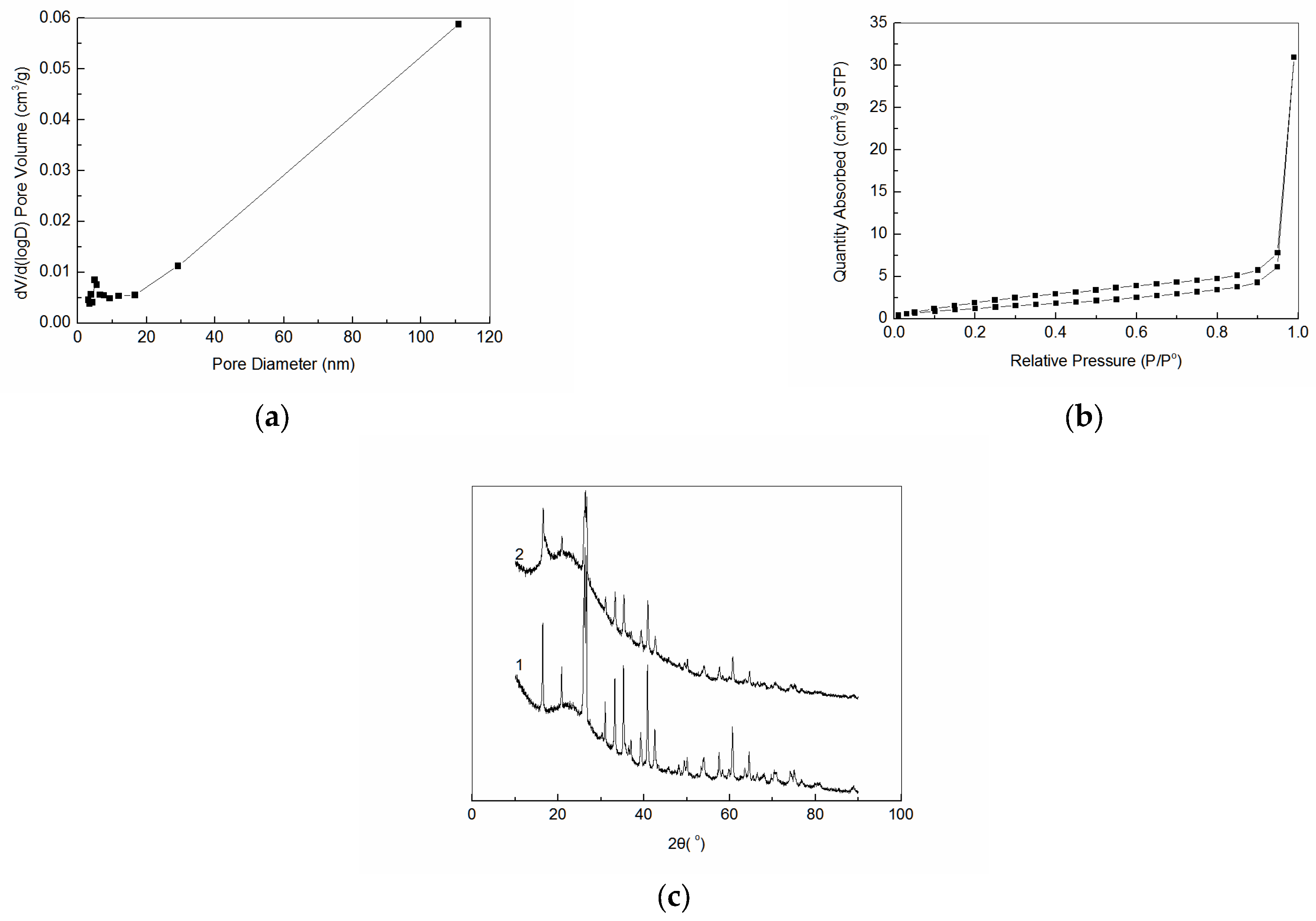
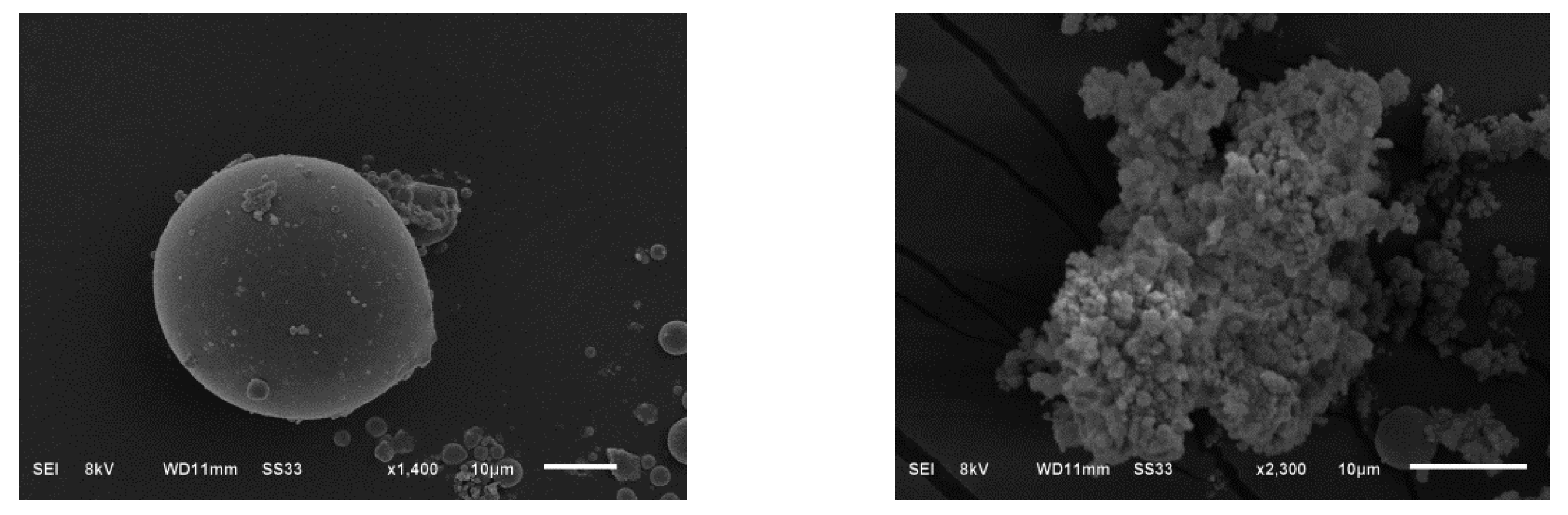
3.1. Characterization of Amidoxime-Modified Polyacrylonitrile/Fly Ash Composite
3.2. Effect of Initial pH on Adsorption
3.3. Effect of Coexisting Ions on Adsorption of Zn2+
3.4. Effect of Contact Time on Adsorption
3.5. Adsorption Kinetics
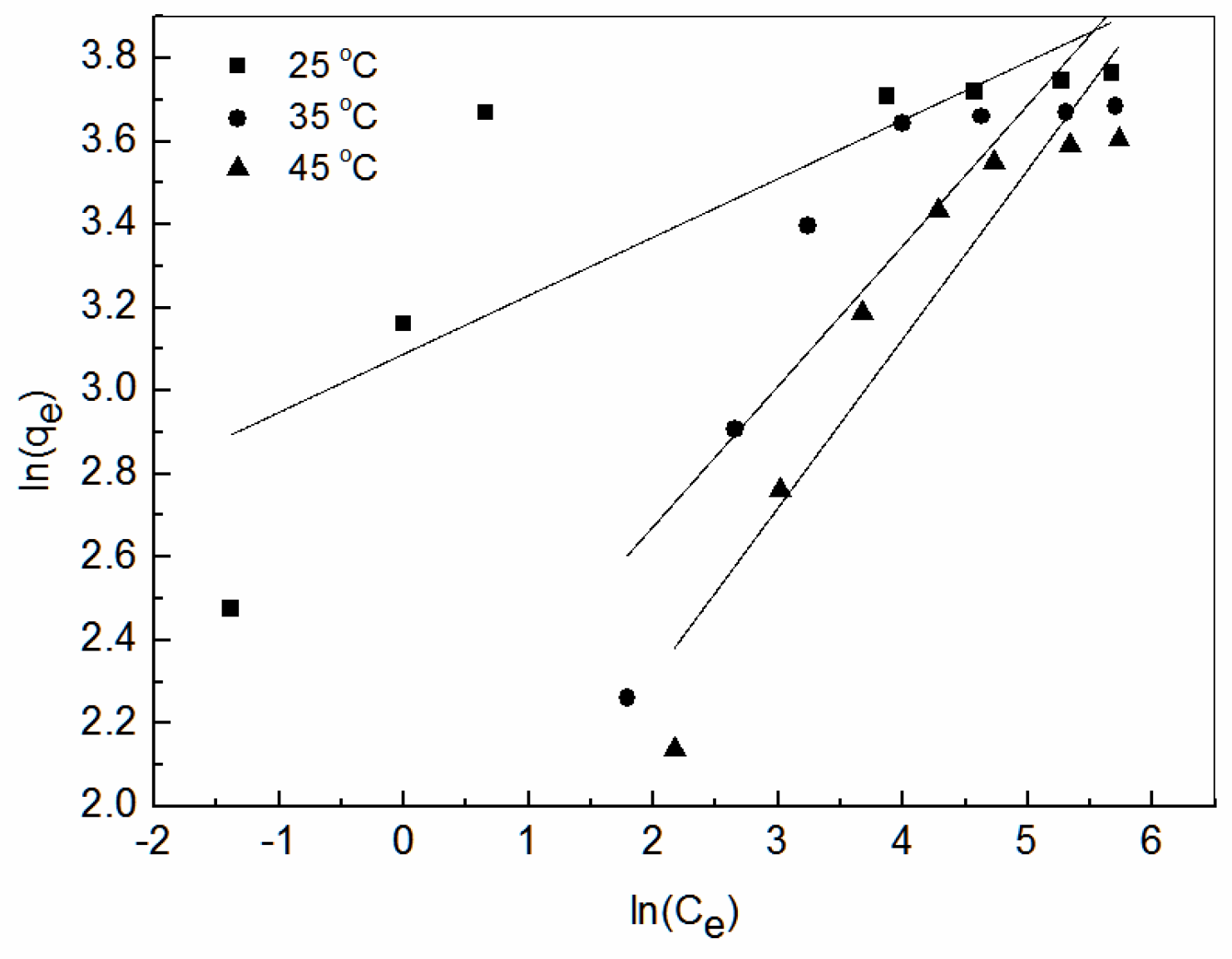
3.6. Adsorption Isotherm
3.7. Thermodynamics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baltakys, K.; Iljina, A.; Eisinas, A. Gyrolite adsorption of Zn2+ ions in acidic and alkaline solutions. Mater. Sci. 2015, 21, 123–128. [Google Scholar] [CrossRef]
- Contreras-Bustos, R.; Espejel-Ayala, F.; Cercado-Quezada, B.; Jiménez-Becerril, J.; Jiménez-Reyes, M. Adsorption of Zn2+ from solutions on manganese oxide obtained via ozone precipitation reaction. Pol. J. Chem. Technol. 2016, 18, 46–50. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Li, W.; Ou, D.; Li, C.; Hou, M.; Li, H.; Liu, Y. Enhanced adsorption of Zn2+ by salinity-aided aerobic granular sludge: Performance and binding mechanism. J. Environ. Manag. 2019, 242, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Idowu, A.A.; Temilade, F.A.; Peter, A.; Vahidhabanu, S.; Babu, B.R. Agro waste material as ecofriendly adsorbent for the removal of Zn(II): Isotherm, kinetic, thermodynamic and optimization studies. Desalination Water Treat. 2019, 155, 250–258. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Gong, Y.; Zhao, D.; Dang, Z.; Lin, Z. Enhanced removal of zinc and cadmium from water using carboxymethyl cellulose-bridged chlorapatite nanoparticles. Chemosphere 2021, 263, 128038. [Google Scholar] [CrossRef] [PubMed]
- Medha, I.; Chandra, S.; Vanapalli, K.R.; Samal, B.; Bhattacharya, J.; Das, B.K. (3-Aminopropyl)triethoxysilane and iron rice straw biochar composites for the sorption of Cr (VI) and Zn (II) using the extract of heavy metals contaminated soil. Sci. Total Environ. 2021, 771, 144764. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, W. Adsorption and purification of zinc in mining wastewater using microbial immobilization technology. Asian J. Chem. 2013, 25, 6811–6814. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, Y.X.; Chen, Y.L.; Qian, H. Recycle of Ag+ and Zn2+ with magnetic adsorbent in process of its purification from wastewater. Clean-Soil Air Water 2014, 42, 71–80. [Google Scholar] [CrossRef]
- Mishra, U.; Paul, S.; Bandyopadhaya, M. Removal of zinc ions from wastewater using industrial waste sludge: A novel approach. Environ. Prog. Sustain. Energy 2013, 32, 576–586. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Fu, J.; Ding, X.; Zhao, J. Zn2+ adsorption from wastewater using a chitosan/β-cyclodextrin-based composite membrane. J. Food Biochem. 2020, 44, e13483. [Google Scholar] [CrossRef]
- Sun, J.-M.; Li, F.; Huang, J.-C. Optimum pH for Cr6+ Co-removal with mixed Cu2+, Zn2+, and Ni2+ precipitation. Ind. Eng. Chem. Res. 2006, 45, 1557–1562. [Google Scholar] [CrossRef]
- Rötting, T.S.; Ayora, C.; Carrera, J. Improved passive treatment of high Zn and Mn concentrations using caustic magnesia (MgO): Particle size effects. Environ. Sci. Technol. 2008, 42, 9370–9377. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Tang, Q.; Meng, Y.; Liu, J.; Feng, T.; Zhao, X.; Zhu, S.; Yu, W.; Yang, B. Reversible “Off–On” fluorescence of Zn2+-passivated carbon dots: Mechanism and potential for the detection of EDTA and Zn2+. Langmuir 2018, 34, 7767–7775. [Google Scholar] [CrossRef]
- Valverde, J.L.; Lucas, A.; Gonzalez, M.; Rodriguez, J.F. Ion-exchange equilibria of Cu2+, Cd2+, Zn2+, and Na+ ions on the cationic exchanger amberlite IR-120. J. Chem. Eng. Data 2001, 46, 1404–1409. [Google Scholar] [CrossRef]
- Borba, C.E.; Silva, E.A.; Spohr, S.; Santos, G.H.F.; Guirardello, R. Ion exchange equilibrium prediction for the system Cu2+-Zn2+-Na+. J. Chem. Eng. Data 2010, 55, 1333–1341. [Google Scholar] [CrossRef]
- Valverde, J.L.; de Lucas, A.; Carmona, M.; González, M.; Rodríguez, J.F. Equilibrium data of the exchange of Cu2+, Cd2+ and Zn2+ ions for H+ on the cationic exchanger Lewatit TP-207. J. Chem. Technol. Biotechnol. 2004, 79, 1371–1375. [Google Scholar] [CrossRef]
- Malakootian, M.; Mirzaienia, F.; Malakootian, M. Removal efficiency of Cu2+ and Zn2+ from industrial wastewater by using microbial desalination cell. J. Water Chem. Technol. 2019, 41, 334–339. [Google Scholar] [CrossRef]
- Passos, H.; Cruz, B.; Schaeffer, N.; Patinha, C.; da Silva, E.F.; Coutinho, J.A.P. Selective sequential recovery of zinc and copper from acid mine drainage. ACS Sustain. Chem. Eng. 2021, 9, 3647–3657. [Google Scholar] [CrossRef]
- Liu, L.; Liu, S.; Peng, H.; Yang, Z.; Zhao, L.; Tang, A. Surface charge of mesoporous calcium silicate and its adsorption characteristics for heavy metal ions. Solid State Sci. 2020, 99, 106072. [Google Scholar] [CrossRef]
- Bandura, A.V.; Sofo, J.O.; Kubicki, J.D. Adsorption of Zn2+ on the (110) surface of TiO2 (Rutile): A density functional molecular dynamics study. J. Phys. Chem. C 2011, 115, 9608–9614. [Google Scholar] [CrossRef]
- Zeng, R.; Tang, W.; Liu, X.; Ding, C.; Gong, D. Adsorption of Zn2+ from aqueous solutions by si-substituted carbonated hydroxylapatite: Equilibrium, kinetics, and mechanisms. Environ. Prog. Sustain. Energy 2018, 37, 2073–2081. [Google Scholar] [CrossRef]
- Ide, Y.; Ochi, N.; Ogawa, M. Effective and selective adsorption of Zn2+ from seawater on a layered silicate. Angew. Chem. 2010, 123, 680–682. [Google Scholar] [CrossRef]
- Paton-Morales, P.; Talens-Alesson, F.I. Effect of competitive adsorption of Zn2+ on the flocculation of lauryl sulfate micelles by Al3+. Langmuir 2002, 18, 8295–8301. [Google Scholar] [CrossRef]
- Tang, W.; Wang, X.; Zeng, G.; Liang, J.; Li, X.; Xing, W.; He, D.; Tang, L.; Liu, Z. Electro-assisted adsorption of Zn(II) on activated carbon cloth in batch-flow mode: Experimental and theoretical investigations. Environ. Sci. Technol. 2019, 53, 2670–2678. [Google Scholar] [CrossRef] [PubMed]
- Seyedein Ghannad, S.M.R.; Lotfollahi, M.N. Preparation of granular activated carbons from composite of powder activated carbon and modified beta-zeolite and application to heavy metals removal. Water Sci. Technol. 2018, 77, 1591–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Zhang, R.; Li, K.; Li, B.; Tang, C. Adsorption of copper and zinc on activated carbon prepared from Typha latifolia L. Clean-Soil Air Water 2015, 43, 79–85. [Google Scholar] [CrossRef]
- Luo, X.; Lei, X.; Cai, N.; Xie, X.; Xue, Y.; Yu, F. Removal of heavy metal ions from water by magnetic cellulose-based beads with embedded chemically modified magnetite nanoparticles and activated carbon. ACS Sustain. Chem. Eng. 2016, 4, 3960–3969. [Google Scholar] [CrossRef]
- Chouyyok, W.; Shin, Y.; Davidson, J.; Samuels, W.D.; LaFemina, N.H.; Rutledge, R.D.; Fryxell, G.E.; Sangvanich, T.; Yantasee, W. Selective removal of copper(II) from natural waters by nanoporous sorbents functionalized with chelating diamines. Environ. Sci. Technol. 2010, 44, 6390–6395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Martín, J.; López-Garzón, R.; Godino-Salido, M.L.; Cuesta-Martos, R.; Gutiérrez-Valero, M.D.; Arranz-Mascarós, P.; Stoeckli-Evans, H. Adsorption of Zn2+ and Cd2+ from aqueous solution onto a carbon sorbent containing a pyrimidine-polyamine conjugate as ion receptor. Eur. J. Inorg. Chem. 2005, 2005, 3093–3103. [Google Scholar] [CrossRef]
- Ji, X.D.; Ma, Y.Y.; Peng, S.H.; Gong, Y.Y.; Zhang, F. Simultaneous removal of aqueous Zn(2+), Cu(2+), Cd(2+), and Pb(2+) by zeolites synthesized from low-calcium and high-calcium fly ash. Water Sci. Technol. 2017, 76, 2106–2119. [Google Scholar] [CrossRef] [PubMed]
- Shubin, A.A.; Zhidomirov, G.M.; Yakovlev, A.L.; van Santen, R.A. Comparative quantum chemical study of stabilization energies of Zn2+ ions in different zeolite structures. J. Phys. Chem. B 2001, 105, 4928–4935. [Google Scholar] [CrossRef]
- Nuryanthi, N.; Syahputra, A.R.; Oktaviani, O.; Puspitasari, T.; Pangerteni, D.S.; Kurniawan, R.; Astuti, D.A.T.; Darwis, D. Preparation of zeolite-g-polyacrylamide using radiation induced grafting and its adsorption isotherms study on several heavy metal ions. Macromol. Symp. 2020, 391, 1900139. [Google Scholar] [CrossRef]
- Igberase, E.; Osifo, P.; Ofomaja, A. Adsorption of metal ions by microwave assisted grafting of cross-linked chitosan beads. Equilibrium, isotherm, thermodynamic and desorption studies. Appl. Organomet. Chem. 2017, 32, e4131. [Google Scholar] [CrossRef]
- Puspitasari, T.; Oktaviani, O.; Pangerteni, D.S.; Nurfilah, E.; Darwis, D. Study of metal ions removal from aqueous solution by using radiation crosslinked chitosan-co-poly(acrylamide)-based adsorbent. Macromol. Symp. 2015, 353, 168–177. [Google Scholar] [CrossRef]
- Yang, T.-C.; Li, C.-F.; Chou, S.-S.; Chou, C.-C. Adsorption of metal cations by water-soluble N-alkylated disaccharide chitosan derivatives. J. Appl. Polym. Sci. 2005, 98, 564–570. [Google Scholar] [CrossRef]
- Yildirim, M.Ş.; Bíçer, Y.; Yildiz, C. Utilization of fly ash and polypropylene wastes in the production of a new porous composite material. J. Porous Mater. 1996, 3, 189–191. [Google Scholar] [CrossRef]
- Patel, H. Environmental valorisation of bagasse fly ash: A review. RSC Adv. 2020, 10, 31611–31621. [Google Scholar] [CrossRef]
- Dhariwal, A. Laboratory investigations on environmental pollutant materials: Plastics and flyash. Indian J. Environ. Prot. 2010, 30, 943–947. [Google Scholar]
- Varis, O.; Kummu, M. The Major Central Asian River Basins: An Assessment of Vulnerability. Int. J. Water Resour. Dev. 2012, 28, 433–452. [Google Scholar] [CrossRef]
- Deng, X.; Qi, L.; Zhang, Y. Experimental study on adsorption of hexavalent chromium with microwave-assisted alkali modified fly ash. Water Air Soil Pollut. 2018, 229, 18–23. [Google Scholar] [CrossRef]
- Oyehan, T.A.; Olabemiwo, F.A.; Tawabini, B.S.; Saleh, T.A. The capacity of mesoporous fly ash grafted with ultrathin film of polydiallyldimethyl ammonium for enhanced removal of phenol from aqueous solutions. J. Clean. Prod. 2020, 263, 121280. [Google Scholar] [CrossRef]
- Tang, J.; Su, M.; Wu, Q.; Wei, L.; Wang, N.; Xiao, E.; Zhang, H.; Wei, Y.; Liu, Y.; Ekberg, C.; et al. Highly efficient recovery and clean-up of four heavy metals from MSWI fly ash by integrating leaching, selective extraction and adsorption. J. Clean. Prod. 2019, 234, 139–149. [Google Scholar] [CrossRef]
- HuiI, K.; Chao, C.; Kot, S. Removal of mixed heavy metal ions in wastewater by zeolite 4A and residual products from recycled coal fly ash. J. Hazard. Mater. 2005, 127, 89–101. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, P. Novel Magnetic Fly Ash/Poly(acrylic acid) Composite Microgel for Selective Adsorption of Pb(II) Ion: Synthesis and Evaluation. Ind. Eng. Chem. Res. 2014, 53, 2924–2931. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, P. Covalently crosslinked fly ash/poly(acrylic acid-co-acrylamide) composite microgels as novel magnetic selective adsorbent for Pb2+ ion. J. Colloid Interface Sci. 2014, 426, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Sruthi, P.R.; Anas, S. An overview of synthetic modification of nitrile group in polymers and applications. J. Polym. Sci. 2020, 58, 1039–1061. [Google Scholar] [CrossRef]
- Tian, H.; Jiao, L.; Dong, D. Rapid determination of trace cadmium in drinking water using laser-induced breakdown spectroscopy coupled with chelating resin enrichment. Sci. Rep. 2019, 9, 10443. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Ding, C. Optimization of ultrasonic synthesis of N-succinyl-chitosan and adsorption of Zn2+ from aqueous solutions. Desalination Water Treat. 2013, 52, 7856–7865. [Google Scholar] [CrossRef]
- Miyake, Y.; Ishida, H.; Tanaka, S.; Kolev, S.D. Theoretical analysis of the pseudo-second order kinetic model of adsorption. Application to the adsorption of Ag(I) to mesoporous silica microspheres functionalized with thiol groups. Chem. Eng. J. 2013, 218, 350–357. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R. Application of Elovich Equation to the Kinetics of Phosphate Release and Sorption in Soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Ruthven, D.M. Principles of Adsorption and Adsorption Processes; John Wiley and Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Chatzopoulos, D.; Varma, A.; Irvine, R.L. Activated carbon adsorption and desorption of toluene in the aqueous phase. AIChE J. 1993, 39, 2027–2041. [Google Scholar] [CrossRef]
- Wang, C.; Yang, R.; Wang, H. Synthesis of ZIF-8/fly ash composite for adsorption of Cu2+, Zn2+ and Ni2+ from aqueous solutions. Materials 2020, 13, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karanac, M.; Đolić, M.; Veljovic, D.; Rajaković-Ognjanović, V.; Veličković, Z.; Pavićević, V.; Marinković, A. The removal of Zn2+, Pb2+, and As(V) ions by lime activated fly ash and valorization of the exhausted adsorbent. Waste Manag. 2018, 78, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Purbasari, A.; Ariyanti, D.; Sumardiono, S. Preparation and application of fly ash-based geopolymer for heavy metal removal. In AIP Conference Proceedings; AIP Publishing LLC: Woodbury, CA, USA, 2019; Volume 2197, p. 050006(1–5). [Google Scholar] [CrossRef]






| Kinetic Model | Pseudo-First Order Kinetic | Pseudo-Second Order Kinetic | Elovich Equation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qe,exp (mg/g) | k1 (min−1) | qe,cal (mg/g) | R2 | k2 g/(min·mg) | qe,cal (mg/g) | R2 | α | β | R2 |
| 39.81 | 0.0536 | 1.0644 | 0.4453 | 0.0250 | 39.92 | 0.9985 | 2297 | 0.2803 | 0.9785 |
| Adsorbent | Adsorption Capacity (mg/g) | Reference |
|---|---|---|
| ZIF-8/fly ash composite | 197 | [52] |
| lime activated fly ash | 33.13 | [53] |
| fly ash-based geopolymer | 35.18 | [54] |
| modified fly ash | 13.38 | [55] |
| amidoxime-modified polyacrylonitrile/fly ash composite | 43.08 | This work |
| T (°C) | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| qe,cal | b | R2 | K | n | R2 | |
| 25 | 43.08 | 0.6090 | 0.9997 | 21.96 | 7.115 | 0.6189 |
| 35 | 41.93 | 0.0762 | 0.9971 | 7.38 | 2.961 | 0.7441 |
| 45 | 40.65 | 0.0366 | 0.9970 | 4.49 | 2.462 | 0.8617 |
| T (°C) | b | ΔG0 (kJ/mol) | ΔH0 (kJ/mol) | ΔS0 (J/(mol·K)) |
|---|---|---|---|---|
| 25 | 609 | −15.885 | −116.88 | −334.27 |
| 35 | 76.2 | −11.096 | ||
| 45 | 36.6 | −9.518 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Song, X.; Ma, J.; Yu, H.; Liu, G.; Chen, F. Preparation and Characterization of a Novel Amidoxime-Modified Polyacrylonitrile/Fly Ash Composite Adsorbent and Its Application to Metal Wastewater Treatment. Int. J. Environ. Res. Public Health 2022, 19, 856. https://doi.org/10.3390/ijerph19020856
Sun Y, Song X, Ma J, Yu H, Liu G, Chen F. Preparation and Characterization of a Novel Amidoxime-Modified Polyacrylonitrile/Fly Ash Composite Adsorbent and Its Application to Metal Wastewater Treatment. International Journal of Environmental Research and Public Health. 2022; 19(2):856. https://doi.org/10.3390/ijerph19020856
Chicago/Turabian StyleSun, Yan, Xiaojun Song, Jing Ma, Haochen Yu, Gangjun Liu, and Fu Chen. 2022. "Preparation and Characterization of a Novel Amidoxime-Modified Polyacrylonitrile/Fly Ash Composite Adsorbent and Its Application to Metal Wastewater Treatment" International Journal of Environmental Research and Public Health 19, no. 2: 856. https://doi.org/10.3390/ijerph19020856








