The Roles of Different Fractions in Freshwater Biofilms in the Photodegradation of Methyl Orange and Bisphenol A in Aqueous Solutions
Abstract
1. Introduction
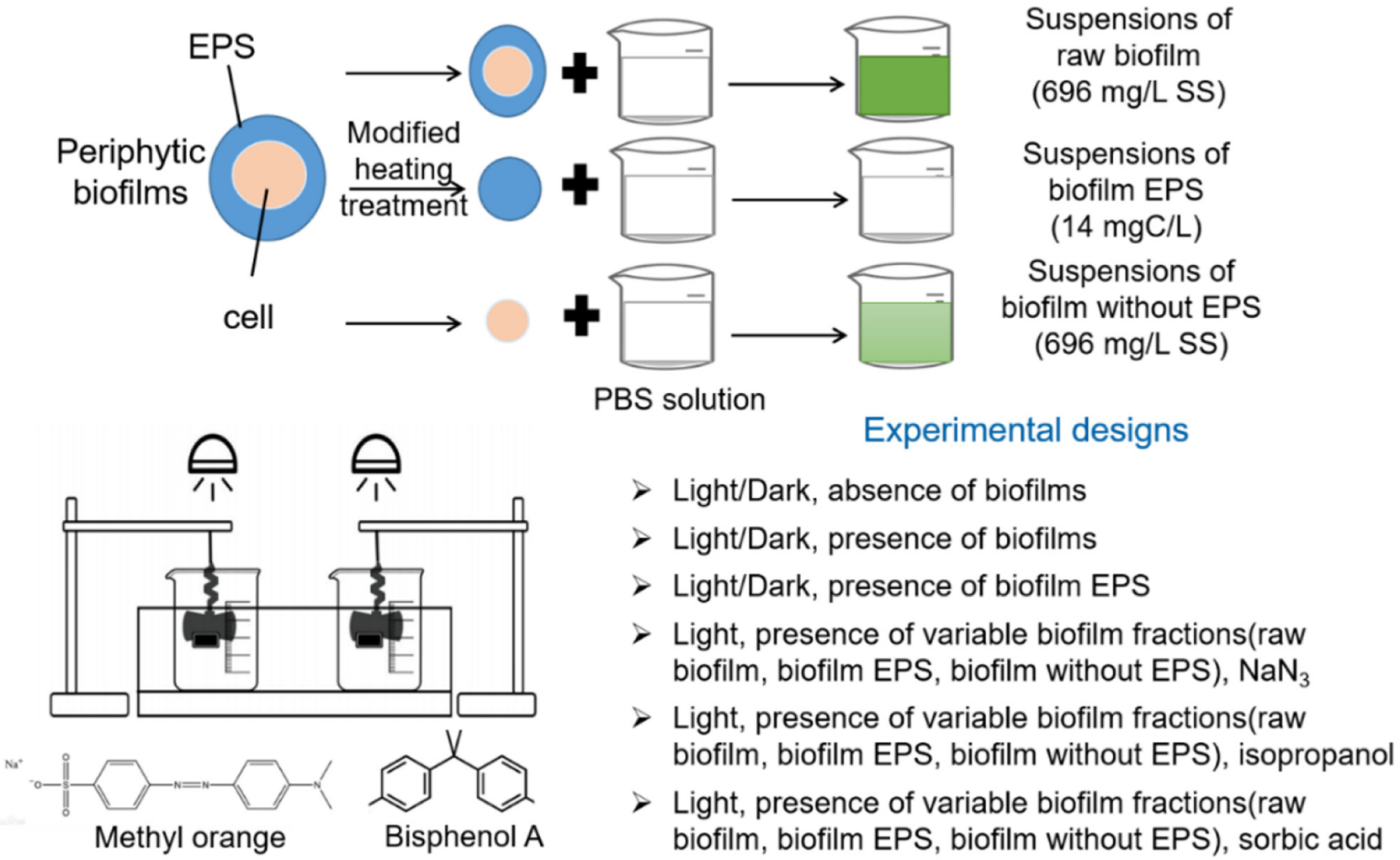
2. Materials and Methods
2.1. Sampling of Freshwater Biofilms
2.2. Chemicals, EPS Extraction and Characterization
2.3. Characterization of EPS Fractions
2.4. Photochemical Reaction Experiments
2.5. Reactive Species Detection
2.6. Analytical Methods
3. Results and Discussion
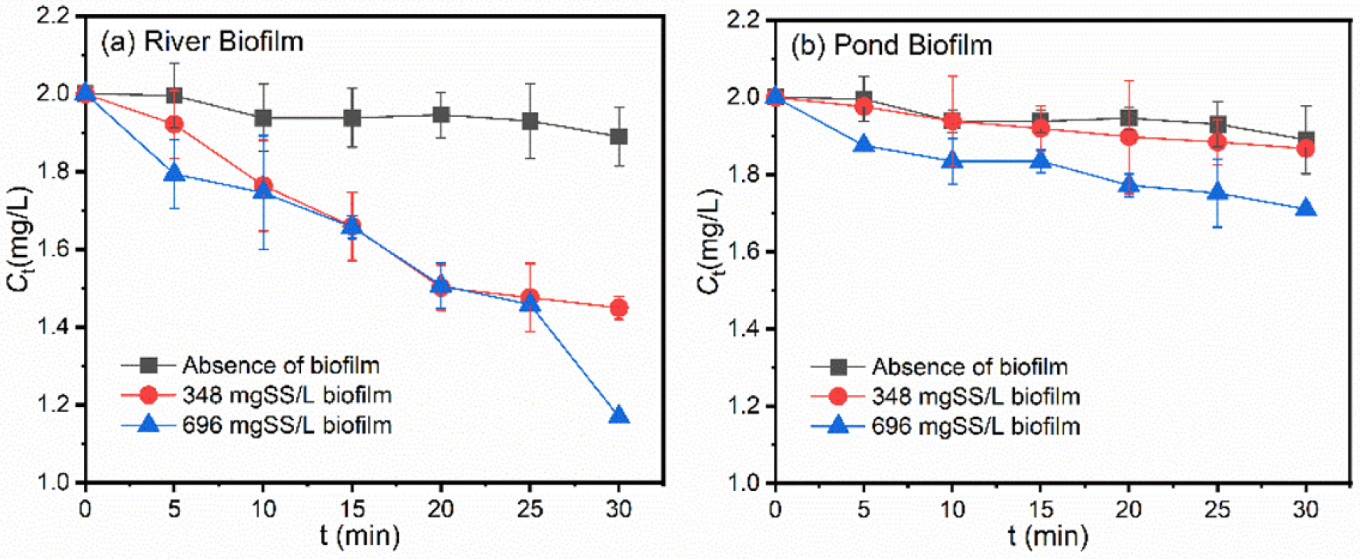
3.1. Photodegradation of Pollutants in the Presence of Freshwater Biofilms
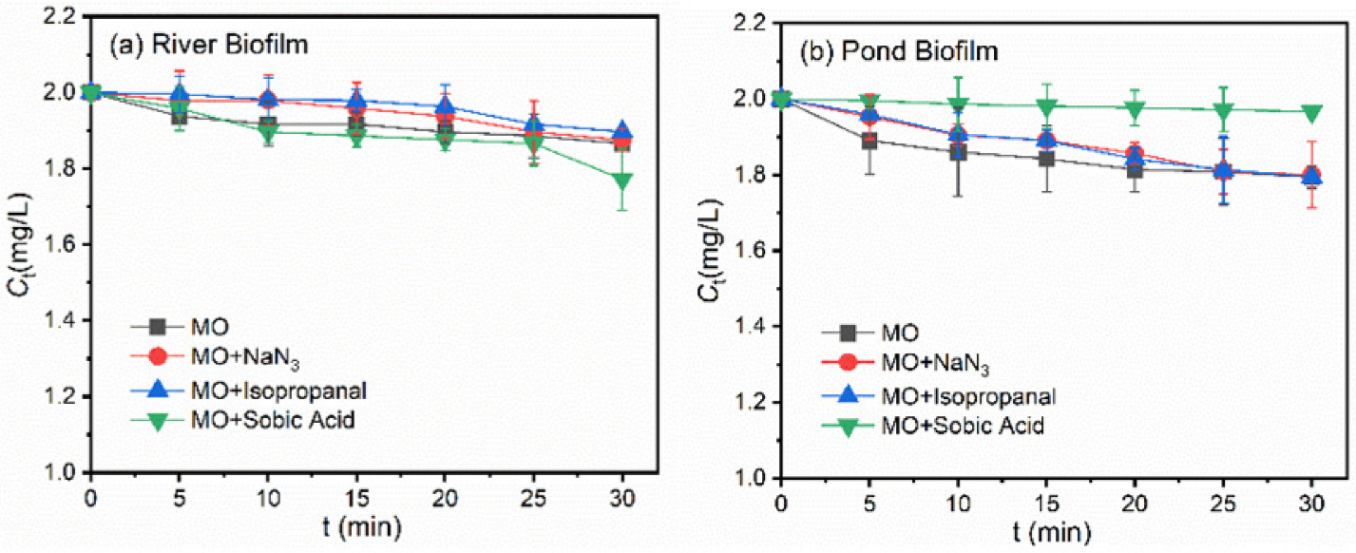
3.2. Roles of ROS during Photodegradation of Pollutants in the Presence of Biofilms
3.3. Characterization of EPS Derived from Freshwater Biofilms
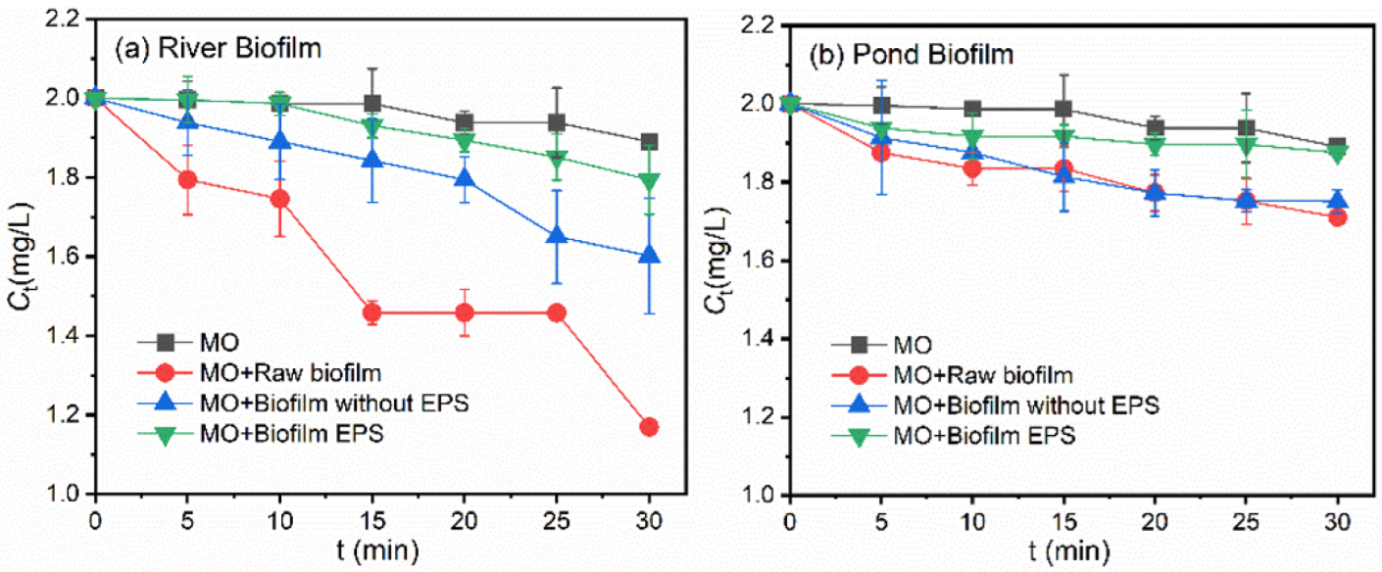
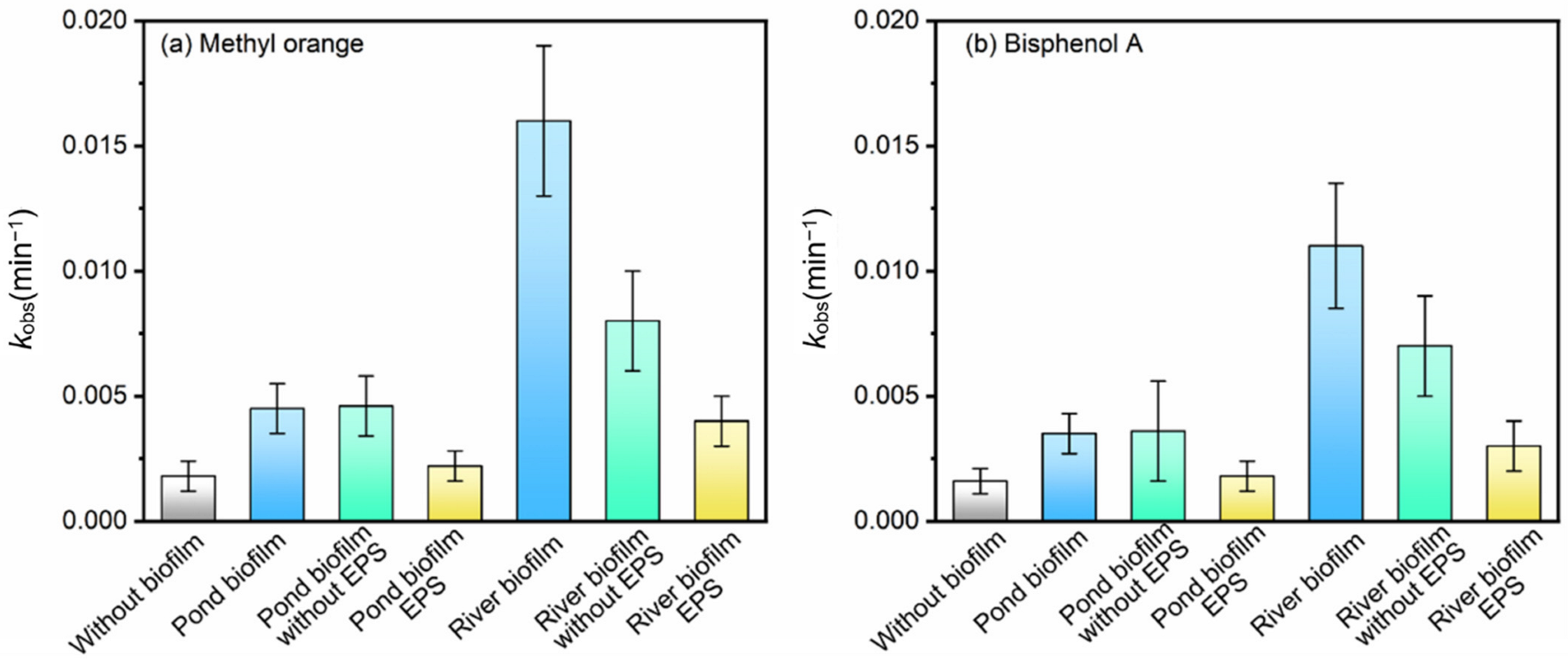
3.4. Roles of Different Fractions in Biofilms in the Photodegradation of Pollutants
3.5. Photosensitizing Mechanism of Different Biofilm Fractions in Pollutant Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Im, J.; Loffler, F.E. Fate of Bisphenol A in Terrestrial and Aquatic Environments. Environ. Sci. Technol. 2016, 50, 8403–8416. [Google Scholar] [CrossRef] [PubMed]
- Pallotti, F.; Pelloni, M.; Gianfrilli, D.; Lenzi, A.; Lombardo, F.; Paoli, D. Mechanisms of Testicular Disruption from Exposure to Bisphenol A and Phtalates. J. Clin. Med. 2020, 9, 471. [Google Scholar] [CrossRef] [PubMed]
- Naz, M.; Rafiq, A.; Ikram, M.; Haider, A.; Ahmad, S.O.A.; Haider, J.; Naz, S. Elimination of dyes by catalytic reduction in the absence of light: A review. J. Mater. Sci. 2021, 56, 15572–15608. [Google Scholar] [CrossRef]
- Pavithra, K.G.; Kumar, P.S.; Jaikumar, V.; Rajan, P.S. Removal of colorants from wastewater: A review on sources and treatment strategies. J. Ind. Eng. Chem. 2019, 75, 1–19. [Google Scholar] [CrossRef]
- Battin, T.J.; Besemer, K.; Bengtsson, M.M.; Romani, A.M.; Packmann, A.I. The ecology and biogeochemistry of stream biofilms. Nat. Rev. Microbiol. 2016, 14, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Bonnineau, C.; Artigas, J.; Chaumet, B.; Dabrin, A.; Fabure, J.; Ferrari, B.J.D.; Lebrun, J.D.; Margoum, C.; Mazzella, N.; Miege, C.; et al. Role of Biofilms in Contaminant Bioaccumulation and Trophic Transfer in Aquatic Ecosystems: Current State of Knowledge and Future Challenges. Rev. Environ. Contam. Toxicol. 2021, 253, 115–153. [Google Scholar]
- Wu, Y.; Xia, L.; Yu, Z.; Shabbir, S.; Kerr, P.G. In situ bioremediation of surface waters by periphytons. Bioresour. Technol. 2014, 151, 367–372. [Google Scholar] [CrossRef]
- Zhu, N.; Wang, S.; Tang, C.; Duan, P.; Yao, L.; Tang, J.; Wong, P.K.; An, T.; Dionysiou, D.D.; Wu, Y. Protection Mechanisms of Periphytic Biofilm to Photocatalytic Nanoparticle Exposure. Environ. Sci. Technol. 2019, 53, 1585–1594. [Google Scholar] [CrossRef]
- Wang, L.T.; Hua, X.Y.; Zhang, L.W.; Song, N.; Dong, D.M.; Guo, Z.Y. Influence of organic carbon fractions of freshwater biofilms on the sorption for phenanthrene and ofloxacin: The important role of aliphatic carbons. Sci. Total Environ. 2019, 685, 818–826. [Google Scholar] [CrossRef]
- Dong, D.M.; Zhang, Y.; Hua, X.Y.; Jiang, X.; Liang, D.P.; Guo, Z.Y. Effect of Dissolved Organic Matter on the Generation of H2O2 in Natural Biofilm Systems Under Illumination. Chem. J. Chin. Univ. Chin. 2019, 40, 800–808. [Google Scholar] [CrossRef]
- Joshi, R.P.; Thagard, S.M. Streamer-Like Electrical Discharges in Water: Part I. Fundamental Mechanisms. Plasma Chem. Plasma Process. 2013, 33, 1–15. [Google Scholar] [CrossRef]
- Joshi, R.P.; Thagard, S.M. Streamer-Like Electrical Discharges in Water: Part II. Environmental Applications. Plasma Chem. Plasma Process. 2013, 33, 17–49. [Google Scholar] [CrossRef]
- Baena-Nogueras, R.M.; Gonzalez-Mazo, E.; Lara-Martin, P.A. Degradation kinetics of pharmaceuticals and personal care products in surface waters: Photolysis vs biodegradation. Sci. Total Environ. 2017, 590, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.Y.; Li, M.; Su, Y.L.; Dong, D.M.; Guo, Z.Y.; Liang, D.P. The degradation of linear alkylbenzene sulfonate (LAS) in the presence of light and natural biofilms: The important role of photosynthesis. J. Hazard. Mater. 2012, 229, 450–454. [Google Scholar] [CrossRef]
- Katam, K.; Shimizu, T.; Soda, S.; Bhattacharyya, D. Performance evaluation of two trickling filters removing LAS and caffeine from wastewater: Light reactor (algal-bacterial consortium) vs dark reactor (bacterial consortium). Sci. Total Environ. 2020, 707, 135987. [Google Scholar] [CrossRef]
- Lyautey, E.; Jackson, C.R.; Cayrou, J.; Rols, J.L.; Garabetian, F. Bacterial community succession in natural river biofilm assemblages. Microb. Ecol. 2005, 50, 589–601. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Tian, Y.J.; Zou, J.R.; Feng, L.; Zhang, L.Q.; Liu, Y.Z. Chlorella vulgaris enhance the photodegradation of chlortetracycline in aqueous solution via extracellular organic matters (EOMs): Role of triplet state EOMs. Water Res. 2019, 149, 35–41. [Google Scholar] [CrossRef]
- Zepp, R.G.; Schlotzhauer, P.F. Influence of algae on photolysis rates of chemicals in water. Environ. Sci. Technol. 1983, 17, 462–468. [Google Scholar] [CrossRef]
- Tian, Y.J.; Wei, L.X.; Yin, Z.; Feng, L.; Zhang, L.R.; Liu, Y.Z.; Zhang, L.Q. Photosensitization mechanism of algogenic extracellular organic matters (EOMs) in the photo-transformation of chlortetracycline: Role of chemical constituents and structure. Water Res. 2019, 164, 114940. [Google Scholar] [CrossRef]
- Zhou, H.; Lian, L.; Yan, S.; Song, W. Insights into the photo-induced formation of reactive intermediates from effluent organic matter: The role of chemical constituents. Water Res. 2017, 112, 120–128. [Google Scholar] [CrossRef] [PubMed]
- McNeill, K.; Canonica, S. Triplet state dissolved organic matter in aquatic photochemistry: Reaction mechanisms, substrate scope, and photophysical properties. Environ. Sci. Process. Impacts 2016, 18, 1381–1399. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Li, Y.; Zhang, P.S.; Zhang, S.J.; Li, P.; Wang, P.F.; Wang, C. Sorption removal of phthalate esters and bisphenols to biofilms from urban river: From macroscopic to microcosmic investigation. Water Res. 2019, 150, 261–270. [Google Scholar] [CrossRef]
- Wang, L.F.; Chen, W.; Song, X.C.; Li, Y.; Zhang, W.L.; Zhang, H.J.; Niu, L.H. Cultivation substrata differentiate the properties of river biofilm EPS and their binding of heavy metals: A spectroscopic insight. Environ. Res. 2020, 182, 109052. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.W.; Dong, D.M.; Hua, X.Y.; Guo, Z.Y. Inhibitory effects of extracellular polymeric substances on ofloxacin sorption by natural biofilms. Sci. Total Environ. 2018, 625, 178–184. [Google Scholar] [CrossRef]
- Burdon, F.J.; Bai, Y.; Reyes, M.; Tamminen, M.; Staudacher, P.; Mangold, S.; Singer, H.; Rasanen, K.; Joss, A.; Tiegs, S.D.; et al. Stream microbial communities and ecosystem functioning show complex responses to multiple stressors in wastewater. Glob. Chang. Biol. 2020, 26, 6363–6382. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Wang, Y.T.; Li, Y.; Zhang, W.L.; Zhang, H.J.; Niu, L.H.; Habibul, N. Benthic Biofilm Bacterial Communities and Their Linkage with Water-Soluble Organic Matter in Effluent Receivers. Int. J. Environ. Res. Public Health 2022, 19, 1994. [Google Scholar] [CrossRef]
- Miao, L.Z.; Yu, Y.; Adyel, T.M.; Wang, C.Q.; Liu, Z.L.; Liu, S.Q.; Huang, L.Y.; You, G.X.; Meng, M.; Qu, H.; et al. Distinct microbial metabolic activities of biofilms colonizing microplastics in three freshwater ecosystems. J. Hazard. Mater. 2021, 403, 123577. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Frolund, B.; Palmgren, R.; Keiding, K.; Nielsen, P.H. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res. 1996, 30, 1749–1758. [Google Scholar] [CrossRef]
- Zhou, S.F.; Liao, Z.Y.; Zhang, B.P.; Hou, R.; Wang, Y.; Zhou, S.G.; Zhang, Y.F.; Ren, Z.J.; Yuan, Y. Photochemical Behavior of Microbial Extracellular Polymeric Substances in the Aquatic Environment. Environ. Sci. Technol. 2021, 55, 15090–15099. [Google Scholar] [CrossRef]
- Hu, B.; Wang, P.F.; Wang, C.; Bao, T.L. Photogeochemistry of particulate organic matter in aquatic systems: A review. Sci. Total Environ. 2022, 806, 150467. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Carlos, L.; Martire, D.O.; Gonzalez, M.C.; Gomis, J.; Bernabeu, A.; Amat, A.M.; Arques, A. Photochemical fate of a mixture of emerging pollutants in the presence of humic substances. Water Res. 2012, 46, 4732–4740. [Google Scholar] [CrossRef] [PubMed]
- Peuravuori, J.; Pihlaja, K. Molecular size distribution and spectroscopic properties of aquatic humic substances. Anal. Chim. Acta 1997, 337, 133–149. [Google Scholar] [CrossRef]
- Chen, W.; Qian, C.; Zhou, K.G.; Yu, H.Q. Molecular Spectroscopic Characterization of Membrane Fouling: A Critical Review. Chem 2018, 4, 1492–1509. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence excitation—Emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Golanoski, K.S.; Fang, S.; Del Vecchio, R.; Blough, N.V. Investigating the Mechanism of Phenol Photooxidation by Humic Substances. Environ. Sci. Technol. 2012, 46, 3912–3920. [Google Scholar] [CrossRef]
- Zhang, J.W.; Fu, D.F.; Wu, J.L. Photodegradation of Norfloxacin in aqueous solution containing algae. J. Environ. Sci. 2012, 24, 743–749. [Google Scholar] [CrossRef]
- Fabure, J.; Dufour, M.; Autret, A.; Uher, E.; Fechner, L.C. Impact of an urban multi-metal contamination gradient: Metal bioaccumulation and tolerance of river biofilms collected in different seasons. Aquat. Toxicol. 2015, 159, 276–289. [Google Scholar] [CrossRef]
- Wirth, S.M.; Lowry, G.V.; Tilton, R.D. Natural Organic Matter Alters Biofilm Tolerance to Silver Nanoparticles and Dissolved Silver. Environ. Sci. Technol. 2012, 46, 12687–12696. [Google Scholar] [CrossRef] [PubMed]
- Bowler, P.; Murphy, C.; Wolcott, R. Biofilm exacerbates antibiotic resistance: Is this a current oversight in antimicrobial stewardship? Antimicrob. Resist. Infect. Control. 2020, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Proia, L.; Lupini, G.; Osorio, V.; Perez, S.; Barcelo, D.; Schwartz, T.; Amalfitano, S.; Fazi, S.; Romani, A.M.; Sabater, S. Response of biofilm bacterial communities to antibiotic pollutants in a Mediterranean river. Chemosphere 2013, 92, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.W.; Mah, T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]



| Sample | Carbon-Containing Functional Group Contribution (Area, %) | ||
|---|---|---|---|
| C-(C/H) (i.e., 284.8 eV) | C-(O/N) (i.e., 286.43 eV) | O-C=O (i.e., 288.66 eV) | |
| River biofilm EPS | 78.49 | 12.27 | 9.24 |
| Pond biofilm EPS | 72.71 | 16.98 | 10.31 |
| Sample | Alkyl (%) 0–45 ppm | Methoxyl (%) 45–63 ppm | Carbohydrate (%) 63–93 ppm | Aryl (%) 93–148 ppm | O-Aryl (%) 148–165 ppm | Carboxyl (%) 165–190 ppm | Carbonyl (%) 190–220 ppm | Aromatic Carbon a | Aliphatic Carbon b | Polar Carbon c |
|---|---|---|---|---|---|---|---|---|---|---|
| River biofilm EPS | 34.6 | 12.5 | 38.1 | 3.8 | 4.1 | 6.6 | 0.3 | 7.9 | 85.2 | 61.6 |
| Pond biofilm EPS | 41.3 | 14 | 37.2 | 2.1 | 0 | 5.4 | 0 | 2.1 | 92.5 | 56.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, H.; Wang, L.; Zeng, G.; Wang, L.; Li, Y. The Roles of Different Fractions in Freshwater Biofilms in the Photodegradation of Methyl Orange and Bisphenol A in Aqueous Solutions. Int. J. Environ. Res. Public Health 2022, 19, 12995. https://doi.org/10.3390/ijerph192012995
Yin H, Wang L, Zeng G, Wang L, Li Y. The Roles of Different Fractions in Freshwater Biofilms in the Photodegradation of Methyl Orange and Bisphenol A in Aqueous Solutions. International Journal of Environmental Research and Public Health. 2022; 19(20):12995. https://doi.org/10.3390/ijerph192012995
Chicago/Turabian StyleYin, Haojie, Lingling Wang, Guangshu Zeng, Longfei Wang, and Yi Li. 2022. "The Roles of Different Fractions in Freshwater Biofilms in the Photodegradation of Methyl Orange and Bisphenol A in Aqueous Solutions" International Journal of Environmental Research and Public Health 19, no. 20: 12995. https://doi.org/10.3390/ijerph192012995
APA StyleYin, H., Wang, L., Zeng, G., Wang, L., & Li, Y. (2022). The Roles of Different Fractions in Freshwater Biofilms in the Photodegradation of Methyl Orange and Bisphenol A in Aqueous Solutions. International Journal of Environmental Research and Public Health, 19(20), 12995. https://doi.org/10.3390/ijerph192012995






