Levels, Distribution and Health Risk Assessment of Organochlorine Pesticides in Agricultural Soils from the Pearl River Delta of China
Abstract
:1. Instruction
2. Materials and Method
2.1. The Situation of the Pearl River Delta Region of China
2.2. Sample Collection and Preparation
2.3. Sample Analysis
2.4. Human Health Risk Assessment
2.5. Quality Assurance and Quality Control
2.6. Data Processing and Statistical Analysis
3. Results and Discussion
3.1. Residual Status of OCPs in the Pearl River Delta
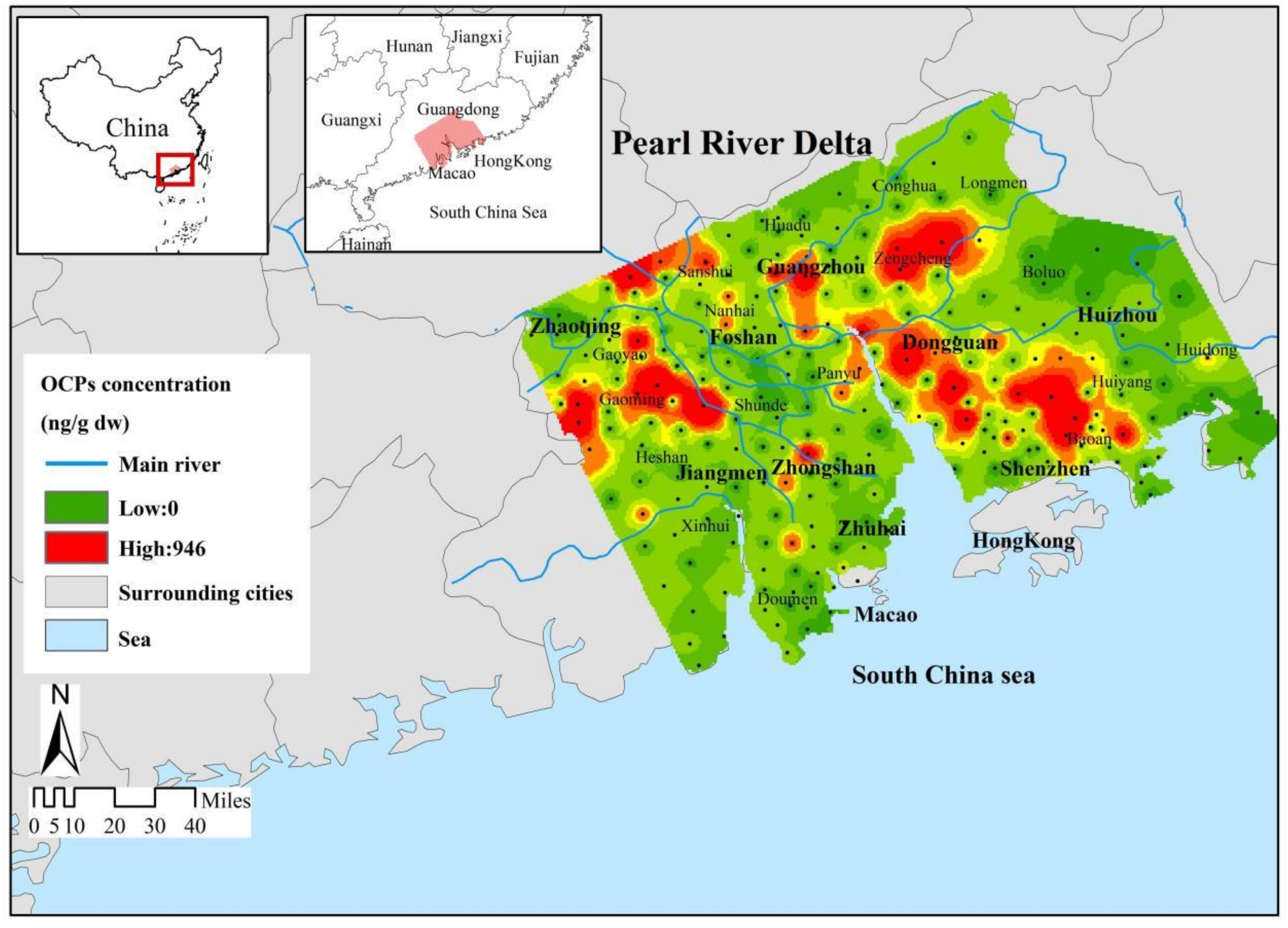
3.2. Spatial Distribution Characteristics of OCPs in the Pearl River Delta
3.3. Vertical Distribution Characteristics of OCP in Soil Profiles
3.4. Source Identification of OCPs in the Pearl River Delta
3.4.1. HCHs
3.4.2. DDTs
3.4.3. Other OCPs
3.5. Correlations among Soil Properties and Pollutants
3.6. Human Health Risk Assessment
3.6.1. Non-Carcinogenic Health Risk Evaluation
3.6.2. Carcinogenic Health Risk Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martyniuk, C.J.; Mehinto, A.C.; Denslow, N.D. Organochlorine pesticides: Agrochemicals with potent endocrine-disrupting properties in fish. Mol. Cell Endocrinol. 2020, 507, 110764. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.F.; Olmos, B.; Granada, A.; López-Espinosa, M.J.; Molina-Molina, J.M.; Fernandez, J.M.; Cruz, M.; Olea-Serrano, F.; Olea, N. Human exposure to endocrine-disrupting chemicals and prenatal risk factors for cryptorchidism and hypospadias: A nested case-control study. Environ. Health Perspect. 2007, 115, 8. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.J.; Xu, J.; Zhang, R.Y.; Yu, J. The association between environmental endocrine disruptors and cardiovascular diseases: A systematic review and meta-analysis. Environ. Res. 2020, 187, 109464. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.Z. Determination of chloramphenicol, enrofloxacin and 29 pesticides residues in bovine milk by liquid chromatography-tandem mass spectrometry. Chemosphere 2011, 83, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Keswani, C.; Dilnashin, H.; Birla, H.; Roy, P.; Tyagi, R.K.; Singh, D.; Rajput, V.D.; Minkina, T.; Singh, S.P. Global footprints of organochlorine pesticides: A pan-global survey. Environ. Geochem. Health 2022, 44, 149–177. [Google Scholar] [CrossRef] [PubMed]
- Aichner, B.; Bussian, B.; Lehnik-Habrink, P.; Hein, S. Levels and spatial distribution of persistent organic pollutants in the environment: A case study of German Forest soils. Environ. Sci. Technol. 2013, 47, 12703–12714. [Google Scholar] [CrossRef] [PubMed]
- Barber, J.L.; Sweetman, A.J.; Van Wijk, D.; Jones, K.C. Hexachlorobenzene in the global environment: Emissions, levels, distribution, trends and processes. Sci. Total Environ. 2005, 349, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Fommei, E.; Turci, R.; Ripoli, A.; Balzan, S.; Bianchi, F.; Morelli, L.; Coi, A. Evidence for persistent organochlorine pollutants in the human adrenal cortex. J. Appl. Toxico. 2017, 37, 1091–1097. [Google Scholar] [CrossRef]
- Sun, J.T.; Pan, L.L.; Tsang, D.C.W.; Zhan, Y.; Zhu, L.Z.; Li, X.D. Organic contamination and remediation in the agricultural soils of China: A critical review. Sci. Total Environ. 2018, 615, 724–740. [Google Scholar] [CrossRef]
- Pokhrel, B.; Gong, P.; Wang, X.P.; Chen, M.K.; Wang, C.F.; Gao, S.P. Distribution, sources, and air-soil exchange of OCPs, PCBs and PAHs in urban soils of Nepal. Chemosphere 2018, 200, 532–541. [Google Scholar] [CrossRef]
- Zhang, J.J.; Wang, Y.; Hua, D.L. Occurrence, distribution and possible sources of organochlorine pesticides in peri-urban vegetable soils of Changchun, Northeast China. Hum. Eco Risk Assess. Int. J. 2017, 23, 2033–2045. [Google Scholar] [CrossRef]
- Kafaei, R.; Arfaeinia, H.; Savari, A.; Mahmoodi, M.; Rezaei, M.; Rayani, M.; Sorial, G.A.; Fattahi, N.; Ramavandi, B. Organochlorine pesticides contamination in agricultural soils of southern Iran. Chemosphere 2020, 240, 124983. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.G.; Feng, Q.H.; He, Q.S.; Huang, Y.M.; Zhang, Y.; Jiang, G.; Zhao, W.; Gao, B.; Lin, K.; Xu, Z.H. Sources, atmospheric transport and deposition mechanism of organochlorine pesticides in soils of the Tibetan Plateau. Sci. Total Environ. 2017, 577, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.P.; Wu, Y.N.; Yin, S.A.; Li, J.G.; Zhao, Y.F.; Zhang, L.; Chen, H.J.; Liu, Y.P.; Yang, X.; Li, X.W. National survey of the levels of persistent organochlorine pesticides in the breast milk of mothers in China. Environ. Pollut. 2011, 159, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.F. Long-Rang Atmospheric Transport and Transformations of Organochlorine Pesticides (OCPs) in the Qinghai-Tibet Plateau; China University of Geosciences: Wuhan, China, 2018. [Google Scholar]
- Sun, J.T.; Pan, L.L.; Zhan, Y.; Lu, H.N.; Tsang, D.C.W.; Liu, W.X.; Wang, X.L.; Li, X.D.; Zhu, L.Z. Contamination of phthalate esters, organochlorine pesticides and polybrominated diphenyl ethers in agricultural soils from the Yangtze River Delta of China. Sci. Total Environ. 2016, 544, 670–676. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Mid-Atlantic RISK Assessment; Untied States Environmental Protection Agency: Washington, DC, USA, 2015.
- Dou, L.; Yang, G.Y. Distribution Characteristics and Risk Assessment of Organochlorine Pesticides in Surface Soil of Pearl River Delta Economic Zone. Environ. Sci. 2015, 36, 2954–2963. [Google Scholar]
- Zhang, H.Y.; Gao, R.T.; Jiang, S.R. Spatial Variability of Organochlorine Pesticides (DDTs and HCHs) in Surface Soils of Farmland in Beijing, China. Sci. Agric. Sin. 2006, 39, 1403–1410. [Google Scholar]
- An, Q.; Dong, Y.H.; Wang, H. Residues and distribution character of organochlorine pesticides in soils in Nanjing area. Acta Sci. Circumstantiae 2005, 25, 470–474. [Google Scholar]
- Zhang, H.B.; Luo, Y.M.; Zhao, Q.G. Hong Kong soil reserches Ⅳ Contents and compositions of organochlorines in soil. Acta Pedol. Sin. 2006, 43, 220–225. [Google Scholar]
- Chen, L.G.; Ran, Y.; Xing, B.S.; Mai, B.X.; He, J.H.; Wei, X.G.; Fu, J.M.; Sheng, G.Y. Contents and sources of polycyclic aromatic hydrocarbons and organochlorine pesticides invegetable soils of Guangzhou, China. Chemosphere 2005, 60, 879–890. [Google Scholar] [CrossRef]
- Gong, Z.M.; Tao, S.; Xu, F.L.; Dawson, R.; Liu, W.X.; Cui, Y.H.; Cao, J.; Wang, X.J.; Shen, W.R.; Zhang, W.J.; et al. Level and distribution of DDT in surface soils from Tianjin, China. Chemosphere 2004, 54, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.F.; Feng, J.Y.; Gang, L.I.; Liang, M.Q.; Wang, R.J.; Cai, C. Occurrence and possible sources of organochlorine pesticides in soils of Ningbo, East China. Earth Environ. Sci. Trans. R. Soc. Edinb. 2018, 109, 495–500. [Google Scholar] [CrossRef]
- Qu, C.K.; Albanese, S.; Li, J.J.; Cicchella, D.; Zuzolo, D.; Hope, D.; Cerino, P.; Pizzolante, A.; Doherty, L.A.; Lima, A.; et al. Organochlorine pesticides in the soils from Benevento provincial territory, southern Italy: Spatial distribution, air-soil exchange and implications for environmental health. Sci. Total Environ. 2019, 674, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Tesi, J.N.; Tesi, G.O.; Ossai, J.C.; Agbozu, I.E. Organochlorine pesticides (OCPs) in agricultural soils of Southern Nigeria: Spatial distribution, source identification, ecotoxicological and human health risks assessment. Environ. Forensics 2020. [Google Scholar] [CrossRef]
- Yun, S.M.; Yoon, J.k.; Kim, J.I.; Kim, I.J.; Kim, H.K.; Chung, H.M.; Kim, D.J.; Noh, H.J. Evaluation of residual level and distribution characteristics of organochlorine pesticides in agricultural soils in South Korea. Environ. Sci Pollut. Res. 2022, 29, 46003–46017. [Google Scholar] [CrossRef]
- Nyihirani, F.; Qu, C.K.; Yuan, Z.; Zhang, Y.C.; Mbululo, Y.; Janneh, M.; Qi, S.H. Level, source, and distribution of organochlorine pesticides (OCPs) in agricultural soils of Tanzania. Environ. Monit. Assess. 2022, 194, 19. [Google Scholar] [CrossRef]
- Qu, C.K.; Albanese, S.; Chen, W.; Lima, A.; Doherty, L.A.; Piccolo, A.; Arienzo, M.; Qi, S.H.; De Vivo, B. The status of organochlorine pesticide contamination in the soils of the Campanian Plain, southern Italy, and correlations with soil properties and cancer risk. Environ. Pollut. 2016, 216, 500–511. [Google Scholar] [CrossRef]
- Mishra, K.; Sharma, R.C.; Kumar, S. Contamination levels and spatial distribution of organochlorine pesticides in soils from India. Ecotoxicol. Environ. Saf. 2012, 76, 215–225. [Google Scholar] [CrossRef]
- Ge, J.; Woodward, L.A.; Li, Q.X.; Wang, J. Composition, distribution and risk assessment of organochlorine pesticides in soils from the Midway Atoll, North Pacific Ocean. Sci. Total Environ. 2013, 452, 421–426. [Google Scholar] [CrossRef]
- Calvelo Pereira, R.; Monterroso, C.; Martínez Cortizas, A.; Macías, F. Analysis of composition, distribution and origin of hexachlorocyclohexane residues in agricultural soils from NW Spain. Sci. Total Environ. 2010, 408, 5583–5591. [Google Scholar] [CrossRef]
- Tang, Z.W.; Huang, Q.F.; Nie, Z.Q.; Yang, Y.F.; Yang, J.; Qu, D.; Cheng, J.L. Levels and distribution of organochlorine pesticides and hexachlorobutadiene in soils and terrestrial organisms from a former pesticide-producing area in Southwest China. Stoch. Environ. Res. Risk Assess. 2016, 30, 1249–1262. [Google Scholar] [CrossRef]
- Niu, L.L.; Xu, C.; Yao, Y.J.; Liu, K.; Yang, F.X.; Tang, M.L.; Liu, W.P. Status, Influences and Risk Assessment of Hexachlorocyclohexanes in Agricultural Soils Across China. Environ. Sci. Technol. 2013, 47, 12140–12147. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.F.; Wang, X.T.; Jia, Y.; Wang, F.; Wu, M.H.; Sheng, G.Y.; Fu, J.M. Occurrence, distribution and possible sources of organochlorine pesticides in agricultural soil of Shanghai, China. J. Hazard. Mater. 2009, 170, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.F.; Qi, S.H.; Qu, C.K.; Li, H.; Chen, W.W.; Zhang, L.; Hu, T.; Shi, L. Distribution Characteristics and Risk Assessment of Organochlorine Pesticides in Soil from Jiufeng Mountain Range in Fujian, China. Environ. Sci. 2014, 35, 2691–2697. [Google Scholar]
- Wang, Y.W.; Wang, T.; Li, A.; Fu, J.J.; Wang, P.; Zhang, Q.H.; Jiang, G.B. Selection of bioindicators of polybrominated diphenyl ethers, polychlorinated biphenyls, and organochlorine pesticides in mollusks in the Chinese Bohai Sea. Environ. Sci. Technol. 2008, 42, 7159–7165. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, W.J.; Hu, T.P.; Xing, X.L.; Mao, Y.; Shi, M.M.; Xu, A.; Su, Y.W.; Li, X.Y.; Yu, H.K.; et al. Status of organochlorine pesticide pollution on surface soil of Huixian wetland in Guilin, China. J. Agro-Environ. Sci. 2021, 40, 371–381. [Google Scholar]
- Hitch, R.K.; Day, H.R. Unusual persistence of DDT in some western USA soil. Bull. Environ. Contam. Toxicol. 1992, 48, 259–264. [Google Scholar] [CrossRef]
- Zhu, S.Y. Residues and Soil-Air Exchange of Chlorinated POPs with Their Associated Human Health Risks from Typical Agricultural Area and Industrial Area in the Hangzhou City; Zhejiang University: Zhejiang, China, 2017. [Google Scholar]
- Harner, T.; Wideman, J.L.; Jantunen, L.M.; Bidleman, T.F.; Parkhurst, M.J. Residues of organochlorine pesticides in Alabama soils. Environ. Pollut. 1999, 106, 323–332. [Google Scholar] [CrossRef]
- Jia, H.L.; Li, Y.F.; Wang, D.G.; Cai, D.J.; Yang, M.; Ma, J.M.; Hu, J.X. Endosulfan in China 1-gridded usage inventories. Environ. Sci. Pollut. Res. 2009, 16, 295–301. [Google Scholar] [CrossRef]
- Ketyam, B.; Imsilp, K.; Poapolathep, A.; Poapolathep, S.; Jermnak, U.; Phaochoosak, N.; Tanhan, P. Health risk associated with the consumption of duck egg containing endosulfan residues. Environ. Monit. Assess. 2016, 188, 270. [Google Scholar] [CrossRef]
- Westbom, R.; Hussen, A.; Megersa, N.; Retta, N.; Mathiasson, L.; Björklund, E. Assessment of organochlorine pesticide pollution in Upper Awash Ethiopian state farm soils using selective pressurised liquid extraction. Chemosphere 2008, 72, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Zhang, G.; Li, J.; Sivakumar, A.; Jones, K.C. Occurrence and sources of selected organochlorine pesticides in the soil of seven major Indian cities: Assessment of air–soil exchange. Environ. Pollut. 2015, 204, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Bidleman, T.F.; Jantunen, L.M.M.; Helm, P.A.; Brorström-Lundén, E.; Juntto, S. Chlordane enantiomers and temporal trends of chlordane isomers in arctic air. Environ. Sci. Technol. 2002, 36, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.P. Contaminations and Health Risks of Organopesticides in Surface Water of Hangzhou; Zhejiang University: Zhejiang, China, 2004. [Google Scholar]
- Gao, J.; Zhou, H.F.; Pan, G.Q.; Wang, J.Z.; Chen, B.Q. Factors influencing the persistence of organochlorine pesticides in surface soil from the region around the Hongze Lake, China. Sci. Total Environ. 2013, 443, 7–13. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, Y.L.; Dawson, R.W.; Shi, Y.J.; Wang, T.Y. Classification and ordination of DDT and HCH in soil samples from the Guanting Reservoir, China. Chemosphere 2005, 60, 762–769. [Google Scholar] [CrossRef]
- Alawi, M.; Khalili, F.; Da’as, K. Interaction behavior of organochlorine pesticides with dissolved Jordanian humic acid. Arch. Environ. Contam. Toxicol. 1995, 28, 513–518. [Google Scholar] [CrossRef]
- Sun, Y.; Chang, X.P.; Zhao, L.X.; Zhou, B.; Weng, L.P.; Li, Y.T. Comparative study on the pollution status of organochlorine pesticides (OCPs) and bacterial community diversity and structure between plastic shed and open-field soils from northern China. Sci. Total Environ. 2020, 741, 139620. [Google Scholar] [CrossRef]
- Borisover, M.D.; Graber, E.R. Specific interactions of organic compounds with soil organic carbon. Chemosphere 1997, 34, 1761–1776. [Google Scholar] [CrossRef]
- Kannan, K.; Battula, S.; Loganathan, B.G.; Hong, C.S.; Lam, W.H.; Villeneuve, D.L.; Sajwan, K.; Giesy, J.P.; Aldous, K.M. Trace organic contaminants, including toxaphene and trifluralin, in cotton field soils from Georgia and South Carolina, USA. Arch. Environ. Contam. Toxicol. 2003, 45, 30–36. [Google Scholar] [CrossRef]
- Ali, N.; Khan, S.; Li, Y.Y.; Zheng, N.G.; Yao, H.Y. Influence of biochars on the accessibility of organochlorine pesticides and microbial community in contaminated soils. Sci. Total Environ. 2019, 647, 551–560. [Google Scholar] [CrossRef]


| Compound | Min ng/g | Max ng/g | Mean ng/g | Detection Rate /% |
|---|---|---|---|---|
| α-HCH | ND a | 37.7 | 1.09 | 32.5 |
| γ-HCH | ND | 89.0 | 1.40 | 35.8 |
| β-HCH | ND | 11.8 | 0.70 | 31.3 |
| δ-HCH | ND | 68.3 | 1.33 | 32.1 |
| ΣHCHs | ND | 110 | 4.52 | 64.2 |
| o,p’-DDD | ND | 165 | 2.33 | 15.0 |
| o,p’-DDE | ND | 174 | 3.80 | 23.8 |
| p,p’-DDE | ND | 97.6 | 2.06 | 28.3 |
| o,p’-DDT | ND | 489 | 15.2 | 27.9 |
| p,p’-DDD | ND | 58.6 | 0.34 | 5.42 |
| p,p’-DDT | ND | 580 | 10.5 | 27.5 |
| ΣDDTs | ND | 743 | 34.2 | 65.4 |
| Aldrin | ND | 5.80 | 0.04 | 0.83 |
| Dieldrin | ND | 12.6 | 0.17 | 2.50 |
| Endrin | ND | 283 | 2.81 | 3.33 |
| Mirex | ND | 29.2 | 0.51 | 5.42 |
| α-Endosulfan | ND | 288 | 13.0 | 22.5 |
| β-Endosulfan | ND | ND | - | - |
| cis-chlordane | ND | 20.4 | 0.63 | 9.17 |
| Trans-Chlordane | ND | 8.70 | 0.08 | 1.25 |
| heptachlor epoxide isomer A | ND | 113 | 3.89 | 21.3 |
| heptachlor epoxide isomer B | ND | 102 | 0.43 | 0.42 |
| Cis-Nonachlor | ND | 90.4 | 0.63 | 2.92 |
| trans-Nonachlor | ND | ND | - | - |
| HCB | ND | 41.5 | 0.40 | 4.58 |
| ΣOCPs | ND | 946 | 65.5 | 89.6 |
| Regions | Year of Sampling | HCHs ng/g (Mean) | DDTs ng/g (Mean) | References |
|---|---|---|---|---|
| Beijing | 2003 | 0.64~32.32 (1.47) | 1.42~5910.80 (77.18) | [19] |
| Nanjing | 2002–2003 | 2.7~130.6 (13.6) | 6.3~1050.7 (64.1) | [20] |
| Hong Kong | 2002–2003 | 2.5~11 (6.19) | ND~5.7 (0.52) | [21] |
| Guangzhou | 1999, 2002 | 0.19~42.3 (4.39) | 3.58~831 (81.4) | [22] |
| Tianjin | 2001 | 1.3~1094.6 (45.8) | 0.071~972.24 (56.01) | [23] |
| Yangtze River Delta | 2014 | 0.37~30.3 (2.46) | 0.13~3515 (56.2) | [16] |
| Pearl River Delta | 2008 | ND~41.62 (1.87) | ND~383.41 (12.30) | [18] |
| Ningbo | —— | 2.7~28.2 (4.6) | 2.2~566.6 (55.6) | [24] |
| Southern Italy | 2015 | ND~0.72 (0.065) | ND~16.4 (1.22) | [25] |
| Southern Nigeria | —— | ND~156 (15.5) | —— | [26] |
| Korea | 2017 | ND~14.51 (0.2) | ND~2187.18 (23.18) | [27] |
| Tanzania | 2019 | ND~1.54 (0.234) | ND~47.44 (2.29) | [28] |
| Campanian Plain | 2011 | 0.03~17.3 (1.38) | 0.08~1231 (107) | [29] |
| Dibrugarh | 2009-2010 | 178~1701 (705) | 75~2296 (757) | [30] |
| Midway Atoll | 2006 | ND~127 (23.5) | 1.4~643 (191) | [31] |
| Galicia, NW Spain | 2003 | ND~2305 (200) | —— | [32] |
| Pearl River Delta | 2019 | ND~110 (4.52) | ND~743 (34.2) | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, S.; Huang, J.; Zhou, H.; Cao, C.; Ai, T.; Xing, H.; Sun, J. Levels, Distribution and Health Risk Assessment of Organochlorine Pesticides in Agricultural Soils from the Pearl River Delta of China. Int. J. Environ. Res. Public Health 2022, 19, 13171. https://doi.org/10.3390/ijerph192013171
Yao S, Huang J, Zhou H, Cao C, Ai T, Xing H, Sun J. Levels, Distribution and Health Risk Assessment of Organochlorine Pesticides in Agricultural Soils from the Pearl River Delta of China. International Journal of Environmental Research and Public Health. 2022; 19(20):13171. https://doi.org/10.3390/ijerph192013171
Chicago/Turabian StyleYao, Siyu, Jiahui Huang, Haijun Zhou, Cuiting Cao, Tao Ai, Huanhuan Xing, and Jianteng Sun. 2022. "Levels, Distribution and Health Risk Assessment of Organochlorine Pesticides in Agricultural Soils from the Pearl River Delta of China" International Journal of Environmental Research and Public Health 19, no. 20: 13171. https://doi.org/10.3390/ijerph192013171






