The Influence of Biostimulants Used in Sustainable Agriculture for Antifungal Protection on the Chemical Composition of Winter Wheat Grain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
- Soil application (kgha−1 year−1)—189 N, 70 P2O5, 105 K2O, 135 SO3;
- Foliar application (gha−1 year−1)—59 N, 260 P2O5, 338.5 K2O, 669.5 MgO, 1450.4 SO3, 23.65 Fe, 2.95 B, 19.55 Cu, 88.2 Mn, 9.25 Zn, 0.79 Mo.
2.2. Soil Conditions
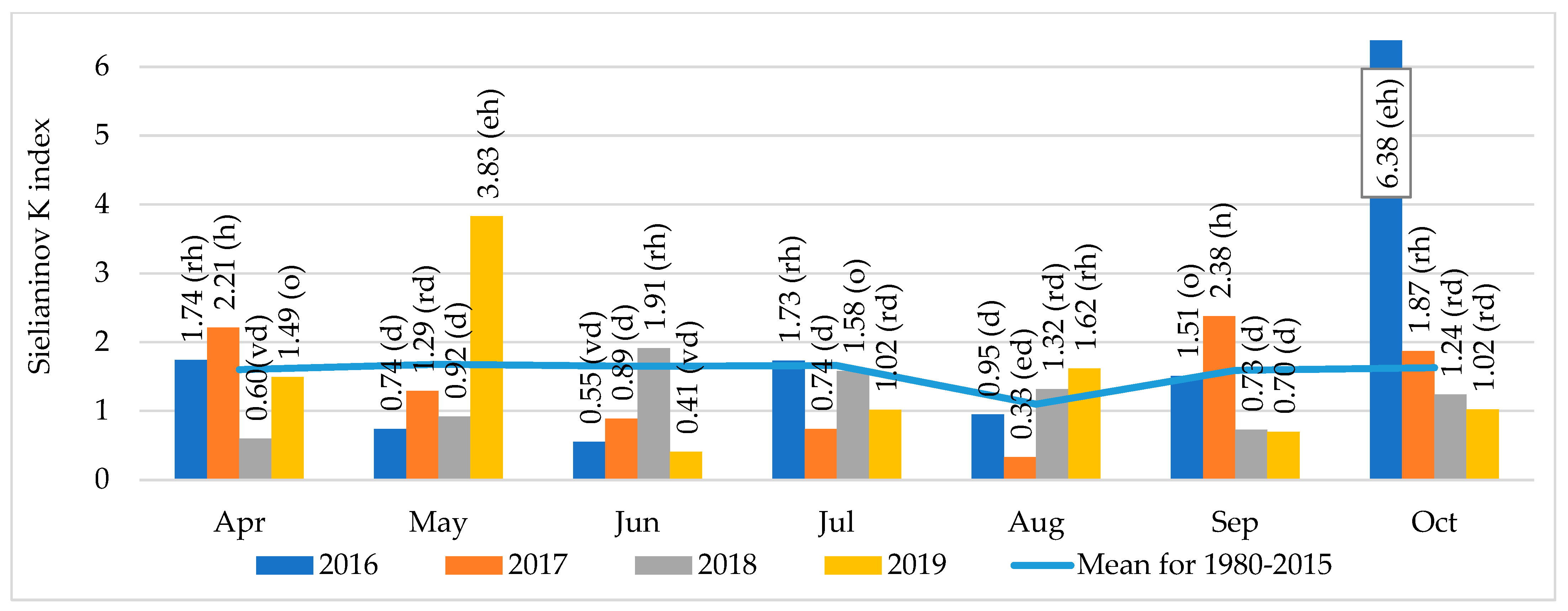
2.3. Weather Conditions
2.4. Analysis of the Chemical Composition of the Grain
2.5. Statistical Analysis
3. Results and Discussion
- 810–975 cm−1 (stretching vibration of C-C group);
- 920–960 cm−1 (vibration of C-C group of amylose);
- 1190 cm−1 (vibration of C-C group of sugars);
- 1260 cm−1 (vibration of III-row amide—protein);
- 1382–1338 cm−1 vibration of the secondary structure of proteins (α-helix);
- 1455 cm−1 (vibration of C-H group of proteins and sugars);
- 1550 cm−1 (vibration of protein groups included in amylopectins)
- 1637 cm−1 (vibration of protein groups included in amylopectins);
- 1740 cm−1 (vibration of C-O group of proteins and fats);
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 8 August 2022).
- Jarecki, W.; Czernicka, M. Yield and quality of winter wheat (Triticum aestivum L.) depending on multi-component foliar fertilization. J. Elem. 2022, 27, 559–567. [Google Scholar] [CrossRef]
- Zargar, M.; Polityko, P.; Pakina, E.; Bayat, M.; Vandyshev, V.; Kavhiza, N.; Kiselev, E. Productivity, quality and economics of four spring wheat (Triticum aestivum L.) cultivars as affected by three cultivation technologies. Agron. Res. 2018, 16, 2254–2264. [Google Scholar] [CrossRef]
- Brouns, F.; van Rooy, G.; Shewry, P.; Rustgi, S.; Jonkers, D. Adverse reactions to wheat or wheat components. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1437–1452. [Google Scholar] [CrossRef] [PubMed]
- Zörb, C.; Ludewig, U.; Hawkesford, M.J. Perspective on wheat yield and quality with reduced nitrogen supply. Trends Plant Sci. 2018, 23, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Horvat, D.; Dvojković, K.; Novoselović, D.; Tucak, M.; Andrić, L.; Magdić, D.; Drezner, G. Response of wheat yield and protein-related quality on late-season urea application. Agronomy 2022, 12, 886. [Google Scholar] [CrossRef]
- Shewry, P.R. Wheat. J. Exp. Bot. 2009, 60, 1537–1553. [Google Scholar] [CrossRef]
- Jańczak-Pieniążek, M.; Buczek, J.; Bobrecka-Jamro, D.; Szpunar-Krok, E.; Tobiasz-Salach, R.; Jarecki, W. Morphophysiology, productivity and quality of soybean (Glycine max (L.) Merr.) cv. Merlin in response to row spacing and seeding systems. Agronomy 2021, 11, 403. [Google Scholar] [CrossRef]
- Gasanova, I.; Yerashova, M.; Astakhova, Y.; Drumova, O. Influence of mineral fertilizers and other agrotechnical cultivation methods on yield and grain protein content of winter wheat. Am. J. Agric. For. 2021, 9, 89–94. [Google Scholar] [CrossRef]
- Kulyk, M.I.; Rozhkov, A.O.; Kalinichenko, O.V.; Taranenko, A.O.; Onopriienko, O.V. Effect of winter wheat variety, hydrothermal coefficient (HTC) and thousand kernel weight (TKW) on protein content, grain and protein yield. Agron. Res. 2020, 18, 3. [Google Scholar] [CrossRef]
- Ljubičić, N.; Popović, V.; Ćirić, V.; Kostić, M.; Ivošević, B.; Popović, D.; Pandžić, M.; El Musafah, S.; Janković, S. Multivariate interaction analysis of winter wheat grown in environment of limited soil conditions. Plants 2021, 10, 604. [Google Scholar] [CrossRef]
- Golba, J.; Studnicki, M.; Gozdowski, D.; Mądry, W.; Rozbicki, J. Influence of genotype, crop management, and environment on winter wheat grain yield determination based on components of yield. Crop Sci. 2018, 58, 660–669. [Google Scholar] [CrossRef]
- Ashraf, M. Stress-Induced Changes in Wheat Grain Composition and Quality. Crit. Rev. Food Sci. Nutr. 2014, 54, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Dolferus, R.; Ji, X.; Richards, R. Abiotic stress and control of grain number in cereals. Plant Sci. 2011, 181, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, M.; Hammond-Kosack, K.E.; Solomon, P.S. A review of wheat diseases—A field perspective. Mol. Plant Pathol. 2018, 19, 1523–1536. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Toward a sustainable agriculture through plant biostimulants: From experimental data to practical applications. Agronomy 2020, 10, 1461. [Google Scholar] [CrossRef]
- Mukherjee, D. Microbial Interventions in Soil and Plant Health for Improving Crop Efficiency. In Microbial Interventions in Agriculture and Environment; Singh, D., Prabha, R., Eds.; Springer: Singapore, 2009; pp. 17–47. [Google Scholar] [CrossRef]
- Corsi, S.; Ruggeri, G.; Zamboni, A.; Bhakti, P.; Espen, L.; Ferrante, A.; Noseda, M.; Varanini, Z.; Scarafoni, A. A bibliometric analysis of the scientific literature on biostimulants. Agronomy 2022, 12, 1257. [Google Scholar] [CrossRef]
- Szczepanek, M.; Wszelaczyńska, E.; Pobereżny, J. Effect of seaweed biostimulant application in spring wheat. AgroLife Sci. J. 2018, 7, 131–136. Available online: https://www.cabdirect.org/cabdirect/abstract/20183195796 (accessed on 15 August 2022).
- Lamparski, R.; Szczepanek, M. Effect of bioregulator Kelpak application in spring wheat on the occurrence of phytophagous insects. Prog. Plant Prot. 2013, 53, 1. (In Polish) [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hort. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Du Jardin, P.; Xu, L.; Geelen, D. Agricultural functions and action mechanisms of plant biostimulants (PBs): An Introduction. In The Chemical Biology of Plant Biostimulants; Geelen, D., Xu, L., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2020; pp. 1–29. [Google Scholar] [CrossRef]
- Xu, L.; Galeen, D. Developing biostimulants from agro-food and industrial by-products. Front. Plant Sci. 2018, 9, 1567. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Caradonia, F.; Battaglia, V.; Righi, L.; Pascali, G.; La Torre, A. Plant biostimulant regulatory framework: Prospects in europe and current situation at international level. J. Plant Growth Regul. 2019, 38, 438–448. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Ambrosini, S.; Sega, D.; Santi, C.; Zamboni, A.; Varanini, Z.; Pandolfini, T. Evaluation of the potential use of a collagen-based protein hydrolysate as a plant multi-stress protectant. Front. Plant Sci. 2021, 12, 63. [Google Scholar] [CrossRef]
- Sharma, H.S.S.; Selby, C.; Carmichael, E.; McRoberts, C.; Rao, J.R.; Ambrosino, P.; Chiurazzi, M.; Pucci, M.; Martin, T. Physicochemical analyses of plant biostimulant formulations and characterisation of commercial products by instrumental techniques. Chem. Biol. Technol. 2016, 3, 13. [Google Scholar] [CrossRef]
- Marketsandmarkets. Global Biostimulants Market (2021–2026) by Active Ingredient, Application Method, Crop Type, Form, Geography and the Impact of COVID-19 with Ansoff Analysis. 2021, p. 138. Available online: https://www.marketresearch.com/Infogence-Marketing-Advisory-Services-v4010/Global-Biostimulants-Active-Ingredient-Application-14533191/ (accessed on 16 August 2022).
- Piot, O.; Autran, J.-C.; Manfait, M. Spatial distribution of protein and phenolic constituents in wheat grain as probed by confocal Raman microspectroscopy. J. Cereal Sci. 2000, 32, 57–71. [Google Scholar] [CrossRef]
- Stawoska, I.; Wesełucha-Birczyńska, A.; Skoczowski, A.; Dziurka, M.; Waga, J. FT-Raman spectroscopy as a tool to study the secondary structures of wheat gliadin proteins. Molecules 2021, 26, 5388. [Google Scholar] [CrossRef]
- Yaseen, T.; Sun, D.-W.; Cheng, J.-H. Raman imaging for food quality and safety evaluation: Fundamentals and applications. Trends Food Sci. Technol. 2017, 62, 177–189. [Google Scholar] [CrossRef]
- Qin, J.; Chao, K.; Kim, M.S. Raman chemical imaging system for food safety and quality inspection. Trans. ASABE 2010, 53, 1873–1882. [Google Scholar] [CrossRef]
- Pačuta, V.; Rašovský, M.; Michalska-Klimczak, B.; Wyszyński, Z. Grain yield and quality traits of durum wheat (Triticum durum Desf.) treated with seaweed- and humic acid-based biostimulants. Agronomy 2021, 11, 1270. [Google Scholar] [CrossRef]
- Uzunova, A.N.; Popova, L. Effect of salicylic acod on leaf anatomy and chloroplast ultrastructure of barley plants. Photosynthetica 2000, 38, 243–250. [Google Scholar] [CrossRef]
- Durango, D.; Pulgarin, N.; Echeverri, F.; Escobar, G.; Quiñones, W. Effect of salicylic acid and structurally related compounds in the accumulation of phytoalexins in cotyledons of common bean (Phaseolus vulgaris L.) cultivars. Molecules 2013, 18, 10609–10628. [Google Scholar] [CrossRef] [PubMed]
- World Reference Base for Soil Resources 2014. Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. FAO. Available online: http://www.fao.org/fileadmin/templates/nr/images/resources/pdf_documents/wrb2007_red.pdf (accessed on 29 December 2021).
- Skowera, B.; Jędrszczyk, E.; Kopcińska, J.; Ambroszczyk, A.M.; Kołton, A. The effects of hydrothermal conditions during vegetation period on fruit quality of processing tomatoes. Pol. J. Environ. Stud. 2014, 23, 195–202. Available online: http://www.pjoes.com/The-Effects-of-Hydrothermal-Conditions-r-nduring-Vegetation-Period-on-Fruit-Quality,89183,0,2.html (accessed on 16 August 2022).
- Huang, M.; Wang, Q.; Zhu, Q.; Qin, J.; Huang, G. Review of seed quality and safety tests using optical sensing technologies. Seed Sci. Technol. 2015, 43, 337–366. [Google Scholar] [CrossRef]
- Yang, G.; Wang, Q.; Liu, C.; Wang, X.; Fan, S.; Huang, W. Rapid and visual detection of the main chemical compositions in maize seeds based on Raman hyperspectral imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 200, 186–194. [Google Scholar] [CrossRef]
- Czaja, T.; Sobota, A.; Szostak, R. Quantification of ash and moisture in wheat flour by Raman Spectroscopy. Foods 2020, 9, 280. [Google Scholar] [CrossRef]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and infrared spectroscopy of carbohydrates: A review. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 317–335. [Google Scholar] [CrossRef]
- Ciołek, A.; Makarska, E.; Wesołowski, M. Content of selected nutrients in wheat, barley and oat grain from organic and conventional farming. J. Elem. 2012, 17, 181–189. [Google Scholar] [CrossRef]
- Asseng, S.; Martre, P.; Maiorano, A.; Rötter, R.P.; O’Leary, G.J.; Fitzgerald, G.J.; Girousse, C.; Motzo, R.; Giunta, F.; Babar, M.A.; et al. Climate change impact and adaptation for wheat protein. Glob. Change Biol. 2019, 25, 155–173. [Google Scholar] [CrossRef]
- Nuttall, J.G.; O’Leary, G.J.; Panozzo, J.F.; Walker, C.K.; Barlow, K.M.; Fitzgerald, G.J. Models of grain quality in wheat—A review. Field Crops Res. 2017, 202, 136–145. [Google Scholar] [CrossRef]
- Hotea, I.; Dragomirescu, M.; Colibar, O.; Tirziu, E.; Herman, V.; Berbecea, A.; Radulov, I. The influence of climate conditions and meteorological factors on the nutritional value of wheat (Triticum aestivum L.) used for human and animals nutrition, in Romania. IOP Conf. Ser. Earth Environ. Sci. 2021, 906, 012019. [Google Scholar] [CrossRef]
- Cociu, A.I.; Alionte, E. Effect of different tillage systems on grain yield and its quality of winter wheat, maize and soybean under different weather conditions. Rom. Agric. Res. 2017, 34, 1222–4227. Available online: http://www.incda-fundulea.ro/rar.htm (accessed on 15 August 2022).
- Hwang, J.; Choi, N.; Park, A.; Park, J.-Q.; Chung, J.H.; Baek, S.; Cho, S.G.; Baek, S.-J.; Choo, J. Fast and sensitive recognition of various explosive compounds using Raman spectroscopy and principal component analysis. J. Mol. Struct. 2013, 1039, 130–136. [Google Scholar] [CrossRef]
- Shanmukh, S.; Jones, L.; Zhao, Y.P.; Driskell, J.D.; Tripp, R.A.; Dluhy, R.A. Identification and classification of respiratory syncytial virus (RSV) strains by surface-enhanced Raman spectroscopy and multivariate statistical techniques. Anal. Bioanal. Chem. 2008, 390, 1551–1555. [Google Scholar] [CrossRef]



| Plant Protection | Treatment Time (BBCH Scale) | Trade Name | Active Substance (gdm−3) | Terms and Doses | |
|---|---|---|---|---|---|
| Preparation (dm3ha−1) | Active Substance (gha−1) | ||||
| (1) Control (without plant protection) | - | - | - | - | - |
| (2) Intensive | 31–33 | Duett Star 334 SE (BASF) | Fenpropimorph (250) | 1.0 | 250 |
| Epoxiconazole (84) | 84.0 | ||||
| 45–47 | Acanto 250 SC (DuPont) | Azoxystrobin (250) | 0.6 | 150 | |
| Bumper 250 SC (ADAMA) | Propiconazole (250) | 0.7 | 175 | ||
| 65–69 | Mystic 250 EC (Nufarm) | Tebuconazole (250) | 0.9 | 225 | |
| (3) Extensive | 31–33 | Duett Star 334 SE (BASF) | Fenpropimorph (250) | 1.0 | 250 |
| Epoxiconazole (84) | 84.0 | ||||
| 45–47 | Acanto 250 SC (DuPont) | Azoxystrobin (250) | 0.6 | 150 | |
| Bumper 250 SC (ADAMA) | Propiconazole (250) | 0.7 | 175 | ||
| (4) PlanTonic BIO | 31–33 | PlanTonic BIO | No data | 4.0 | No data |
| 45–47 | 4.0 | ||||
| 65–69 | 4.0 | ||||
| (5) PlanTonic BIO + Natural Crop | 31–33 | PlanTonic BIO | 4.0 | ||
| Natural Crop SL | 1.5 | ||||
| 45–47 | PlanTonic BIO | 4.0 | |||
| Natural Crop SL | 1.5 | ||||
| 65–69 | PlanTonic BIO | 4.0 | |||
| Natural Crop SL | 1.5 | ||||
| (6) PlanTonic BIO + BioFol Plex | 31–33 | PlanTonic BIO | 4.0 | ||
| BioFol Plex | 2.0 | ||||
| 45–47 | PlanTonic BIO | 4.0 | |||
| BioFol Plex | 2.0 | ||||
| 65–69 | PlanTonic BIO | 4.0 | |||
| Biostimulators | Biostimulator Charakteristics | |
|---|---|---|
| BioFol Plex (Biostyma, Września, Poland) | Complexed with humic acids | 2.0% Ntot; 0.3% Mg; 5.0% S; 0.15% B; 0.05% Cu; 0.20% Fe; 0.10% Mn; 0.50% Zn; 1.25% C; 5.0% extract from algae; traces of plant hormones, betaine (C5H11NO2), plant-derived amino acids, thiamine |
| Natural Crop SL (Natural Crop, Italy) | Concentrate of peptides and L-amino acids obtained by enzymatic hydrolysis of collagen | 9.0% NO2−-N; 24.5% Corg; total > 50%, free > 2.0% L-amino acids (GLY, PRO, HYP, GLU, ALA, ARG, ASP, SER, HIS, LYS, LEU, VAL, PHE, ILE, THR, TYR, CYS, MET) |
| PlanTonic Bio (OGET Innovations GmbH, Allerheiligen bei Wildon, Austria) | Nettle (Urtica dioica L.) extract, willow (Salix sp.) extract, sunflower (Helianthus annus L.) oil | Full chemical composition of the preparation reserved by the manufacturer |
| Type of Fertiliser | Trade Name | Chemical Composition | Fertiliser Dose (kg ha−1) | Dose (ha−1 Year−1) | Application Term |
|---|---|---|---|---|---|
| Soil-applied | Polifoska 6 | 6% N-NH4+, 20% P2O5, 30% K2O, 7% SO3 | 350 | 21 kg N, 70 kg P2O5, 105 kg K2O, 24.5 kg SO3 | before sowing |
| Saletrosan® 26 | 26% N (including 19% N-NH4+, 7% N-NO3−), 32.5% SO3 | 300 | 88.4 kg N, 110.5 kg SO3 | BBCH 25–27 | |
| Zaksan® | 32% N (including 16% N-NH4+, 16% N-NO3−), | 250 | 80 kg N | BBCH 33–35 | |
| Foliar fertilisers | GranuFol Fosfor | 10.0% N, 41% P2O5, 12% K2O, 2.3% MgO, 2.5% SO3, 0.03% B, 0.03% Cu, 0.16% Fe, 0.07% Mn, 0.002% Mo, 0.07% Zn | 1.0 | 10 g N, 410 g P2O5, 120 g K2O, 23 g MgO, 25 g SO3, 0.3 g B, 0.3 g Cu, 1.6 g Fe, 0.7 g Mn, 0.02 g Mo, 0.7 g Zn | BBCH 31–32 |
| GranuFol CuMan | 43.3% SO3, 5% Cu, 25% Mn | 0.3 | 129.9 g SO3, 15 g Cu, 75 g Mn | ||
| Wuxal mikro | 5% N, 10% K2O, 3% MgO, 5.2% S, 0.3% B, 0.5% Cu, 1% Fe, 1.5% Mn, 0.01% Mo, 1% Zn | 0.5 | 39 g N, 78.5 g K2O, 23.5 g MgO, 40.5 g S, 2.35 g B, 3.95 g Cu, 7.85 g Fe, 11.8 g Mn, 0.75 g Mo, 7.85 g Zn | ||
| Granufol Mag | 20% MgO, 41% SO3, 0.42% Fe | 1.5 | 600 g MgO, 1230 g SO3, 12.6 g Fe | BBCH 31–32 BBCH 54–55 | |
| GranuFol Potas | 10.0% N, 12% P2O5, 41% K2O, 2.3% MgO, 2.5% SO3, 0.03% B, 0.03% Cu, 0.16% Fe, 0.07% Mn, 0.002% Mo, 0.07% Zn | 1.0 | 10 g N, 120 g P2O5, 410 g K2O, 23 g MgO, 25 g SO3, 0.3 g B, 0.3 g Cu, 1.6 g Fe, 0.7 g Mn, 0.02 g Mo, 0.7 g Zn | BBCH 54–55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szpunar-Krok, E.; Depciuch, J.; Drygaś, B.; Jańczak-Pieniążek, M.; Mazurek, K.; Pawlak, R. The Influence of Biostimulants Used in Sustainable Agriculture for Antifungal Protection on the Chemical Composition of Winter Wheat Grain. Int. J. Environ. Res. Public Health 2022, 19, 12998. https://doi.org/10.3390/ijerph192012998
Szpunar-Krok E, Depciuch J, Drygaś B, Jańczak-Pieniążek M, Mazurek K, Pawlak R. The Influence of Biostimulants Used in Sustainable Agriculture for Antifungal Protection on the Chemical Composition of Winter Wheat Grain. International Journal of Environmental Research and Public Health. 2022; 19(20):12998. https://doi.org/10.3390/ijerph192012998
Chicago/Turabian StyleSzpunar-Krok, Ewa, Joanna Depciuch, Barbara Drygaś, Marta Jańczak-Pieniążek, Katarzyna Mazurek, and Renata Pawlak. 2022. "The Influence of Biostimulants Used in Sustainable Agriculture for Antifungal Protection on the Chemical Composition of Winter Wheat Grain" International Journal of Environmental Research and Public Health 19, no. 20: 12998. https://doi.org/10.3390/ijerph192012998





