Mixture Risk Assessment of Complex Real-Life Mixtures—The PANORAMIX Project
Abstract
1. Introduction
2. Chemical Mixture Drivers across the Environment-Food-Human Continuum
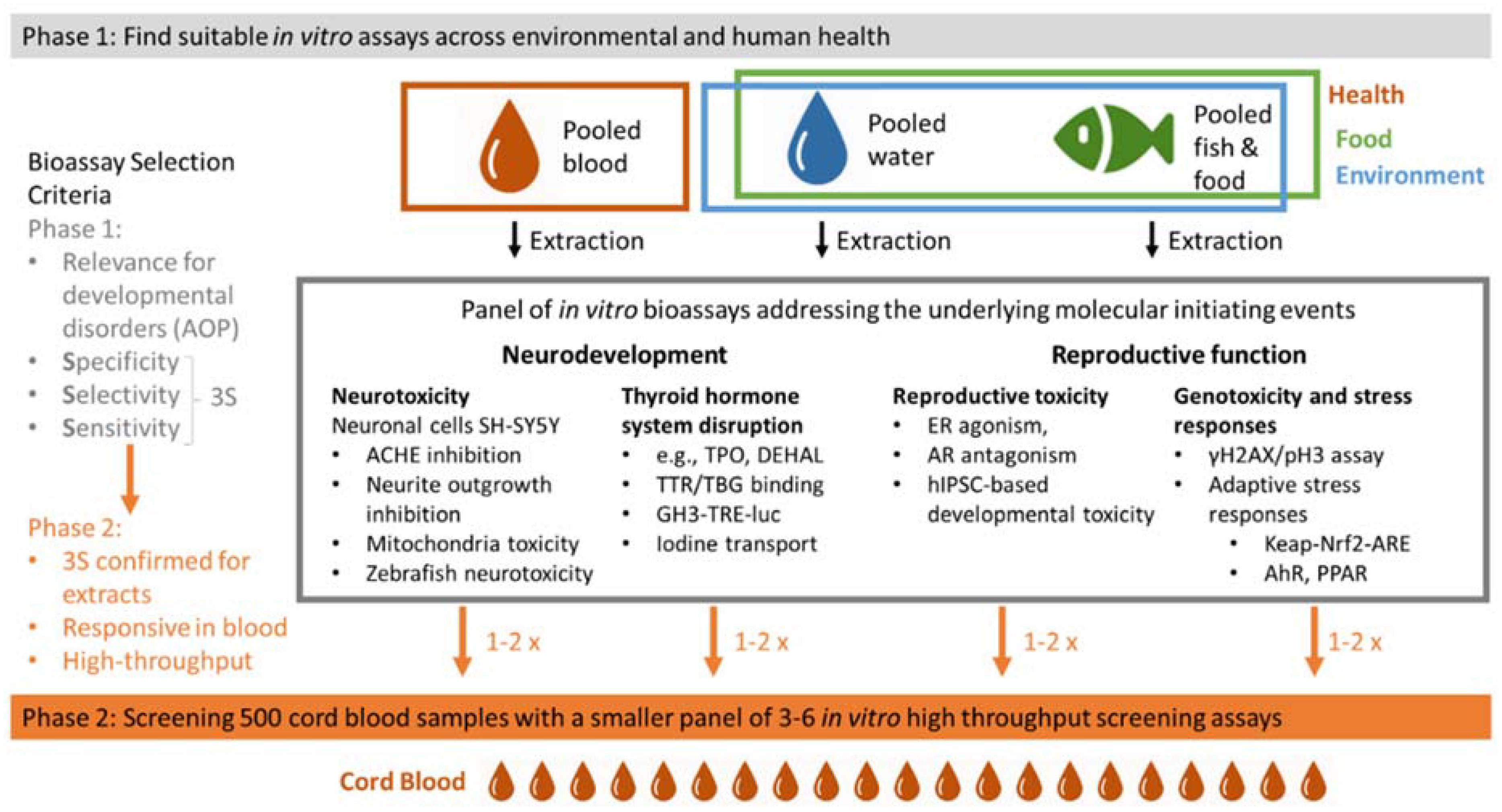
3. Chemical Mixture Drivers in Human Cord Blood
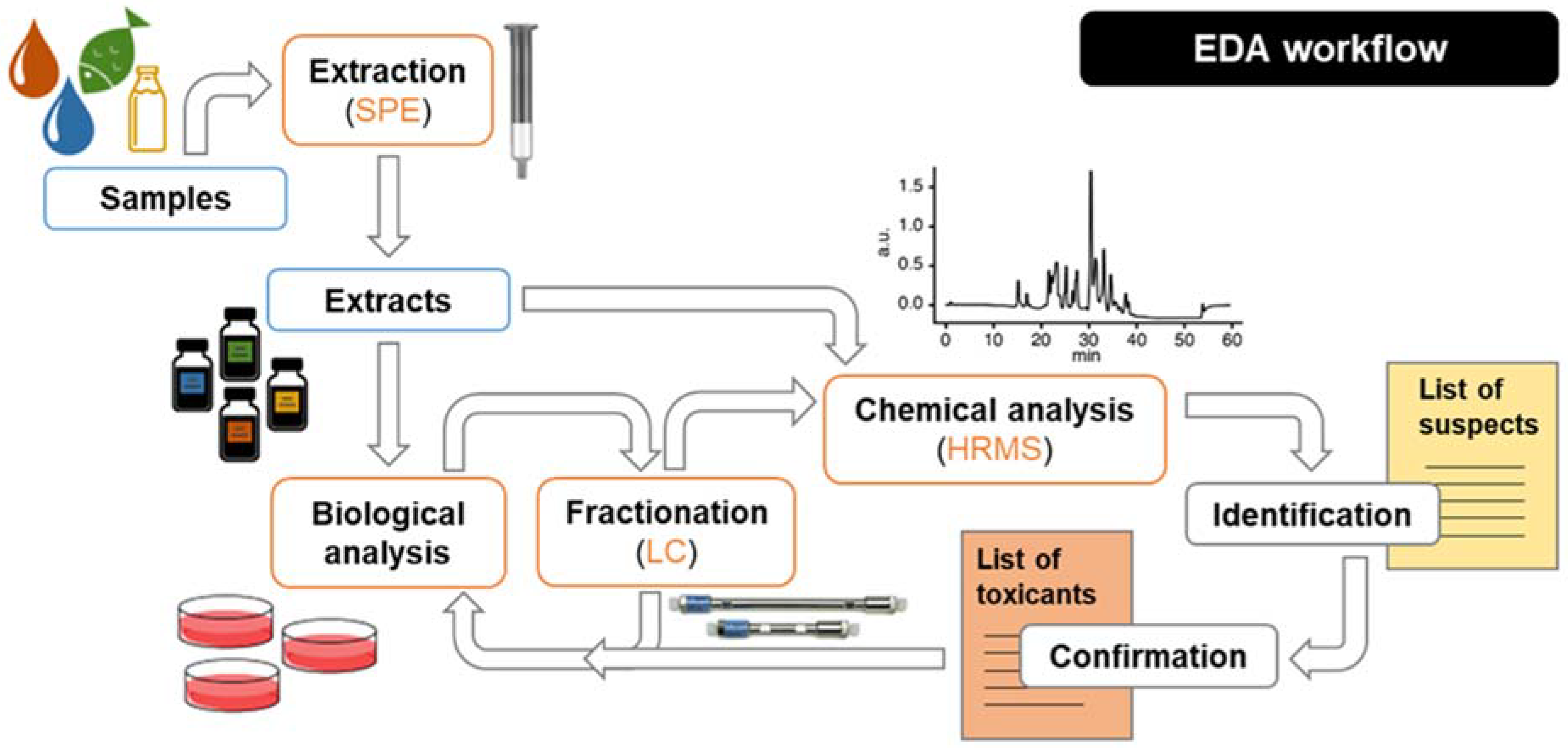
4. State of the Art of Mixture Risk Assessment
5. Pragmatic Solutions for Mixture Risk Assessment
5.1. Designed Mixture Experiments for Assessing Alignment with or Deviation from Additivity
5.2. Case Studies for Evaluating Mixture Assessment Factors and Safety Margins
5.3. Derivation of Effect-Based Trigger Values
5.4. Integrative Web-Based Chemical Mixture Calculator
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fuller, R.; Landrigan, P.J.; Balakrishnan, K.; Bathan, G.; Bose-O’Reilly, S.; Brauer, M.; Caravanos, J.; Chiles, T.; Cohen, A.; Corra, L.; et al. Pollution and Health: A Progress Update. Lancet Planet. Health 2022, 6, e535–e547. [Google Scholar] [CrossRef]
- Evans, R.M.; Martin, O.V.; Faust, M.; Kortenkamp, A. Should the Scope of Human Mixture Risk Assessment Span Legislative/Regulatory Silos for Chemicals? Sci. Total Environ. 2016, 543, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Apel, P.; Kortenkamp, A.; Koch, H.M.; Vogel, N.; Rüther, M.; Kasper-Sonnenberg, M.; Conrad, A.; Brüning, T.; Kolossa-Gehring, M. Time Course of Phthalate Cumulative Risks to Male Developmental Health over a 27-Year Period: Biomonitoring Samples of the German Environmental Specimen Bank. Environ. Int. 2020, 137, 105467. [Google Scholar] [CrossRef]
- Neale, P.A.; Braun, G.; Brack, W.; Carmona, E.; Gunold, R.; König, M.; Krauss, M.; Liebmann, L.; Liess, M.; Link, M.; et al. Assessing the Mixture Effects in in Vitro Bioassays of Chemicals Occurring in Small Agricultural Streams during Rain Events. Environ. Sci. Technol. 2020, 54, 8280–8290. [Google Scholar] [CrossRef] [PubMed]
- Baumer, A.; Jäsch, S.; Ulrich, N.; Bechmann, I.; Landmann, J.; Stöver, A.; Escher, B.I. Chemical Mixtures in Human Post-Mortem Tissues Assessed by a Combination of Chemical Analysis and in Vitro Bioassays after Extraction with Silicone. Environ. Int. 2021, 157, 106867. [Google Scholar] [CrossRef]
- Tralau, T.; Oelgeschläger, M.; Kugler, J.; Bloch, D.; Braeuning, A.; Burgdorf, T.; Marx-Stoelting, P.; Ritz, V.; Schmeisser, S.; Trubiroha, A.; et al. A Prospective Whole-Mixture Approach to Assess Risk of the Food and Chemical Exposome. Nat. Food 2021, 2, 463–468. [Google Scholar] [CrossRef]
- Parish, S.T.; Aschner, M.; Casey, W.; Corvaro, M.; Embry, M.R.; Fitzpatrick, S.; Kidd, D.; Kleinstreuer, N.C.; Lima, B.S.; Settivari, R.S.; et al. An Evaluation Framework for New Approach Methodologies (NAMs) for Human Health Safety Assessment. Regul. Toxicol. Pharmacol. 2020, 112, 104592. [Google Scholar] [CrossRef]
- Vermeulen, R.; Schymanski, E.L.; Barabási, A.L.; Miller, G.W. The Exposome and Health: Where Chemistry Meets Biology. Science 2020, 367, 392–396. [Google Scholar] [CrossRef]
- Caballero-Casero, N.; Belova, L.; Vervliet, P.; Antignac, J.-P.; Castaño, A.; Debrauwer, L.; López, M.E.; Huber, C.; Klanova, J.; Krauss, M.; et al. Towards harmonised criteria in quality assurance and quality control of suspect and non-target LC-HRMS analytical workflows for screening of emerging contaminants in human biomonitoring. Trends Anal. Chem. 2021, 136, 116201. [Google Scholar] [CrossRef]
- Guo, Z.; Huang, S.; Wang, J.; Feng, Y.-L. Recent advances in non-targeted screening analysis using liquid chromatography—High resolution mass spectrometry to explore new biomarkers for human exposure. Talanta 2020, 219, 121339. [Google Scholar] [CrossRef]
- European Chemicals Agency. New Approach Methodologies in Regulatory Science. In Proceedings of a Scientific Workshop; Helsinki, Finland, 19–20 April 2016, ECHA: Helsinki, Finland, 2016; ISBN 9789294953971. [Google Scholar]
- Gwinn, M.R. New Approach Methodologies (NAMs) and Chemical Risk Assessment. 2020. Available online: https://epa.figshare.com/articles/presentation/New_Approach_Methodologies_NAMs_and_Chemical_Risk_Assessment/12837488 (accessed on 2 June 2022).
- Jolliet, O.; Huang, L.; Hou, P.; Fantke, P. High Throughput Risk and Impact Screening of Chemicals in Consumer Products. Risk Anal. 2021, 41, 627–644. [Google Scholar] [CrossRef]
- Carlson, J.M.; Janulewicz, P.A.; Kleinstreuer, N.C.; Heiger-Bernays, W. Impact of High-Throughput Model Parameterization and Data Uncertainty on Thyroid-Based Toxicological Estimates for Pesticide Chemicals. Environ. Sci. Technol. 2022, 56, 5620–5631. [Google Scholar] [CrossRef] [PubMed]
- Bal-Price, A.; Hogberg, H.T.; Crofton, K.M.; Daneshian, M.; FitzGerald, R.E.; Fritsche, E.; Heinonen, T.; Hougaard Bennekou, S.; Klima, S.; Piersma, A.H.; et al. Recommendation on Test Readiness Criteria for New Approach Methods in Toxicology: Exemplified for Developmental Neurotoxicity. ALTEX 2018, 35, 306–352. [Google Scholar] [CrossRef] [PubMed]
- Harrill, J.A.; Everett, L.J.; Haggard, D.E.; Sheffield, T.; Bundy, J.L.; Willis, C.M.; Thomas, R.S.; Shah, I.; Judson, R.S. High-Throughput Transcriptomics Platform for Screening Environmental Chemicals. Toxicol. Sci. 2021, 181, 68–89. [Google Scholar] [CrossRef]
- Harrill, J.; Shah, I.; Setzer, R.W.; Haggard, D.; Auerbach, S.; Judson, R.; Thomas, R.S. Considerations for Strategic Use of High-Throughput Transcriptomics Chemical Screening Data in Regulatory Decisions. Curr. Opin. Toxicol. 2019, 15, 64–75. [Google Scholar] [CrossRef]
- Escher, B.I.; Stapleton, H.M.; Schymanski, E.L. Tracking Complex Mixtures of Chemicals in Our Changing Environment. Science 2020, 367, 388–392. [Google Scholar] [CrossRef]
- Simonnet-Laprade, C.; Bayen, S.; McGoldrick, D.; McDaniel, T.; Hutinet, S.; Marchand, P.; Vénisseau, A.; Cariou, R.; le Bizec, B.; Dervilly, G. Evidence of Complementarity between Targeted and Non-Targeted Analysis Based on Liquid and Gas-Phase Chromatography Coupled to Mass Spectrometry for Screening Halogenated Persistent Organic Pollutants in Environmental Matrices. Chemosphere 2022, 293, 133615. [Google Scholar] [CrossRef]
- Pourchet, M.; Narduzzi, L.; Jean, A.; Guiffard, I.; Bichon, E.; Cariou, R.; Guitton, Y.; Hutinet, S.; Vlaanderen, J.; Meijer, J.; et al. Non-Targeted Screening Methodology to Characterise Human Internal Chemical Exposure: Application to Halogenated Compounds in Human Milk. Talanta 2021, 225, 121979. [Google Scholar] [CrossRef] [PubMed]
- Léon, A.; Cariou, R.; Hutinet, S.; Hurel, J.; Guitton, Y.; Tixier, C.; Munschy, C.; Antignac, J.-P.; Dervilly-Pinel, G.; le Bizec, B. HaloSeeker 1.0: A User-Friendly Software to Highlight Halogenated Chemicals in Nontargeted High-Resolution Mass Spectrometry Data Sets. Anal. Chem. 2019, 91, 3500–3507. [Google Scholar] [CrossRef]
- Kalloo, G.; Wellenius, G.A.; McCandless, L.; Calafat, A.M.; Sjodin, A.; Sullivan, A.J.; Romano, M.E.; Karagas, M.R.; Chen, A.; Yolton, K.; et al. Chemical Mixture Exposures during Pregnancy and Cognitive Abilities in School-Aged Children. Environ. Res. 2021, 197, 111027. [Google Scholar] [CrossRef] [PubMed]
- Thrupp, T.J.; Runnalls, T.J.; Scholze, M.; Kugathas, S.; Kortenkamp, A.; Sumpter, J.P. The Consequences of Exposure to Mixtures of Chemicals: Something from ‘Nothing’ and ‘a Lot from a Little’ When Fish Are Exposed to Steroid Hormones. Sci. Total Environ. 2018, 619–620, 1482–1492. [Google Scholar] [CrossRef]
- Rider, C.V.; Furr, J.R.; Wilson, V.S.; Gray, L.E. Cumulative Effects of in Utero Administration of Mixtures of Reproductive Toxicants That Disrupt Common Target Tissues via Diverse Mechanisms of Toxicity. Int. J. Androl. 2010, 33, 443–462. [Google Scholar] [CrossRef] [PubMed]
- Schildroth, S.; Wise, L.A.; Wesselink, A.K.; de La Cruz, P.; Bethea, T.N.; Weuve, J.; Fruh, V.; Botelho, J.C.; Sjodin, A.; Calafat, A.M.; et al. Correlates of Persistent Endocrine-Disrupting Chemical Mixtures among Reproductive-Aged Black Women. Environ. Sci. Technol. 2021, 55, 14000–14014. [Google Scholar] [CrossRef]
- Lee, J.; Escher, B.I.; Scholz, S.; Schlichting, R. Inhibition of Neurite Outgrowth and Enhanced Effects Compared to Baseline Toxicity in SH-SY5Y Cells. Arch. Toxicol. 2022, 96, 1039–1053. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Kim, J.-H.; Lee, D.-C.; Lim, H.-J.; Kang, J.-C. Toxic Effects on Oxidative Stress, Neurotoxicity, Stress, and Immune Responses in Juvenile Olive Flounder, Paralichthys Olivaceus, Exposed to Waterborne Hexavalent Chromium. Biology 2022, 11, 766. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Huang, R.; Shi, Q.; Boyd, W.A.; Zhao, J.; Sun, N.; Rice, J.R.; Dunlap, P.E.; Hackstadt, A.J.; Bridge, M.F.; et al. Comprehensive Analyses and Prioritization of Tox21 10K Chemicals Affecting Mitochondrial Function by In-Depth Mechanistic Studies. Environ. Health Perspect. 2018, 126, 077010. [Google Scholar] [CrossRef]
- Teixidó, E.; Kießling, T.R.; Krupp, E.; Quevedo, C.; Muriana, A.; Scholz, S. Automated Morphological Feature Assessment for Zebrafish Embryo Developmental Toxicity Screens. Toxicol. Sci. 2019, 167, 438–449. [Google Scholar] [CrossRef]
- Lauschke, K.; Treschow, A.F.; Rasmussen, M.A.; Davidsen, N.; Holst, B.; Emnéus, J.; Taxvig, C.; Vinggaard, A.M. Creating a Human-Induced Pluripotent Stem Cell-Based NKX2.5 Reporter Gene Assay for Developmental Toxicity Testing. Arch. Toxicol. 2021, 95, 1659–1670. [Google Scholar] [CrossRef]
- Hamers, T.; Kortenkamp, A.; Scholze, M.; Molenaar, D.; Cenijn, P.H.; Weiss, J.M. Transthyretin-Binding Activity of Complex Mixtures Representing the Composition of Thyroid-Hormone Disrupting Contaminants in House Dust and Human Serum. Environ. Health Perspect. 2020, 128, 017015. [Google Scholar] [CrossRef]
- Jayarama-Naidu, R.; Johannes, J.; Meyer, F.; Wirth, E.K.; Schomburg, L.; Köhrle, J.; Renko, K. A Nonradioactive Uptake Assay for Rapid Analysis of Thyroid Hormone Transporter Function. Endocrinology 2015, 156, 2739–2745. [Google Scholar] [CrossRef]
- Leusch, F.D.L.; Aneck-Hahn, N.H.; Cavanagh, J.A.E.; du Pasquier, D.; Hamers, T.; Hebert, A.; Neale, P.A.; Scheurer, M.; Simmons, S.O.; Schriks, M. Comparison of in Vitro and in Vivo Bioassays to Measure Thyroid Hormone Disrupting Activity in Water Extracts. Chemosphere 2018, 191, 868–875. [Google Scholar] [CrossRef]
- Renko, K.; Hoefig, C.S.; Dupuy, C.; Harder, L.; Schwiebert, C.; Köhrle, J.; Schomburg, L. A Nonradioactive DEHAL Assay for Testing Substrates, Inhibitors, and Monitoring Endogenous Activity. Endocrinology 2016, 157, 4516–4525. [Google Scholar] [CrossRef]
- Renko, K.; Schäche, S.; Hoefig, C.S.; Welsink, T.; Schwiebert, C.; Braun, D.; Becker, N.-P.; Köhrle, J.; Schomburg, L. An Improved Nonradioactive Screening Method Identifies Genistein and Xanthohumol as Potent Inhibitors of Iodothyronine Deiodinases. Thyroid 2015, 25, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Vinggaard, A.M.; Bonefeld-Jørgensen, E.C.; Jensen, T.K.; Fernandez, M.F.; Rosenmai, A.K.; Taxvig, C.; Rodriguez-Carrillo, A.; Wielsøe, M.; Long, M.; Olea, N.; et al. Receptor-Based in Vitro Activities to Assess Human Exposure to Chemical Mixtures and Related Health Impacts. Environ. Int. 2021, 146, 106191. [Google Scholar] [CrossRef]
- Kopp, B.; Khoury, L.; Audebert, M. Validation of the γH2AX biomarker for genotoxicity assessment: A review. Arch. Toxicol. 2019, 93, 2103–2114. [Google Scholar] [CrossRef] [PubMed]
- Neale, P.A.; Altenburger, R.; Aït-Aïssa, S.; Brion, F.; Busch, W.; de Aragão Umbuzeiro, G.; Denison, M.S.; du Pasquier, D.; Hilscherová, K.; Hollert, H.; et al. Development of a Bioanalytical Test Battery for Water Quality Monitoring: Fingerprinting Identified Micropollutants and Their Contribution to Effects in Surface Water. Water Res. 2017, 123, 734–750. [Google Scholar] [CrossRef]
- Escher, B.I.; Neale, P.A. Effect-Based Trigger Values for Mixtures of Chemicals in Surface Water Detected with In Vitro Bioassays. Environ. Toxicol. Chem. 2021, 40, 487–499. [Google Scholar] [CrossRef]
- Brack, W.; Ait-Aissa, S.; Burgess, R.M.; Busch, W.; Creusot, N.; di Paolo, C.; Escher, B.I.; Mark Hewitt, L.; Hilscherova, K.; Hollender, J.; et al. Effect-Directed Analysis Supporting Monitoring of Aquatic Environments—An in-Depth Overview. Sci. Total Environ. 2016, 544, 1073–1118. [Google Scholar] [CrossRef]
- Hashmi, M.A.K.; Escher, B.I.; Krauss, M.; Teodorovic, I.; Brack, W. Effect-Directed Analysis (EDA) of Danube River Water Sample Receiving Untreated Municipal Wastewater from Novi Sad, Serbia. Sci. Total Environ. 2018, 624, 1072–1081. [Google Scholar] [CrossRef]
- Hashmi, M.A.K.; Krauss, M.; Escher, B.I.; Teodorovic, I.; Brack, W. Effect-Directed Analysis of Progestogens and Glucocorticoids at Trace Concentrations in River Water. Environ. Toxicol. Chem. 2020, 39, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Oberacher, H.; Sasse, M.; Antignac, J.-P.; Guitton, Y.; Debrauwer, L.; Jamin, E.L.; Schulze, T.; Krauss, M.; Covaci, A.; Caballero-Casero, N.; et al. A European Proposal for Quality Control and Quality Assurance of Tandem Mass Spectral Libraries. Environ. Sci. Eur. 2020, 32, 43. [Google Scholar] [CrossRef]
- Pourchet, M.; Debrauwer, L.; Klanova, J.; Price, E.J.; Covaci, A.; Caballero-Casero, N.; Oberacher, H.; Lamoree, M.; Damont, A.; Fenaille, F.; et al. Suspect and Non-Targeted Screening of Chemicals of Emerging Concern for Human Biomonitoring, Environmental Health Studies and Support to Risk Assessment: From Promises to Challenges and Harmonisation Issues. Environ. Int. 2020, 139, 105545. [Google Scholar] [CrossRef] [PubMed]
- Meijer, J.; Lamoree, M.; Hamers, T.; Antignac, J.-P.; Hutinet, S.; Debrauwer, L.; Covaci, A.; Huber, C.; Krauss, M.; Walker, D.I.; et al. An Annotation Database for Chemicals of Emerging Concern in Exposome Research. Environ. Int. 2021, 152, 106511. [Google Scholar] [CrossRef] [PubMed]
- Jonkers, T.J.H.; Meijer, J.; Vlaanderen, J.J.; Vermeulen, R.C.H.; Houtman, C.J.; Hamers, T.; Lamoree, M.H. High-Performance Data Processing Workflow Incorporating Effect-Directed Analysis for Feature Prioritization in Suspect and Nontarget Screening. Environ. Sci. Technol. 2022, 56, 1639–1651. [Google Scholar] [CrossRef]
- Houtman, C.J.; ten Broek, R.; van Oorschot, Y.; Kloes, D.; van der Oost, R.; Rosielle, M.; Lamoree, M.H. High Resolution Effect-Directed Analysis of Steroid Hormone (Ant)Agonists in Surface and Wastewater Quality Monitoring. Environ. Toxicol. Pharmacol. 2020, 80, 103460. [Google Scholar] [CrossRef]
- Kyhl, H.B.; Jensen, T.K.; Barington, T.; Buhl, S.; Norberg, L.A.; Jørgensen, J.S.; Jensen, D.F.G.; Christesen, H.T.; Lamont, R.F.; Husby, S. The Odense Child Cohort: Aims, Design, and Cohort Profile. Paediatr. Perinat. Epidemiol. 2015, 29, 250–258. [Google Scholar] [CrossRef]
- Batke, M.; Damm, G.; Foth, H.; Freyberger, A.; Gebel, T.; Gundert-Remy, U.; Hengstler, J.; Mangerich, A.; Partosch, F.; Röhl, C.; et al. The EU Chemicals Strategy for Sustainability: Critical Reflections on Proposed Regulatory Changes for Endocrine Disruptors and Mixture Toxicity. Arch. Toxicol. 2022, 96, 1133–1135. [Google Scholar] [CrossRef]
- Kümmerer, K.; Dionysiou, D.D.; Olsson, O.; Fatta-Kassinos, D. A Path to Clean Water. Science 2018, 361, 222–224. [Google Scholar] [CrossRef]
- Sprinkle, R.H.; Payne-Sturges, D.C. Mixture Toxicity, Cumulative Risk, and Environmental Justice in United States Federal Policy, 1980–2016: Why, with Much Known, Was Little Done? Environ. Health 2021, 20, 104. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Guidelines for the Health Risk Assessment of Chemical Mixtures. Fed. Regist. 1986, 51, 34014–34025. [Google Scholar]
- EFSA. Opinion of the Scientific Panel on Plant Protection Products and Their Residues to Evaluate the Suitability of Existing Methodologies and, If Appropriate, the Identification of New Approaches to Assess Cumulative and Synergistic Risks from Pesticides to h. EFSA J. 2008, 6, 705. [Google Scholar] [CrossRef]
- EFSA. Guidance on Harmonised Methodologies for Human Health, Animal Health and Ecological Risk Assessment of Combined Exposure to Multiple Chemicals. EFSA J. 2019, 17, e05634. [Google Scholar] [CrossRef]
- OECD. Considerations for Assessing the Risks of Combined Exposure to Multiple Chemicals Series on Testing and Assessment No. 296; OECD Publishing: Paris, France, 2018. [Google Scholar]
- Trac, L.N.; Sjoholm, K.K.; Birch, H.; Mayer, P. Passive Dosing of Petroleum and Essential Oil UVCBs—Whole Mixture Toxicity Testing at Controlled Exposure. Environ. Sci. Technol. 2021, 55, 6150–6159. [Google Scholar] [CrossRef]
- Bopp, S.; Berggren, E.; Kienzler, A.; van der Linden, S.; Worth, A.; Institute for Health and Consumer Protection. Scientific Methodologies for the Assessment of Combined Effects of Chemicals—A Survey and Literature Review: Use of Novel and Alternative Methods in the Assessment of Effects from Combined Exposure to Multiple Chemicals; Publications Office: Luxembourg, Luxembourg, 2015; ISBN 9789279519253. [Google Scholar]
- OECD. Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides and Biotechnology Guidance on Grouping of Chemicals, Second Edition Series on Testing & Assessment No. 194 JT03356214; OECD: Paris, France, 2014. [Google Scholar]
- SCHER; SCENIHR; SCCS. Toxicity and Assessment of Chemical Mixtures. Eur. Union 2013, 10, 21444. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. US EPA: Guidance on Cumulative Risk Assessment of Pesticide Chemicals That Have a Common Mechansim of Toxicity. 2002. Available online: https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/guidance-cumulative-risk-assessment-pesticide (accessed on 18 May 2022).
- EFSA Panel on Plant Protection Products and their Residues Scientific Opinion on the Identification of Pesticides to Be Included in Cumulative Assessment Groups on the Basis of Their Toxicological Profile. EFSA J. 2013, 11, 3293. [CrossRef]
- More, S.J.; Bampidis, V.; Benford, D.; Bragard, C.; Hernandez-Jerez, A.; Bennekou, S.H.; Halldorsson, T.I.; Koutsoumanis, K.P.; Lambré, C.; Machera, K.; et al. Guidance Document on Scientific Criteria for Grouping Chemicals into Assessment Groups for Human Risk Assessment of Combined Exposure to Multiple Chemicals. EFSA J. 2021, 19, e07033. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.; Luckert, C.; Alarcan, J.; de Sousa, G.; Gioutlakis, M.; Katsanou, E.S.; Konstantinidou, P.; Machera, K.; Milani, E.S.; Peijnenburg, A.; et al. An Adverse Outcome Pathway-Based Approach to Assess Steatotic Mixture Effects of Hepatotoxic Pesticides in Vitro. Food Chem. Toxicol. 2020, 139, 111283. [Google Scholar] [CrossRef]
- Anastassiadou, M.; Choi, J.; Coja, T.; Dujardin, B.; Hart, A.; Hernandez-Jerrez, A.F.; Jarrah, S.; Lostia, A.; Machera, K.; Mangas, I.; et al. Cumulative Dietary Risk Assessment of Chronic Acetylcholinesterase Inhibition by Residues of Pesticides. EFSA J. 2021, 19, e06392. [Google Scholar] [CrossRef]
- Crivellente, F.; Hart, A.; Hernandez-Jerez, A.F.; Hougaard Bennekou, S.; Pedersen, R.; Terron, A.; Wolterink, G.; Mohimont, L. Establishment of Cumulative Assessment Groups of Pesticides for Their Effects on the Thyroid. EFSA J. 2019, 17, e05801. [Google Scholar] [CrossRef]
- Colnot, T.; Melching-Kollmuß, S.; Semino, G.; Dekant, W. A Flow Scheme for Cumulative Assessment of Pesticides for Adverse Liver Effects. Regul. Toxicol. Pharmacol. 2020, 116, 104694. [Google Scholar] [CrossRef]
- Beronius, A.; Zilliacus, J.; Hanberg, A.; Luijten, M.; van der Voet, H.; van Klaveren, J. Methodology for Health Risk Assessment of Combined Exposures to Multiple Chemicals. Food Chem. Toxicol. 2020, 143, 111520. [Google Scholar] [CrossRef] [PubMed]
- Boberg, J.; Bredsdorff, L.; Petersen, A.; Löbl, N.; Jensen, B.H.; Vinggaard, A.M.; Nielsen, E. Chemical Mixture Calculator—A Novel Tool for Mixture Risk Assessment. Food Chem. Toxicol. 2021, 152, 112167. [Google Scholar] [CrossRef] [PubMed]
- Martin, O.V.; Evans, R.M.; Faust, M.; Kortenkamp, A. A Human Mixture Risk Assessment for Neurodevelopmental Toxicity Associated with Polybrominated Diphenyl Ethers Used as Flame Retardants. Environ. Health Perspect. 2017, 125, 087016. [Google Scholar] [CrossRef]
- Kortenkamp, A.; Scholze, M.; Ermler, S.; Priskorn, L.; Jørgensen, N.; Andersson, A.-M.; Frederiksen, H. Combined Exposures to Bisphenols, Polychlorinated Dioxins, Paracetamol, and Phthalates as Drivers of Deteriorating Semen Quality. Environ. Int. 2022, 165, 107322. [Google Scholar] [CrossRef] [PubMed]
- Howdeshell, K.L.; Rider, C.V.; Wilson, V.S.; Furr, J.R.; Lambright, C.R.; Gray, L.E. Dose Addition Models Based on Biologically Relevant Reductions in Fetal Testosterone Accurately Predict Postnatal Reproductive Tract Alterations by a Phthalate Mixture in Rats. Toxicol. Sci. 2015, 148, 488–502. [Google Scholar] [CrossRef]
- Orton, F.; Ermler, S.; Kugathas, S.; Rosivatz, E.; Scholze, M.; Kortenkamp, A. Mixture Effects at Very Low Doses with Combinations of Anti-Androgenic Pesticides, Antioxidants, Industrial Pollutant and Chemicals Used in Personal Care Products. Toxicol. Appl. Pharmacol. 2014, 278, 201–208. [Google Scholar] [CrossRef]
- Christiansen, S.; Scholze, M.; Dalgaard, M.; Vinggaard, A.M.; Axelstad, M.; Kortenkamp, A.; Hass, U. Synergistic Disruption of External Male Sex Organ Development by a Mixture of Four Antiandrogens. Environ. Health Perspect. 2009, 117, 1839–1846. [Google Scholar] [CrossRef]
- Cedergreen, N. Quantifying Synergy: A Systematic Review of Mixture Toxicity Studies within Environmental Toxicology. PLoS ONE 2014, 9, e96580. [Google Scholar] [CrossRef]
- Martin, O.; Scholze, M.; Ermler, S.; McPhie, J.; Bopp, S.K.; Kienzler, A.; Parissis, N.; Kortenkamp, A. Ten Years of Research on Synergisms and Antagonisms in Chemical Mixtures: A Systematic Review and Quantitative Reappraisal of Mixture Studies. Environ. Int. 2021, 146, 106206. [Google Scholar] [CrossRef]
- Cukurluoglu, S.; Muezzinoglu, A. Assessment of Toxicity in Waters Due to Heavy Metals Derived from Atmospheric Deposition Using Vibrio Fischeri. J. Environ. Sci. Health Part A 2013, 48, 57–66. [Google Scholar] [CrossRef]
- Crémazy, A.; Brix, K.V.; Wood, C.M. Chronic Toxicity of Binary Mixtures of Six Metals (Ag, Cd, Cu, Ni, Pb, and Zn) to the Great Pond Snail Lymnaea Stagnalis. Environ. Sci. Technol. 2018, 52, 5979–5988. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Svoboda, K.R.; Lenz, K.A.; Pattison, C.; Ma, H. Toxicity Interactions between Manganese (Mn) and Lead (Pb) or Cadmium (Cd) in a Model Organism the Nematode C. Elegans. Environ. Sci. Pollut. Res. 2018, 25, 15378–15389. [Google Scholar] [CrossRef] [PubMed]
- Escher, B.I.; Braun, G.; Zarfl, C. Exploring the concepts of concentration addition and independent action using a linear low-effect mixture model. Environ. Toxicol. Chem. 2020. 39, 2552–2559. [CrossRef]
- Escher, B.I.; Neale, P.A.; Leusch, F.D.L. Effect-Based Trigger Values for in Vitro Bioassays: Reading across from Existing Water Quality Guideline Values. Water Res. 2015, 81, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Brand, W.; de Jongh, C.M.; van der Linden, S.C.; Mennes, W.; Puijker, L.M.; van Leeuwen, C.J.; van Wezel, A.P.; Schriks, M.; Heringa, M.B. Trigger Values for Investigation of Hormonal Activity in Drinking Water and Its Sources Using CALUX Bioassays. Environ. Int. 2013, 55, 109–118. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escher, B.I.; Lamoree, M.; Antignac, J.-P.; Scholze, M.; Herzler, M.; Hamers, T.; Jensen, T.K.; Audebert, M.; Busquet, F.; Maier, D.; et al. Mixture Risk Assessment of Complex Real-Life Mixtures—The PANORAMIX Project. Int. J. Environ. Res. Public Health 2022, 19, 12990. https://doi.org/10.3390/ijerph192012990
Escher BI, Lamoree M, Antignac J-P, Scholze M, Herzler M, Hamers T, Jensen TK, Audebert M, Busquet F, Maier D, et al. Mixture Risk Assessment of Complex Real-Life Mixtures—The PANORAMIX Project. International Journal of Environmental Research and Public Health. 2022; 19(20):12990. https://doi.org/10.3390/ijerph192012990
Chicago/Turabian StyleEscher, Beate I., Marja Lamoree, Jean-Philippe Antignac, Martin Scholze, Matthias Herzler, Timo Hamers, Tina Kold Jensen, Marc Audebert, Francois Busquet, Dieter Maier, and et al. 2022. "Mixture Risk Assessment of Complex Real-Life Mixtures—The PANORAMIX Project" International Journal of Environmental Research and Public Health 19, no. 20: 12990. https://doi.org/10.3390/ijerph192012990
APA StyleEscher, B. I., Lamoree, M., Antignac, J.-P., Scholze, M., Herzler, M., Hamers, T., Jensen, T. K., Audebert, M., Busquet, F., Maier, D., Oelgeschläger, M., Valente, M. J., Boye, H., Schmeisser, S., Dervilly, G., Piumatti, M., Motteau, S., König, M., Renko, K., ... Vinggaard, A. M. (2022). Mixture Risk Assessment of Complex Real-Life Mixtures—The PANORAMIX Project. International Journal of Environmental Research and Public Health, 19(20), 12990. https://doi.org/10.3390/ijerph192012990






