Adverse Effects of Co-Exposure to Cd and Microplastic in Tigriopus japonicus
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Copepod Culture
2.3. Test Regime in the Laboratory
2.3.1. Ingestion Test
2.3.2. Feeding and Oxygen Consumption Test
2.3.3. Growth and Reproduction Test
2.3.4. Offspring Development
2.3.5. Nutritional Analysis
2.4. Statistical Analysis
3. Results
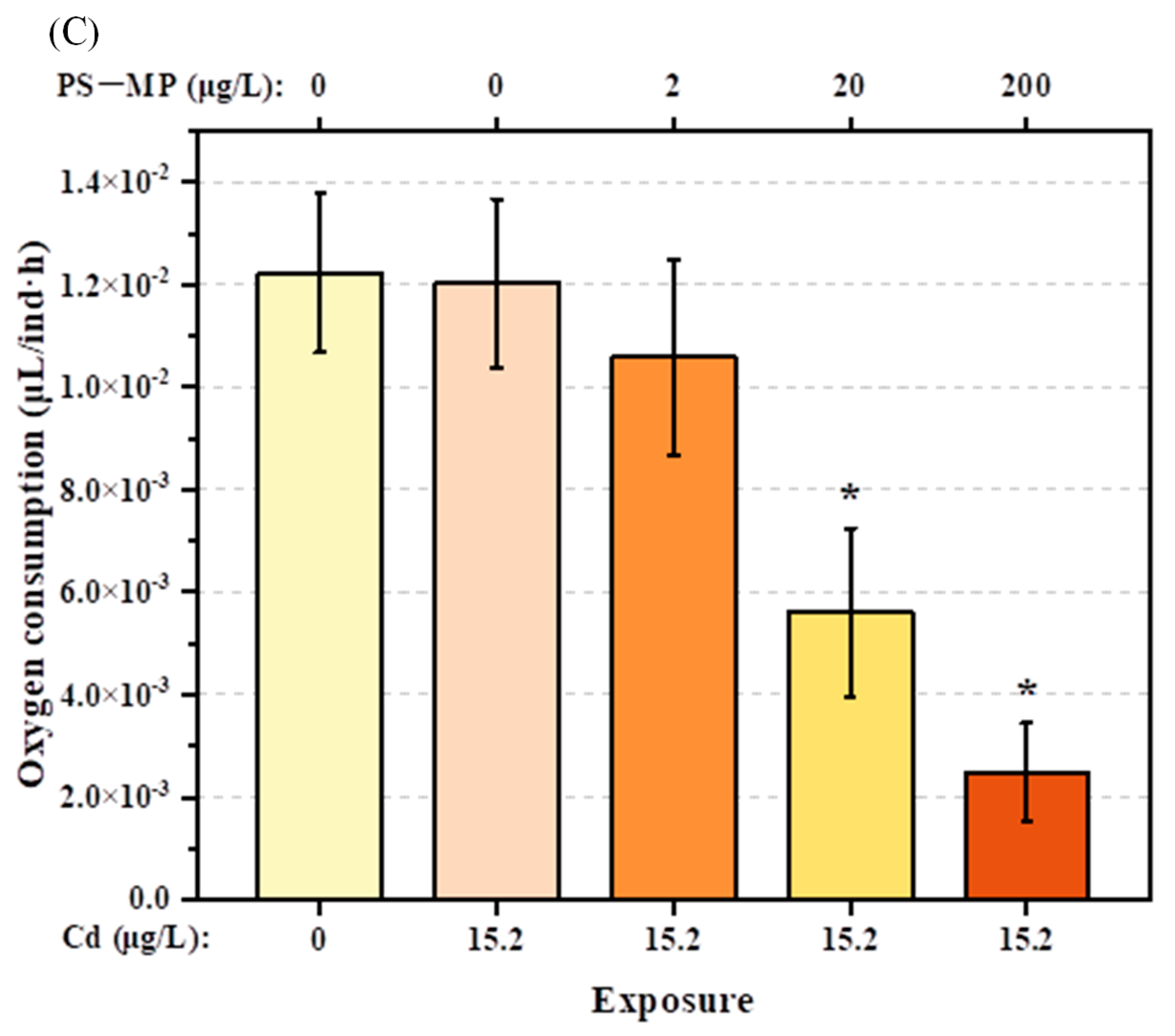
3.1. Impact on Physical Activities
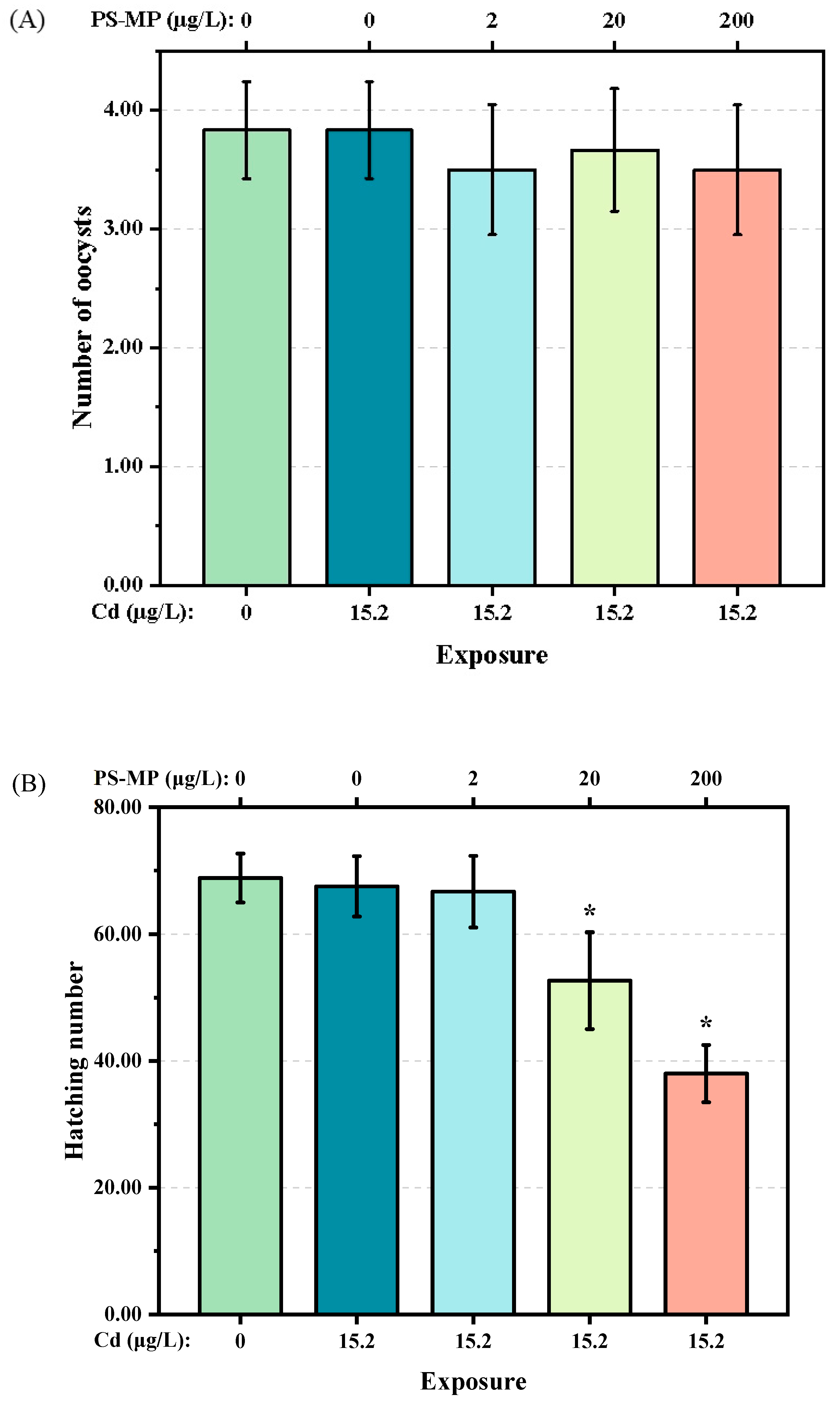
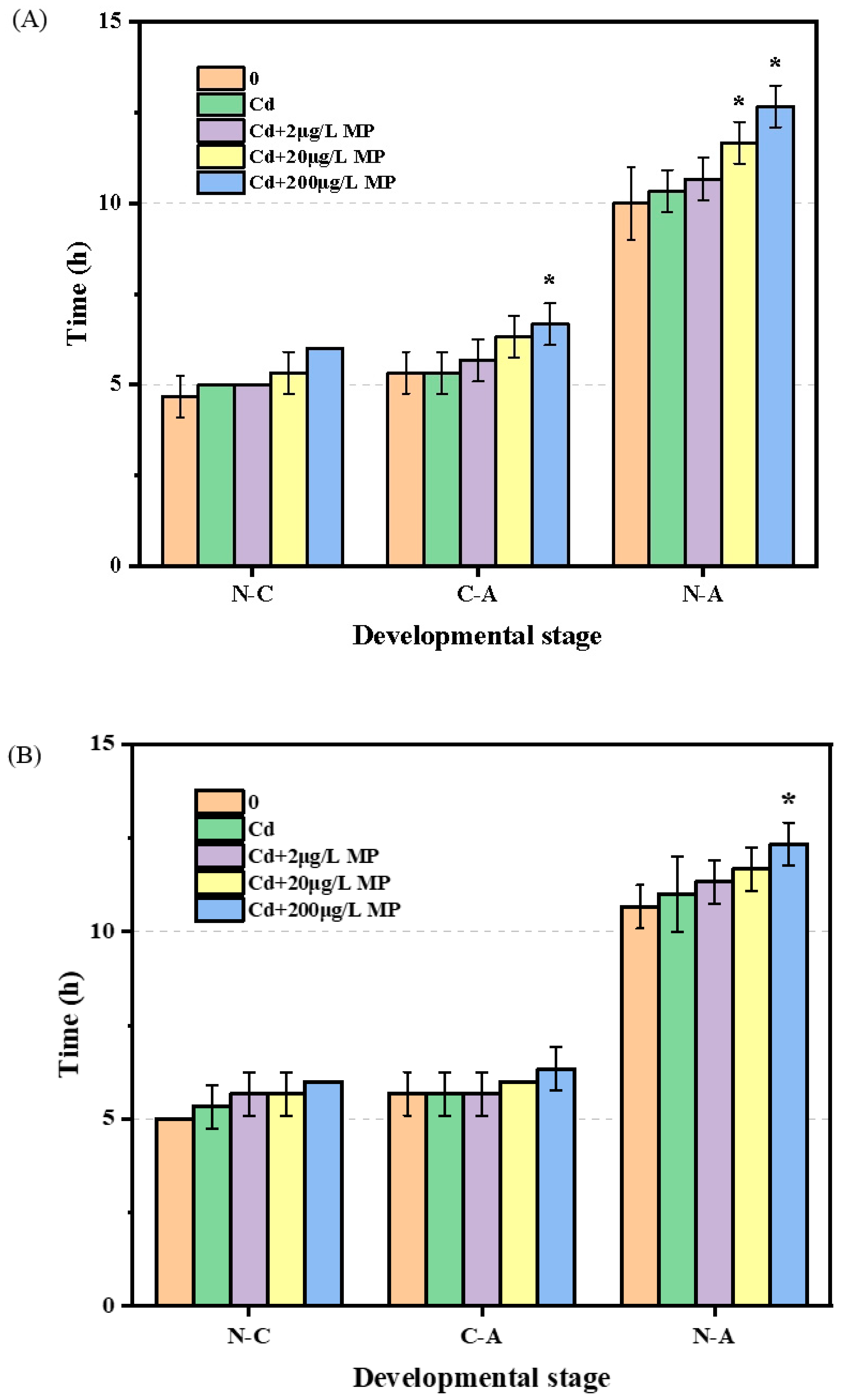
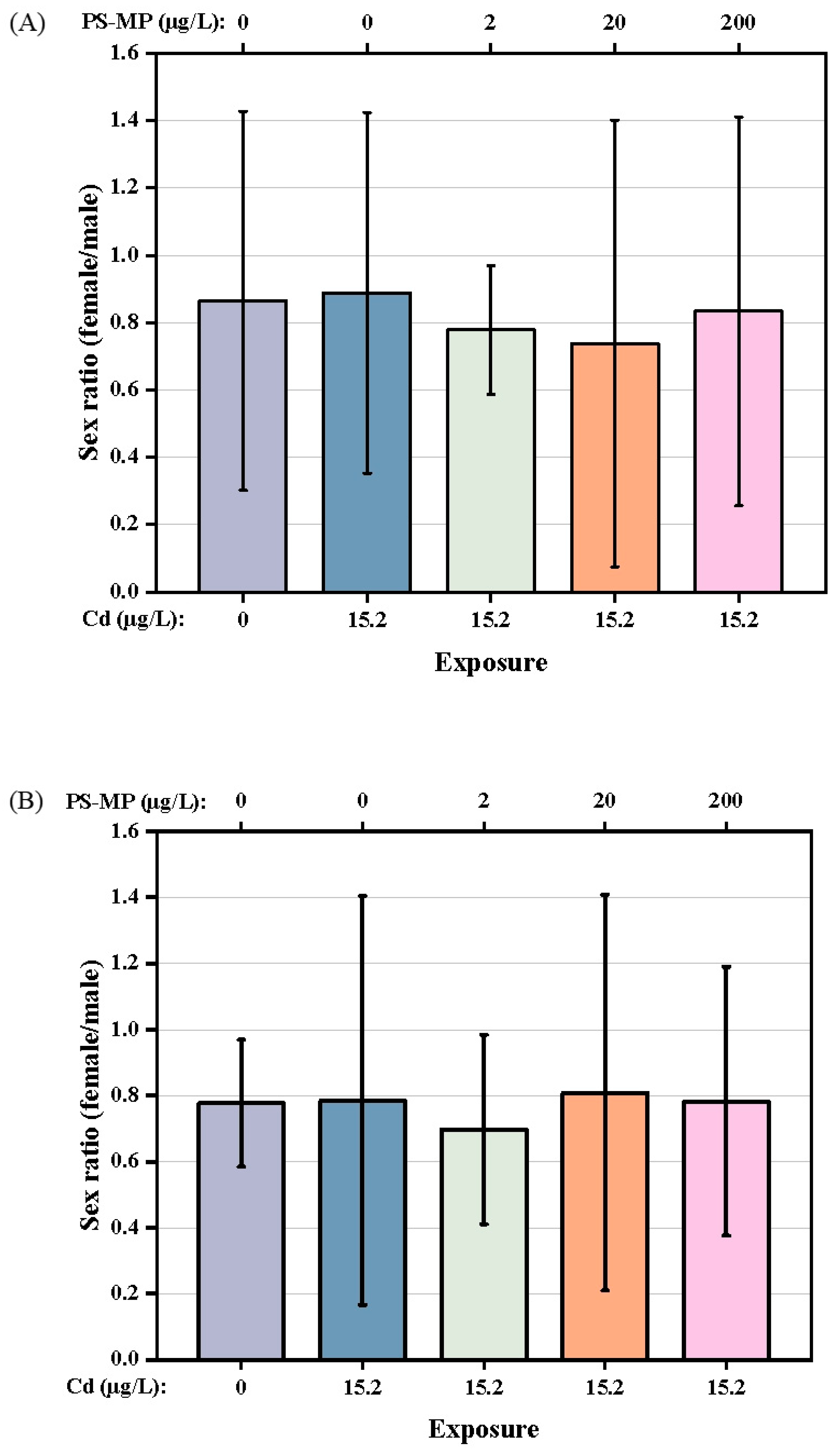
3.2. Impact on the Development of T. japonicas
3.3. Impact on Nutritional Components
4. Discussion
4.1. Physical Impact of PS-Microplastic and Cd
4.2. Impact on Reproductive Capacity of T. japonicus of Cd and PS-Microplastic
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Thompson, R.; Olsen, Y.; Mitchell, R.; Davis, A.; Rowland, S.; John, A.; McGonigle, D.; Russell, A. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Cózar, A.; Martí, E.; Duarte, C.M.; García-de-Lomas, J.; van Sebille, E.; Ballatore, T.J.; Eguíluz, V.M.; González-Gordillo, J.I.; Pedrotti, M.L.; Echevarría, F.; et al. The Arctic Ocean as a Dead End for Floating Plastics in the North Atlantic Branch of the Thermohaline Circulation. Sci. Adv. 2017, 3, 1600582. [Google Scholar] [CrossRef] [PubMed]
- Galloway, T.S.; Cole, M.; Lewis, C. Interactions of Microplastic Debris throughout the Marine Ecosystem. Nat. Ecol. Evol. 2017, 1, 0116. [Google Scholar] [CrossRef]
- Macleod, M.; Arp, H.P.; Tekman, M.B.; Jahnke, A. The Global Threat from Plastic Pollution. Science 2021, 373, 61. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-W.; Shim, W.J.; Kwon, O.Y.; Kang, J.-H. Size-Dependent Effects of Micro Polystyrene Particles in the Marine Copepod Tigriopus japonicus. Environ. Sci. Technol. 2013, 47, 11278–11283. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Chen, B.; Xia, B.; Shi, X.; Qu, K. Polystyrene Microplastics Alter the Behavior, Energy Reserve and Nutritional Composition of Marine Jacopever (Sebastes schlegelii). J. Hazard. Mater. 2018, 360, 97–105. [Google Scholar] [CrossRef]
- Sussarellu, R.; Suquet, M.; Thomas, Y.; Lambert, C.; Fabioux, C.; Pernet, M.E.J.; Le Goïc, N.; Quillien, V.; Mingant, C.; Epelboin, Y.; et al. Oyster Reproduction Is Affected by Exposure to Polystyrene Microplastics. Proc. Natl. Acad. Sci. USA 2016, 113, 201519019. [Google Scholar] [CrossRef]
- Park, E.-J.; Han, J.-S.; Park, E.-J.; Seong, E.; Lee, G.-H.; Kim, D.-W.; Son, H.-Y.; Han, H.-Y.; Lee, B.-S. Repeated-Oral Dose Toxicity of Polyethylene Microplastics and the Possible Implications on Reproduction and Development of the next Generation. Toxicol. Lett. 2020, 324, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yu, P.; Cai, M.; Wu, D.; Zhang, M.; Huang, Y.; Zhao, Y. Polystyrene Nanoplastic Exposure Induces Immobilization, Reproduction, and Stress Defense in the Freshwater Cladoceran Daphnia pulex. Chemosphere 2019, 215, 74–81. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, H.; Wang, Y.; Ren, R.; Qin, X.; Wang, S. Effects of Microplastics and Their Adsorption of Cadmium as Vectors on the Cladoceran Moina monogolica Daday: Implications for Plastic-Ingesting Organisms. J. Hazard. Mater. 2020, 400, 123239. [Google Scholar] [CrossRef]
- D’Costa, A.H. Microplastics in Decapod Crustaceans: Accumulation, Toxicity and Impacts, a Review. Sci. Total Environ. 2022, 832, 154963. [Google Scholar] [CrossRef]
- Martins, A.; Guilhermino, L. Transgenerational Effects and Recovery of Microplastics Exposure in Model Populations of the Freshwater Cladoceran Daphnia magna Straus. Sci. Total Environ. 2018, 631–632, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xiong, H.; Mi, K.; Xue, W.; Wei, W.; Zhang, Y. Toxicity Comparison of Nano-Sized and Micron-Sized Microplastics to Goldfish Carassius Auratus Larvae. J. Hazard. Mater. 2020, 388, 122058. [Google Scholar] [CrossRef]
- Duan, Z.; Duan, X.; Zhao, S.; Wang, X.; Wang, J.; Liu, Y.; Peng, Y.; Gong, Z.; Wang, L. Barrier Function of Zebrafish Embryonic Chorions against Microplastics and Nanoplastics and Its Impact on Embryo Development. J. Hazard. Mater. 2020, 395, 122621. [Google Scholar] [CrossRef] [PubMed]
- Iheanacho, S.C.; Odo, G.E. Neurotoxicity, Oxidative Stress Biomarkers and Haematological Responses in African Catfish (Clarias Gariepinus) Exposed to Polyvinyl Chloride Microparticles. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 232, 108741. [Google Scholar] [CrossRef]
- Xie, X.; Deng, T.; Duan, J.; Xie, J.; Yuan, J.; Chen, M. Exposure to Polystyrene Microplastics Causes Reproductive Toxicity through Oxidative Stress and Activation of the P38 MAPK Signaling Pathway. Ecotoxicol. Environ. Saf. 2020, 190, 110133. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Hamid, N.; Deng, S.; Jia, P.-P.; Pei, D.-S. Individual and Combined Toxicogenetic Effects of Microplastics and Heavy Metals (Cd, Pb, and Zn) Perturb Gut Microbiota Homeostasis and Gonadal Development in Marine Medaka (Oryzias melastigma). J. Hazard. Mater. 2020, 397, 122795. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Wang, S.; Wang, X.; Yu, X.; Hu, M.; Huang, W.; Wang, Y. Nanoplastics Impair the Intestinal Health of the Juvenile Large Yellow Croaker Larimichthys Crocea. J. Hazard. Mater. 2020, 397, 122773. [Google Scholar] [CrossRef] [PubMed]
- Jinhui, S. Effects of Microplastics and Attached Heavy Metals on Growth, Immunity, and Heavy Metal Accumulation in the Yellow Seahorse, Hippocampus Kuda Bleeker. Mar. Pollut. Bull. 2019, 149, 110510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yan, Z.; Lu, G.; Ji, Y. Single and Combined Effects of Microplastics and Roxithromycin on Daphnia magna. Environ. Pollut. 2020, 267, 115560. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhou, W.; Tang, Y.; Shi, W.; Shao, Y.; Ren, P.; Zhang, J.; Xiao, G.; Sun, H.; Liu, G. Microplastics Aggravate the Bioaccumulation of Three Veterinary Antibiotics in the Thick Shell Mussel Mytilus coruscus and Induce Synergistic Immunotoxic Effects. Sci. Total Environ. 2021, 770, 145273. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, Y.; Liu, G.; He, G.; Liu, W. Adsorption Mechanism of Cadmium on Microplastics and Their Desorption Behavior in Sediment and Gut Environments: The Roles of Water PH, Lead Ions, Natural Organic Matter and Phenanthrene. Water Res. 2020, 184, 116209. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zu, B.; Yang, Q.; Huang, Y.; Li, J. Adsorption of Lead and Cadmium by Microplastics and Their Desorption Behavior as Vectors in the Gastrointestinal Environment. J. Environ. Chem. Eng. 2022, 10, 107379. [Google Scholar] [CrossRef]
- Lavers, J.L.; Bond, A.L.; Hutton, I. Plastic Ingestion by Flesh-Footed Shearwaters (Puffinus Carneipes): Implications for Fledgling Body Condition and the Accumulation of Plastic-Derived Chemicals. Environ. Pollut. 2014, 187, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Mammo, F.K.; Amoah, I.D.; Gani, K.M.; Pillay, L.; Ratha, S.K.; Bux, F.; Kumari, S. Microplastics in the Environment: Interactions with Microbes and Chemical Contaminants. Sci. Total Environ. 2020, 743, 140518. [Google Scholar] [CrossRef]
- Yu, J.; Tian, J.-Y.; Xu, R.; Zhang, Z.-Y.; Yang, G.-P.; Wang, X.-D.; Lai, J.-G.; Chen, R. Effects of Microplastics Exposure on Ingestion, Fecundity, Development, and Dimethylsulfide Production in Tigriopus japonicus (Harpacticoida, Copepod). Environ. Pollut. 2020, 267, 115429. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, M.; Chen, X.; Yang, C.; Wu, L. Combined Toxicity of Microplastics and Cadmium on the Zebrafish Embryos (Danio rerio). Sci. Total Environ. 2020, 743, 140638. [Google Scholar] [CrossRef]
- Hinkle, P.; Kinsella, P.; Osterhoudt, K. Cadmium Uptake and Toxicity via Voltage-Sensitive Calcium Channels. J. Biol. Chem. 1987, 262, 16333–16337. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Lu, L.; Zheng, M.; Zhang, X.; Tian, H.; Wang, W.; Ru, S. Polystyrene Microplastics Cause Tissue Damages, Sex-Specific Reproductive Disruption and Transgenerational Effects in Marine Medaka (Oryzias melastigma). Environ. Pollut. 2019, 254, 113024. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, M.; Lu, L.; Li, X.; Zhang, Z.; Ru, S. Adaptation of Life-History Traits and Trade-Offs in Marine Medaka (Oryzias melastigma) after Whole Life-Cycle Exposure to Polystyrene Microplastics. J. Hazard. Mater. 2021, 414, 125537. [Google Scholar] [CrossRef]
- Zidour, M.; Boubechiche, Z.; Pan, Y.-J.; Bialais, C.; Cudennec, B.; Grard, T.; Drider, D.; Flahaut, C.; Ouddane, B.; Souissi, S. Population Response of the Estuarine Copepod Eurytemora Affinis to Its Bioaccumulation of Trace Metals. Chemosphere 2019, 220, 505–513. [Google Scholar] [CrossRef]
- Yu, S.-P.; Chan, B.K.K. Intergenerational Microplastics Impact the Intertidal Barnacle Amphibalanus Amphitrite during the Planktonic Larval and Benthic Adult Stages. Environ. Pollut. 2020, 267, 115560. [Google Scholar] [CrossRef] [PubMed]
- Kadiene, E.U.; Meng, P.-J.; Hwang, J.-S.; Souissi, S. Acute and Chronic Toxicity of Cadmium on the Copepod Pseudodiaptomus Annandalei: A Life History Traits Approach. Chemosphere 2019, 233, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Sun, S.; Han, Y.; Tang, Y.; Zhou, W.; Zhang, W.; Du, X.; Huang, L.; Liu, G. Microplastics Hamper the Fertilization Success of a Broadcast Spawning Bivalve through Reducing Gamete Collision and Gamete Fusion Efficiency. Aquat. Toxicol. 2022, 242, 106049. [Google Scholar] [CrossRef]
- Eltemsah, Y.S.; Bøhn, T. Acute and Chronic Effects of Polystyrene Microplastics on Juvenile and Adult Daphnia magna. Environ. Pollut. 2019, 254, 112919. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.A.; Kozal, J.S.; Jayasundara, N.; Massarsky, A.; Trevisan, R.; Geitner, N.; Wiesner, M.; Levin, E.D.; Di Giulio, R.T. Uptake, Tissue Distribution, and Toxicity of Polystyrene Nanoparticles in Developing Zebrafish (Danio rerio). Aquat. Toxicol. 2018, 194, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Hu, G.; Fan, X.; Jia, H. Sorption Properties of Cadmium on Microplastics: The Common Practice Experiment and A Two-Dimensional Correlation Spectroscopic Study. Ecotoxicol. Environ. Saf. 2020, 190, 110118. [Google Scholar] [CrossRef] [PubMed]




| Treatment | Survival Rate (%) | |
|---|---|---|
| F0 | F1 | |
| Control | 100.00 | 100.00 |
| Cd (15.2 μg/L) | 100.00 | 100.00 |
| Cd (15.2 μg/L) + 2 μg/L MP | 100.00 | 100.00 |
| Cd (15.2 μg/L) + 20 μg/L MP | 100.00 | 96.67 ± 5.77 |
| Cd (15.2 μg/L) + 200 μg/L MP | 93.33 ± 5.77 | 86.67 ± 5.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, W.; Guo, H.; Wang, J.; Han, X.; Cai, W. Adverse Effects of Co-Exposure to Cd and Microplastic in Tigriopus japonicus. Int. J. Environ. Res. Public Health 2022, 19, 13215. https://doi.org/10.3390/ijerph192013215
Shi W, Guo H, Wang J, Han X, Cai W. Adverse Effects of Co-Exposure to Cd and Microplastic in Tigriopus japonicus. International Journal of Environmental Research and Public Health. 2022; 19(20):13215. https://doi.org/10.3390/ijerph192013215
Chicago/Turabian StyleShi, Wenzhuo, Hao Guo, Junqiang Wang, Xuemeng Han, and Wenqian Cai. 2022. "Adverse Effects of Co-Exposure to Cd and Microplastic in Tigriopus japonicus" International Journal of Environmental Research and Public Health 19, no. 20: 13215. https://doi.org/10.3390/ijerph192013215
APA StyleShi, W., Guo, H., Wang, J., Han, X., & Cai, W. (2022). Adverse Effects of Co-Exposure to Cd and Microplastic in Tigriopus japonicus. International Journal of Environmental Research and Public Health, 19(20), 13215. https://doi.org/10.3390/ijerph192013215






