Transport of High-Risk Infectious Substances: Packaging for the Transport of Category A Infectious Specimens in Spain
Abstract
:1. Introduction
- (a)
- The drop test assesses the resistance to free-fall drops from a height of 9 m onto a non-resilient, horizontal, flat, massive, and rigid surface.
- (b)
- The puncture test assesses the resistance to perforation from an impact with a steel rod of 7 kg.
- (c)
- If the external package is made of cardboard/fiberboard, it must be subjected to the water spray test simulating rain fall (two inches per hour) applied to the four sides of the package for 1 h to assess the stability and integrity of the packaging system for 1 h.
- (d)
- The packaging system must withstand a load equivalent to the total mass of identical packages stacked up to a minimum height of 3 m for 24 h.
- (e)
- The primary receptable or the secondary packaging must be capable of withstanding, with no leaking, an internal differential pressure of 95 kPa for 30 min.
2. Materials and Methods
2.1. Packaging
- (a)
- Packaging 1
- (b)
- Packaging 2
- (c)
- Packaging 3
- (d)
- Packaging 4
- (e)
- Packaging 5
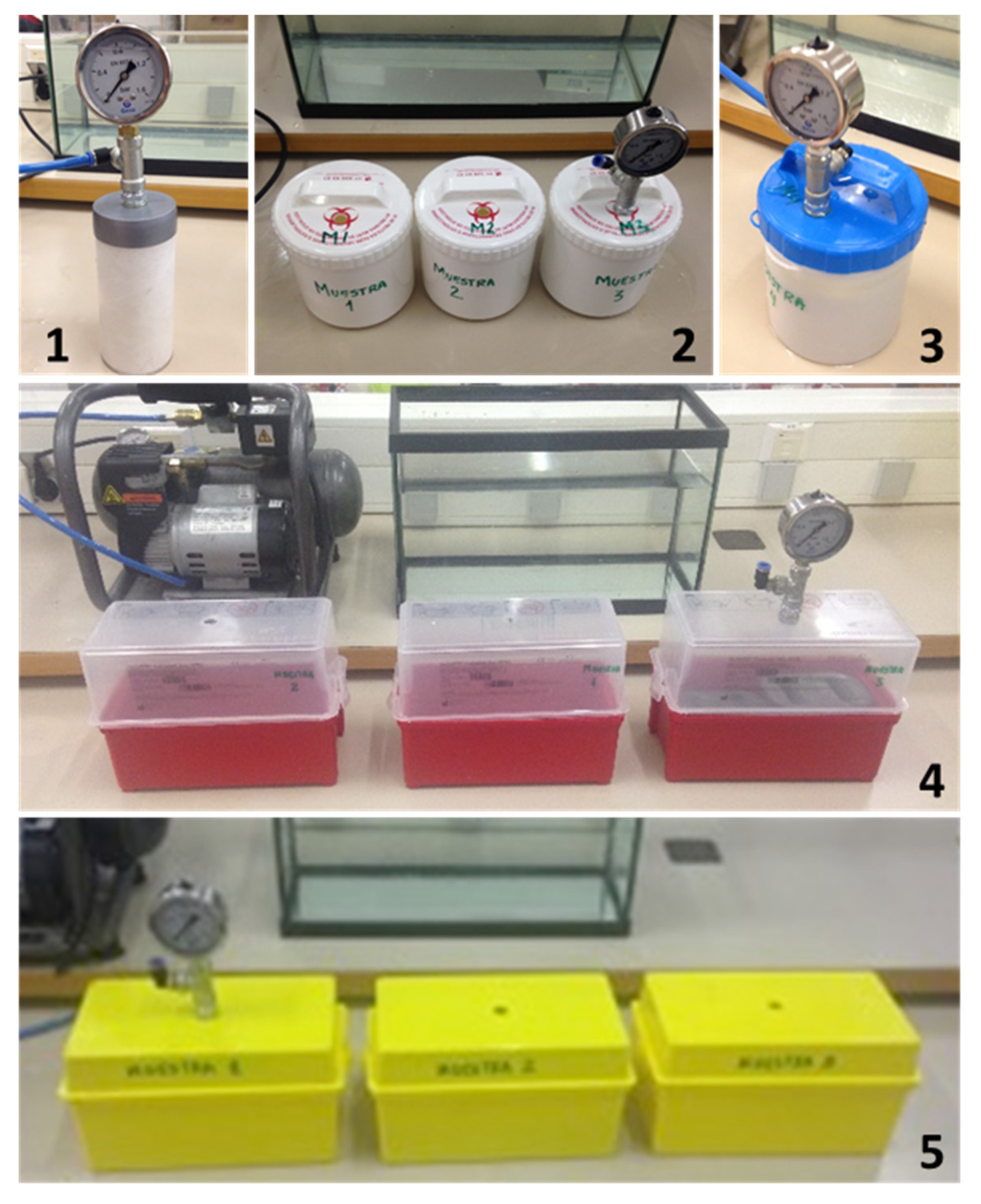
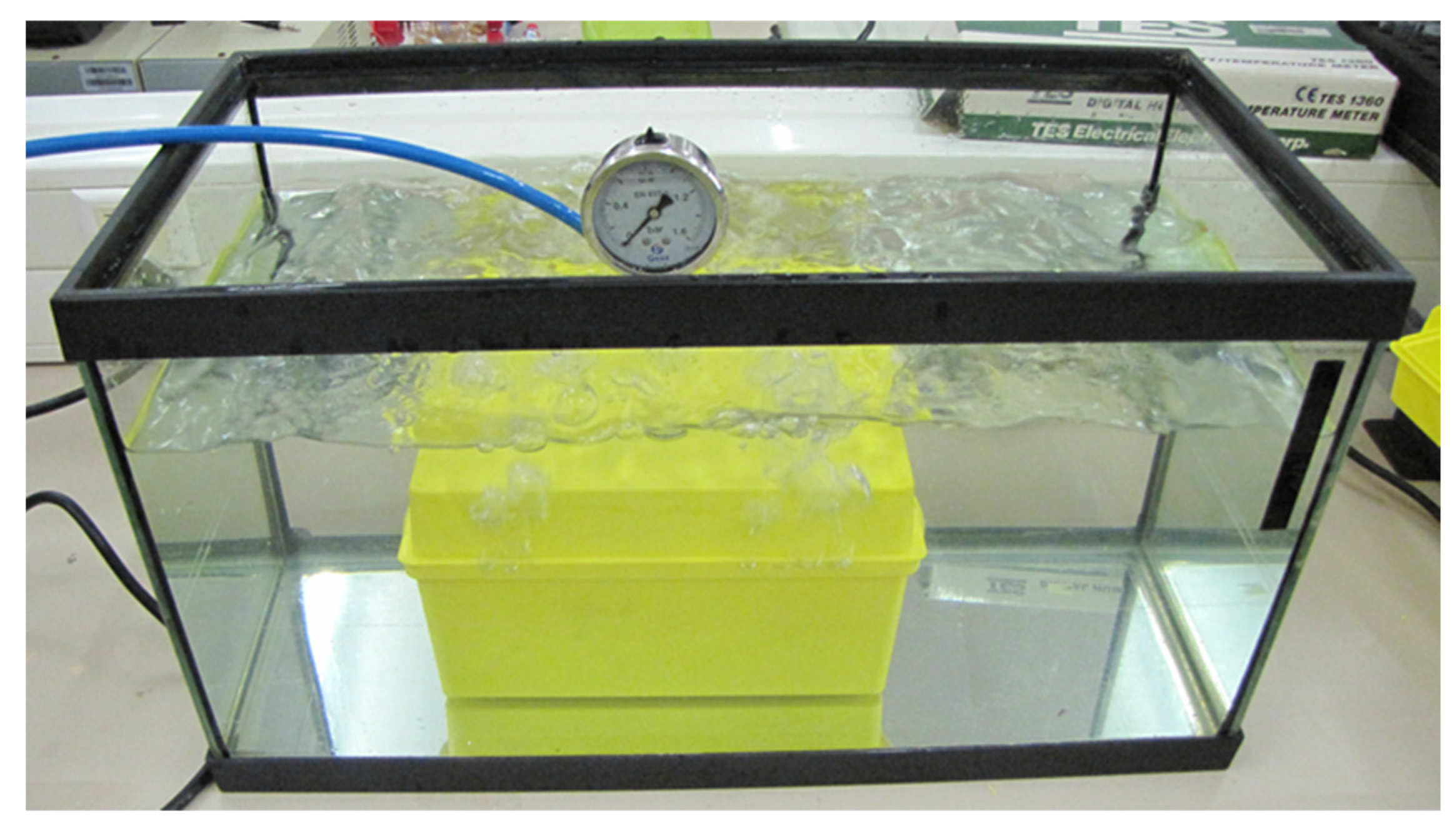
2.2. Pressure Leakproof Test Methodology
3. Results
Pressure Leakproof Test
- (a)
- Packaging 1
- (b)
- Packaging 2
- (c)
- Packaging 3
- (d)
- Packaging 4
- (e)
- Packaging 5
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Guidance on Regulations for the Transport of Infectious Substances 2021–2022; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-9-24001-973-7.
- BOE. Real Decreto 664/1997, de 12 de mayo, sobre la protección de los trabajadores contra los riesgos relacionados con la exposición a agentes biológicos durante el trabajo. Bol. Off. Estado 1997, 124, 16100–16111. [Google Scholar]
- Nisii, C.; Castilletti, C.; Di Caro, A.; Capobianchi, M.R.; Brown, D.; Lloyd, G.; Gunther, S.; Lundkvist, A.; Pletschette, M.; Ippolito, G. The European network of Biosafety-Level-4 laboratories: Enhancing European preparedness for new health threats. Clin. Microbiol. Infect. 2009, 15, 720–726. [Google Scholar] [CrossRef] [Green Version]
- BOE. Texto enmendado de los Anejos A y B del Acuerdo Europeo sobre transporte internacional de mercancías peligrosas por carretera (ADR 2021) con las Enmiendas adoptadas durante las sesiones 105ª, 106ª y 107ª del Grupo de trabajo de transportes de mercancías peligrosas de la Comisión Económica para Europa de las Naciones Unidas (CEPE). Bol. Off. Estado 2021, 88, 41010–42102. [Google Scholar]
- UNECE. ADR—Agreement Concerning the International Carriage of Dangerous Goods by Road. Volume I and II; Report nº ECE/TRANS/300 (vol I and II); United Nations Economic Commission for Europe: New York, NY, USA; Geneve, Switzerland, 2020; ISBN 978-9-21139-179-4. [Google Scholar]
- Montero Guerra, A. Transporte de muestras para Diagnóstico Clínico. Prof. Vet. 2006, 16, 84–89. [Google Scholar]
- Sánchez-Romero, M.I.; Moya, J.M.G.L.; López, J.J.G.; Mira, N.O. Collection, transport and general processing of clinical specimens in Microbiology laboratory. Enferm. Infecc. Microbiol. Clin. Engl. Ed. 2019, 37, 127–134. [Google Scholar] [CrossRef] [PubMed]
- WHO. Laboratory Biosafety Manual, 4th ed.; World Health Organization: Geneva, Switzerland, 2020; ISBN 978-9-240011-311-9.
- UNE-EN ISO 16495:2014; Packaging—Transport Packaging for Dangerous Goods—Test Methods. AENOR: Madrid, Spain, 2014.
- ISO 16495:2022; Packaging—Transport Packaging for Dangerous Goods—Test Methods. International Organization for Standardization: Geneve, Switzerland, 2022.
- Snyder, J.W. Packaging and shipping of infectious substances. Clin. Microbiol. Newsl. 2002, 24, 89–93. [Google Scholar] [CrossRef]
- Adamson, S.; Wilson, R.; James, G. Preparing for Ebolavirus disease: Specimen collection, packaging and transport. Pathology 2015, 47, 403–404. [Google Scholar] [CrossRef] [PubMed]
- Guilinger, T.R.; Gaudioso, J.M.; Aceto, D.G.; Lowe, K.M.; Tucker, M.D.; Salerno, R.M.; Souza, C.A. Development of Self-Remediating Packaging for Safe and Secure Transport of Infectious Substances; (No. SAND2006-6983); Sandia National Lab (SNL-NM): Albuquerque, NM, USA, 2006. [Google Scholar]
- Táboas, J.S.; Cameselle, C.; Mateo, M.; Álvarez, L.; Gouveia, S. Transport infectious substances category A as a high consequence dangerous goods with the potential for misuse in a terrorist event. Int. J. Infect. Dis. 2019, 79, 54–55. [Google Scholar] [CrossRef] [Green Version]
- Graf-Gruber, A.; IATA. Transport of Biological Samples: Air Transport View. In Proceedings of the Second Global Conference of OIE: Reference Laboratory and Collaborating Centres, Paris, France, 21–23 June 2010. [Google Scholar]
- Le, A.B.; Hoboy, S.; Germain, A.; Miller, H.; Thompson, R.; Herstein, J.J.; Jelden, K.C.; Beam, E.L.; Gibbs, S.G.; Lowe, J.J. A pilot survey of the US medical waste industry to determine training needs for safely handling highly infectious waste. Am. J. Infect. Control. 2018, 46, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Gordy, D.; Tashjian, R.S.; Lee, H.; Movassaghi, M.; Yong, W.H. Domestic and international shipping of biospecimens. In Biobanking; Springer Science & Business Media: New York, NY, USA, 2019; pp. 433–443. [Google Scholar]
- SESCAM. SESCAM Pone en Marcha un Nuevo Sistema de Transporte de Muestras Clínicas. Consejería de Sanidad Castilla-La Mancha, 21 August 2008. Available online: https://www.castillalamancha.es/node/184916 (accessed on 8 September 2022).
- El Periódico. Satse Denuncia Irregularidades al Trasladar la Sangre. El Periódico de Aragón, 17 May 2008. Available online: https://www.elperiodicodearagon.com/aragon/2008/05/17/satse-denuncia-irregularidades-trasladar-sangre-47926806.html (accessed on 8 September 2022).
- Girao, F.J. Las Sustancias Infecciosas, aún Sin Vehículos Para Transporte. 2010. Available online: http://frangiraoenelmundo.blogspot.com/2010/05/las-sustancias-infecciosas-aun-sin.html (accessed on 6 September 2022).
- Diario Sanitario. La Sangre Viaja en Taxi. Diario Sanitario, 6 February 2017. Available online: https://diariosanitario.com/la-sangre-viaja-en-taxi (accessed on 8 September 2022).
- Navarra.com. Piden Investigar “Irregularidades” en la Externalización del Transporte del Banco de Sangre de Navarra. Navarra.com–El Español, 18 November 2021. Available online: https://navarra.elespanol.com/articulo/politica/investigar-irregularidad-externalizar-transporte-banco-sangre-navarra/20211118130301385010.html (accessed on 8 September 2022).




| Infectious Substances | UN Number | Packing Instruction | |
|---|---|---|---|
| Category A | Substances containing human pathogens | UN2814 | P620 |
| Substances containing animal pathogens | UN2900 | P620 | |
| Clinical waste | UN3549 | P622 | |
| Category B | Specimens or biological substances | UN3373 | P650 |
| Waste | UN3291 | P621 | |
| Resistance with No Leaking | ||||
|---|---|---|---|---|
| Packaging | Number of Tested Items | Pressure (kPa) | Time (s) | Comments |
| 1 | 3 in water tank 3 out the tank | 12 | 0 | Lid popped off after 4 s |
| 2 | 3 | 0 | 0 | Pressure reached 95 kPa but leaking was observed from the beginning |
| 3 | 6 | 10–52 | 2–10 | 1 item reached 95 kPa for 30 min with no leaking |
| 4 | 3 | 0 | 0 | Lid with no tight closing |
| 5 | 3 | 0 | 0 | Bubbling from the beginning |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, J.H.; Gouveia, S.; Cameselle, C. Transport of High-Risk Infectious Substances: Packaging for the Transport of Category A Infectious Specimens in Spain. Int. J. Environ. Res. Public Health 2022, 19, 12989. https://doi.org/10.3390/ijerph192012989
Sánchez JH, Gouveia S, Cameselle C. Transport of High-Risk Infectious Substances: Packaging for the Transport of Category A Infectious Specimens in Spain. International Journal of Environmental Research and Public Health. 2022; 19(20):12989. https://doi.org/10.3390/ijerph192012989
Chicago/Turabian StyleSánchez, Jorge H., Susana Gouveia, and Claudio Cameselle. 2022. "Transport of High-Risk Infectious Substances: Packaging for the Transport of Category A Infectious Specimens in Spain" International Journal of Environmental Research and Public Health 19, no. 20: 12989. https://doi.org/10.3390/ijerph192012989






