Effects of Biofilms on Trace Metal Adsorption on Plastics in Freshwater Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Plastics and Exposure Device
2.2. Biofilm Incubation and Collection
2.3. DNA Extraction and 16S rRNA Sequencing
2.4. Batch Adsorption Experiments
2.5. Analysis of Adsorption Experiments
3. Results and Discussion
3.1. Characterisation of Plastic Debris
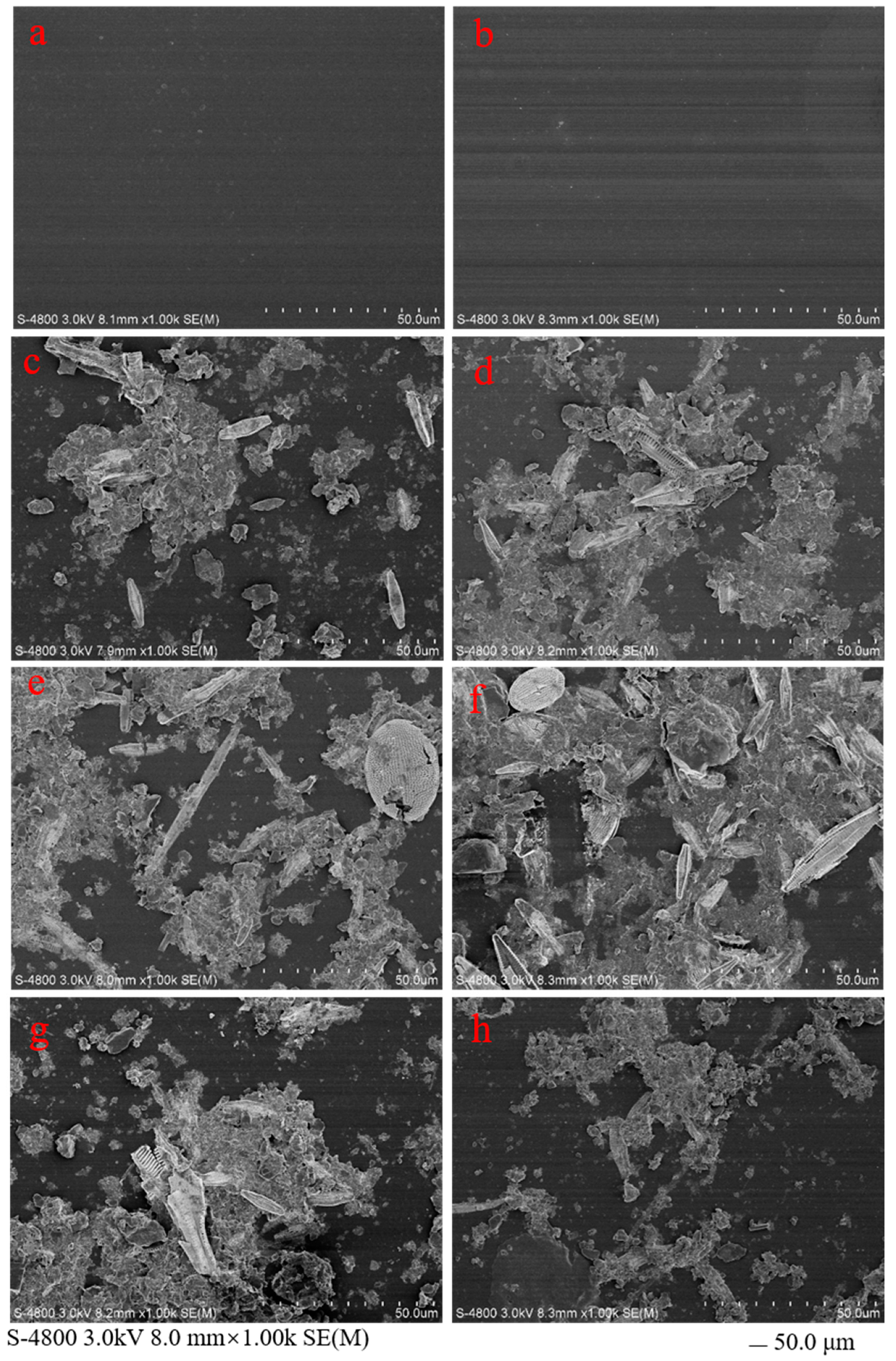
3.1.1. Morphology of Plastic Debris
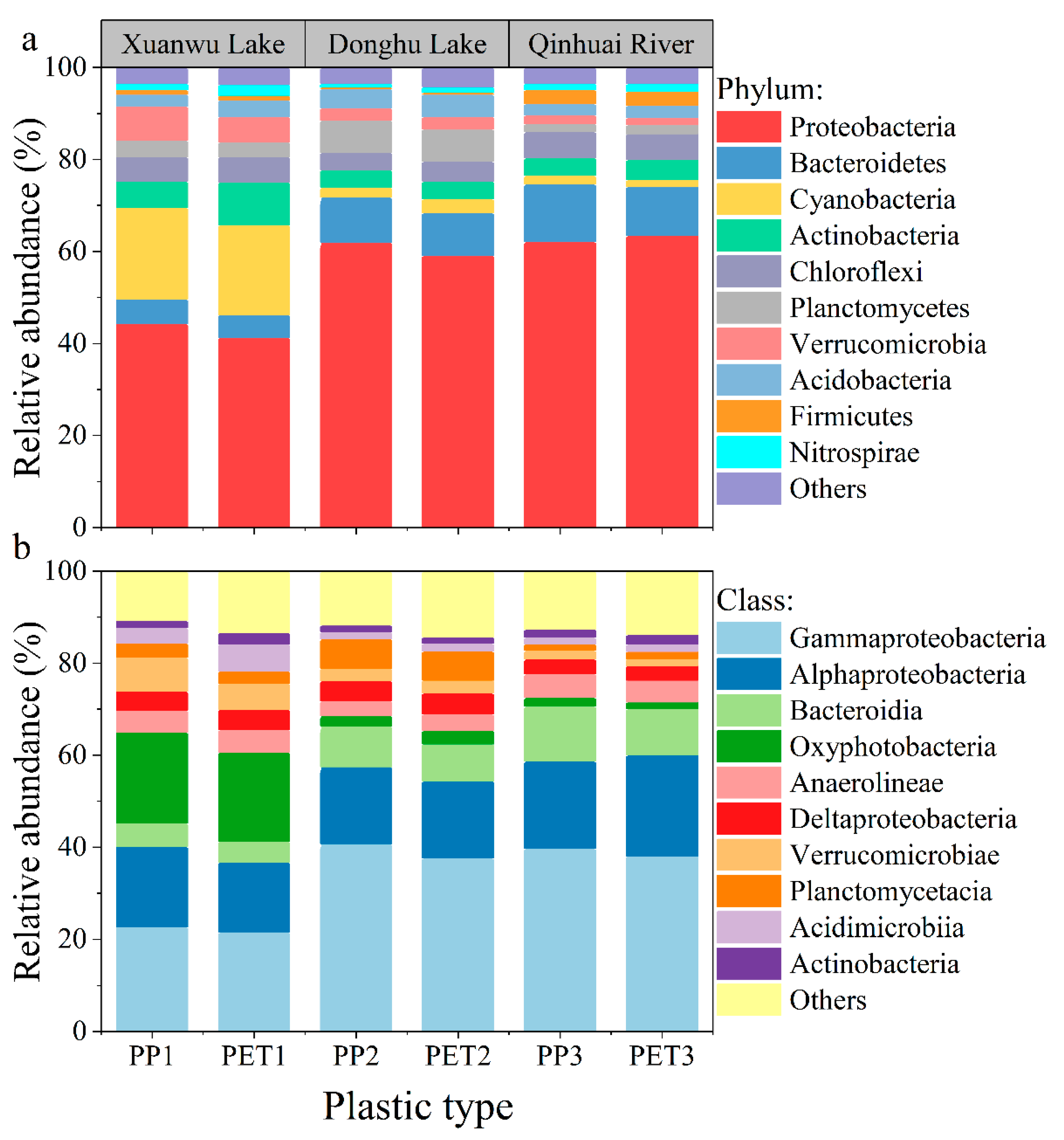
3.1.2. Composition of Microbial Communities in Biofilms on Plastic Debris
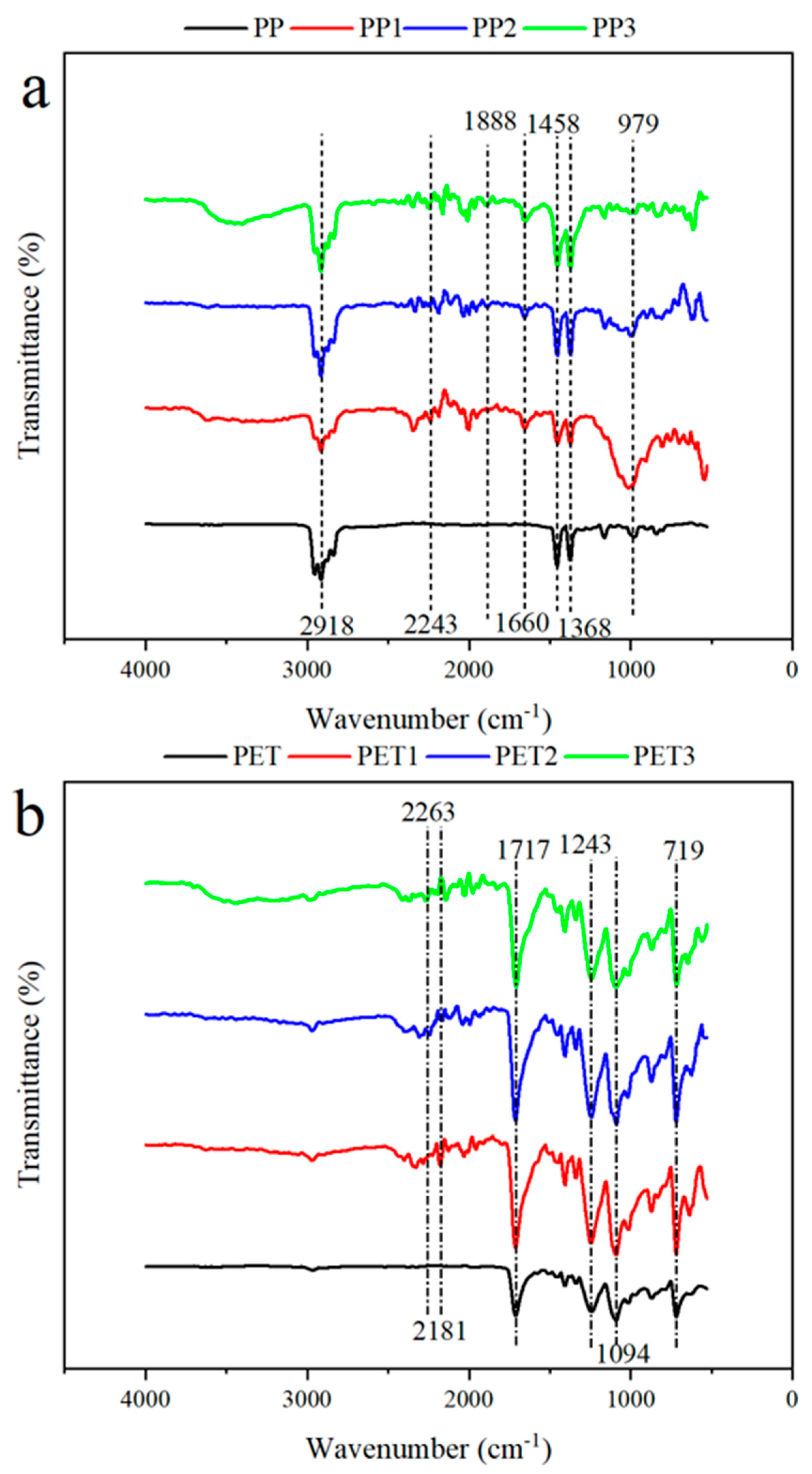
3.1.3. The Functional Groups of Biofilm-Covered Plastics
3.2. Adsorption Isotherms
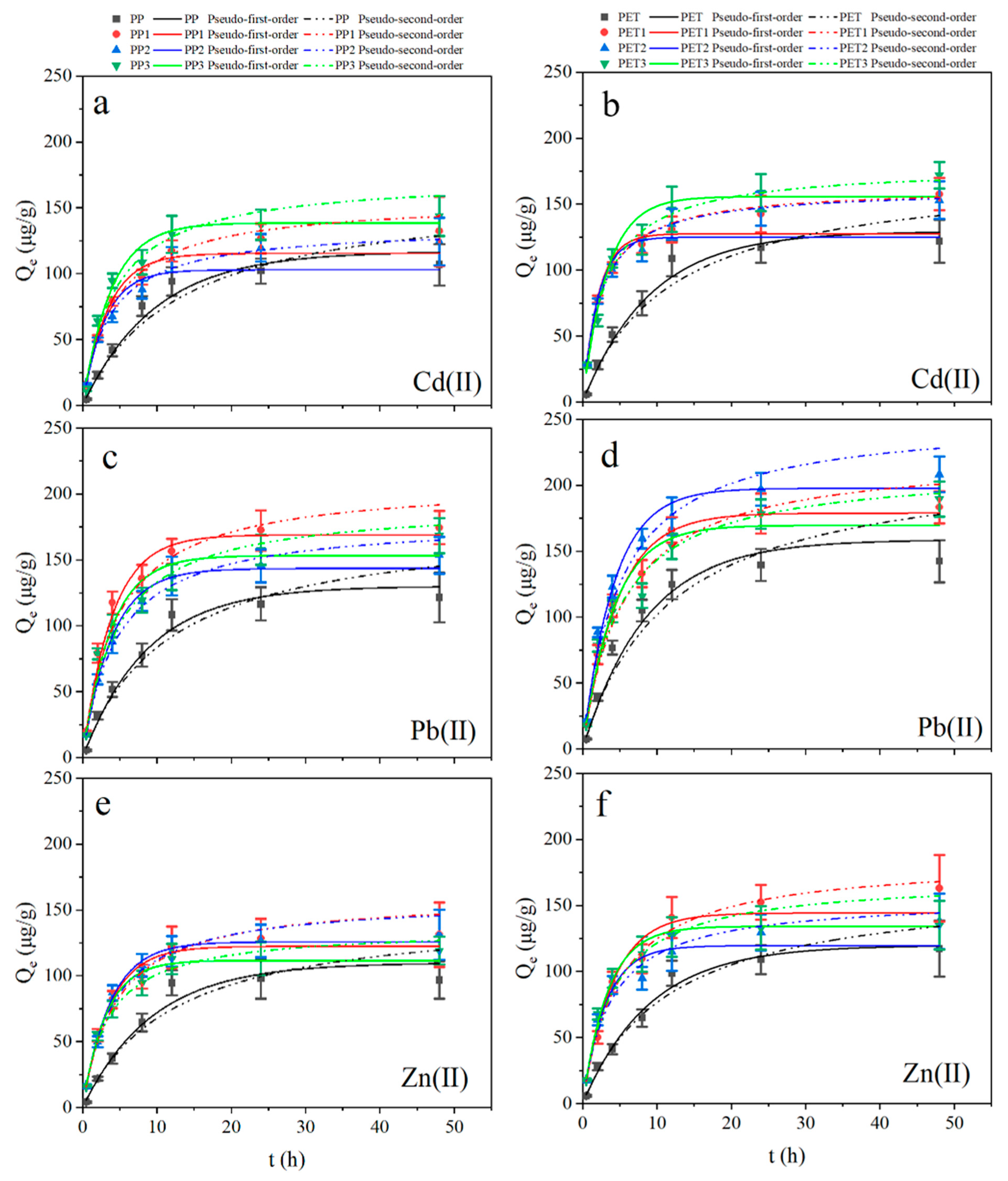
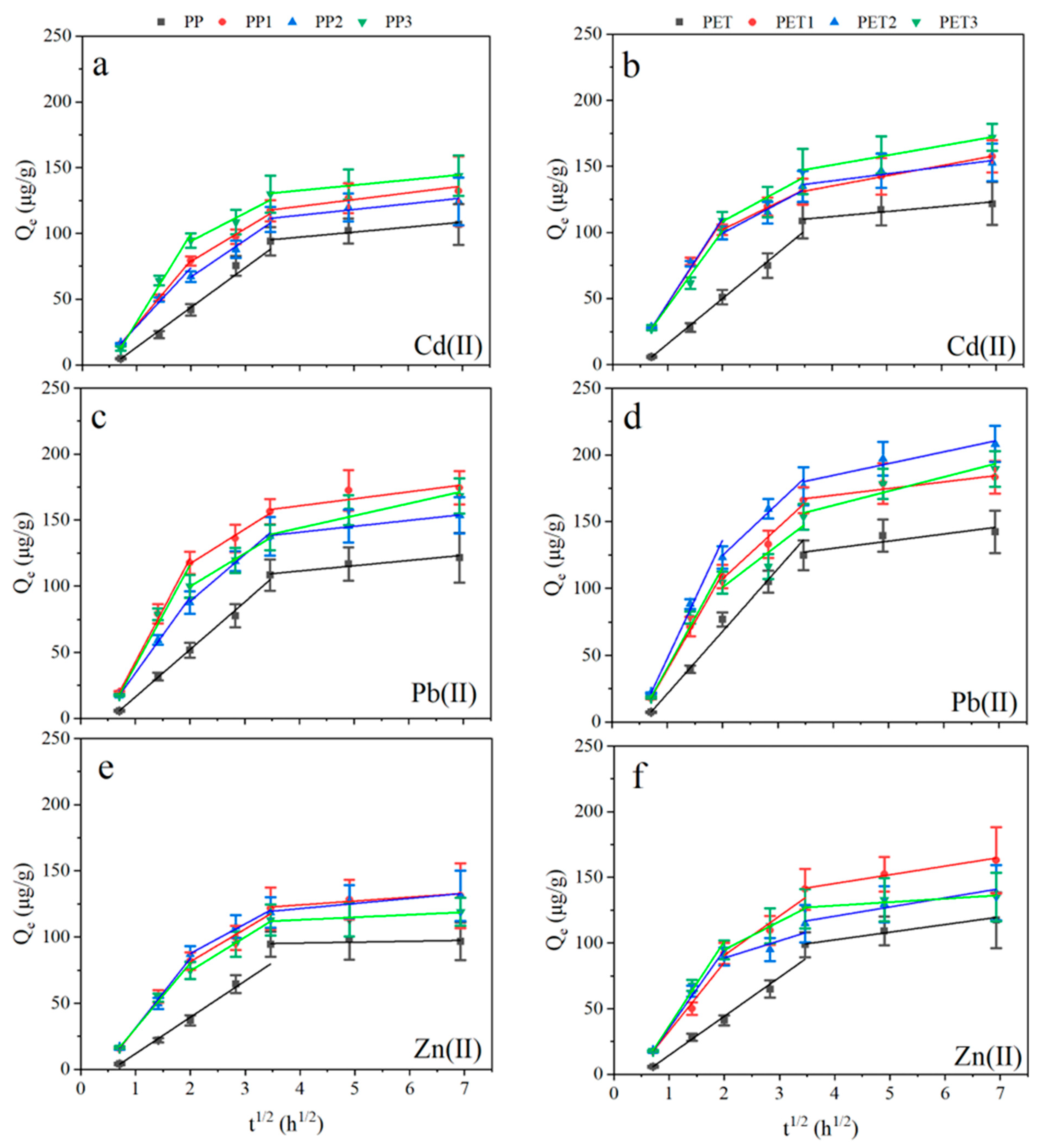
3.3. Adsorption Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrady, A.L. Persistence of Plastic Litter in the Oceans. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 57–72. [Google Scholar]
- Galgani, F.; Hanke, G.; Maes, T. Global Distribution, Composition and Abundance of Marine Litter. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 29–56. [Google Scholar]
- Law, K.L.; Thompson, R.C. Microplastics in the seas. Science 2014, 345, 144–145. [Google Scholar] [CrossRef] [PubMed]
- Beiras, R.; Schönemann, A.M. Currently monitored microplastics pose negligible ecological risk to the global ocean. Sci. Rep. 2020, 10, 22281. [Google Scholar] [CrossRef] [PubMed]
- Burns, E.E.; Boxall, A.B.A. Microplastics in the aquatic environment: Evidence for or against adverse impacts and major knowledge gaps. Environ. Toxicol. Chem. 2018, 37, 2776–2796. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A. Microplastics in the Marine Environment: Distribution, Interactions and Effects. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 245–307. [Google Scholar]
- Wang, J.; Zheng, L.; Li, J. A critical review on the sources and instruments of marine microplastics and prospects on the relevant management in China. Waste Manag. Res. 2018, 36, 898–911. [Google Scholar] [CrossRef]
- Turner, A.; Holmes, L.A. Adsorption of trace metals by microplastic pellets in fresh water. Environ. Chem. 2015, 12, 600–610. [Google Scholar] [CrossRef]
- Ashton, K.; Holmes, L.; Turner, A. Association of metals with plastic production pellets in the marine environment. Mar. Pollut. Bull. 2010, 60, 2050–2055. [Google Scholar] [CrossRef]
- Guan, J.; Qi, K.; Wang, J.; Wang, W.; Wang, Z.; Lu, N.; Qu, J. Microplastics as an emerging anthropogenic vector of trace metals in freshwater: Significance of biofilms and comparison with natural substrates. Water Res. 2020, 184, 116205. [Google Scholar] [CrossRef]
- Zou, J.; Liu, X.; Zhang, D.; Yuan, X. Adsorption of three bivalent metals by four chemical distinct microplastics. Chemosphere 2020, 248, 126064. [Google Scholar] [CrossRef]
- Huang, S.; Song, Q.; Li, Q.; Zhang, H.; Luo, X.; Zheng, Z. Damage of heavy metals to Vallisneria natans (V. natans) and characterization of microbial community in biofilm. Aquat. Toxicol. 2020, 225, 105515. [Google Scholar] [CrossRef]
- Yu, F.; Yang, C.; Zhu, Z.; Bai, X.; Ma, J. Adsorption behavior of organic pollutants and metals on micro/nanoplastics in the aquatic environment. Sci. Total Environ. 2019, 694, 133643. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Lin, L.; Wang, X.; Feng, A.; Yu, A. Pb(II) uptake onto nylon microplastics: Interaction mechanism and adsorption performance. J. Hazard. Mater. 2020, 386, 121960. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Wangjin, X.; Wang, Y.; Meng, G.; Chen, Y. The adsorption behavior of metals in aqueous solution by microplastics effected by UV radiation. J. Environ. Sci. 2020, 87, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhan, X.; Wu, X.; Li, J.; Wang, H.; Gao, S. Effect of weathering on environmental behavior of microplastics: Properties, sorption and potential risks. Chemosphere 2020, 242, 125193. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yuan, J.; Zhou, T.; Zhao, Y.; Yu, F.; Ma, J. Laboratory simulation of microplastics weathering and its adsorption behaviors in an aqueous environment: A systematic review. Environ. Pollut. 2020, 265, 114864. [Google Scholar] [CrossRef]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “Plastisphere”: Microbial Communities on Plastic Marine Debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef]
- Flemming, H.-C. Biofouling and me: My Stockholm syndrome with biofilms. Water Res. 2020, 173, 115576. [Google Scholar] [CrossRef] [PubMed]
- Rummel, C.D.; Jahnke, A.; Gorokhova, E.; Kühnel, D.; Schmitt-Jansen, M. Impacts of Biofilm Formation on the Fate and Potential Effects of Microplastic in the Aquatic Environment. Environ. Sci. Technol. Lett. 2017, 4, 258–267. [Google Scholar] [CrossRef]
- Richard, H.; Carpenter, E.J.; Komada, T.; Palmer, P.T.; Rochman, C.M. Biofilm facilitates metal accumulation onto microplastics in estuarine waters. Sci. Total Environ. 2019, 683, 600–608. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Li, Y.; Li, J.; Wang, F.; Xia, S.; Zhao, J. Biofilm alters tetracycline and copper adsorption behaviors onto polyethylene microplastics. Chem. Eng. J. 2020, 392, 123808. [Google Scholar] [CrossRef]
- Miao, L.; Wang, C.; Adyel, T.M.; Wu, J.; Liu, Z.; You, G.; Meng, M.; Qu, H.; Huang, L.; Yu, Y.; et al. Microbial carbon metabolic functions of biofilms on plastic debris influenced by the substrate types and environmental factors. Environ. Int. 2020, 143, 106007. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Yu, Y.; Adyel, T.M.; Wang, C.; Liu, Z.; Liu, S.; Huang, L.; You, G.; Meng, M.; Qu, H.; et al. Distinct microbial metabolic activities of biofilms colonizing microplastics in three freshwater ecosystems. J. Hazard. Mater. 2021, 403, 123577. [Google Scholar] [CrossRef] [PubMed]
- De Tender, C.; Devriese, L.I.; Haegeman, A.; Maes, S.; Vangeyte, J.; Cattrijsse, A.; Dawyndt, P.; Ruttink, T. Temporal Dynamics of Bacterial and Fungal Colonization on Plastic Debris in the North Sea. Environ. Sci. Technol. 2017, 51, 7350–7360. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Nelson, Y.M.; Lion, L.W.; Shuler, M.L.; Ghiorse, W.C. Adsorption of Pb and Cd onto metal oxides and organic material in natural surface coatings as determined by selective extractions: New evidence for the importance of Mn and Fe oxides. Water Res. 2000, 34, 427–436. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Y.; Liu, G.; He, G.; Liu, W. Adsorption mechanism of cadmium on microplastics and their desorption behavior in sediment and gut environments: The roles of water pH, lead ions, natural organic matter and phenanthrene. Water Res. 2020, 184, 116209. [Google Scholar] [CrossRef]
- Huang, B.; Liu, G.; Wang, P.; Zhao, X.; Xu, H. Effect of Nitric Acid Modification on Characteristics and Adsorption Properties of Lignite. Processes 2019, 7, 167. [Google Scholar] [CrossRef]
- Gong, M.; Yang, G.; Zhuang, L.; Zeng, E.Y. Microbial biofilm formation and community structure on low-density polyethylene microparticles in lake water microcosms. Environ. Pollut. 2019, 252, 94–102. [Google Scholar] [CrossRef]
- McCormick, A.; Hoellein, T.J.; Mason, S.A.; Schluep, J.; Kelly, J.J. Microplastic is an Abundant and Distinct Microbial Habitat in an Urban River. Environ. Sci. Technol. 2014, 48, 11863–11871. [Google Scholar] [CrossRef]
- Jiang, P.; Zhao, S.; Zhu, L.; Li, D. Microplastic-associated bacterial assemblages in the intertidal zone of the Yangtze Estuary. Sci. Total Environ. 2018, 624, 48–54. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wuertz, S. Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Ancion, P.-Y.; Lear, G.; Dopheide, A.; Lewis, G.D. Metal concentrations in stream biofilm and sediments and their potential to explain biofilm microbial community structure. Environ. Pollut. 2013, 173, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Gao, M.; Song, Z.; Qiu, W. As(III) adsorption onto different-sized polystyrene microplastic particles and its mechanism. Chemosphere 2020, 239, 124792. [Google Scholar] [CrossRef]
- Qiongjie, W.; Yong, Z.; Yangyang, Z.; Zhouqi, L.; Jinxiaoxue, W.; Huijuan, C. Effects of biofilm on metal adsorption behavior and microbial community of microplastics. J. Hazard. Mater. 2022, 424, 127340. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Ahamed, T.; Brown, S.P.; Salehi, M. Investigate the role of biofilm and water chemistry on lead deposition onto and release from polyethylene: An implication for potable water pipes. J. Hazard. Mater. 2020, 400, 123253. [Google Scholar] [CrossRef] [PubMed]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
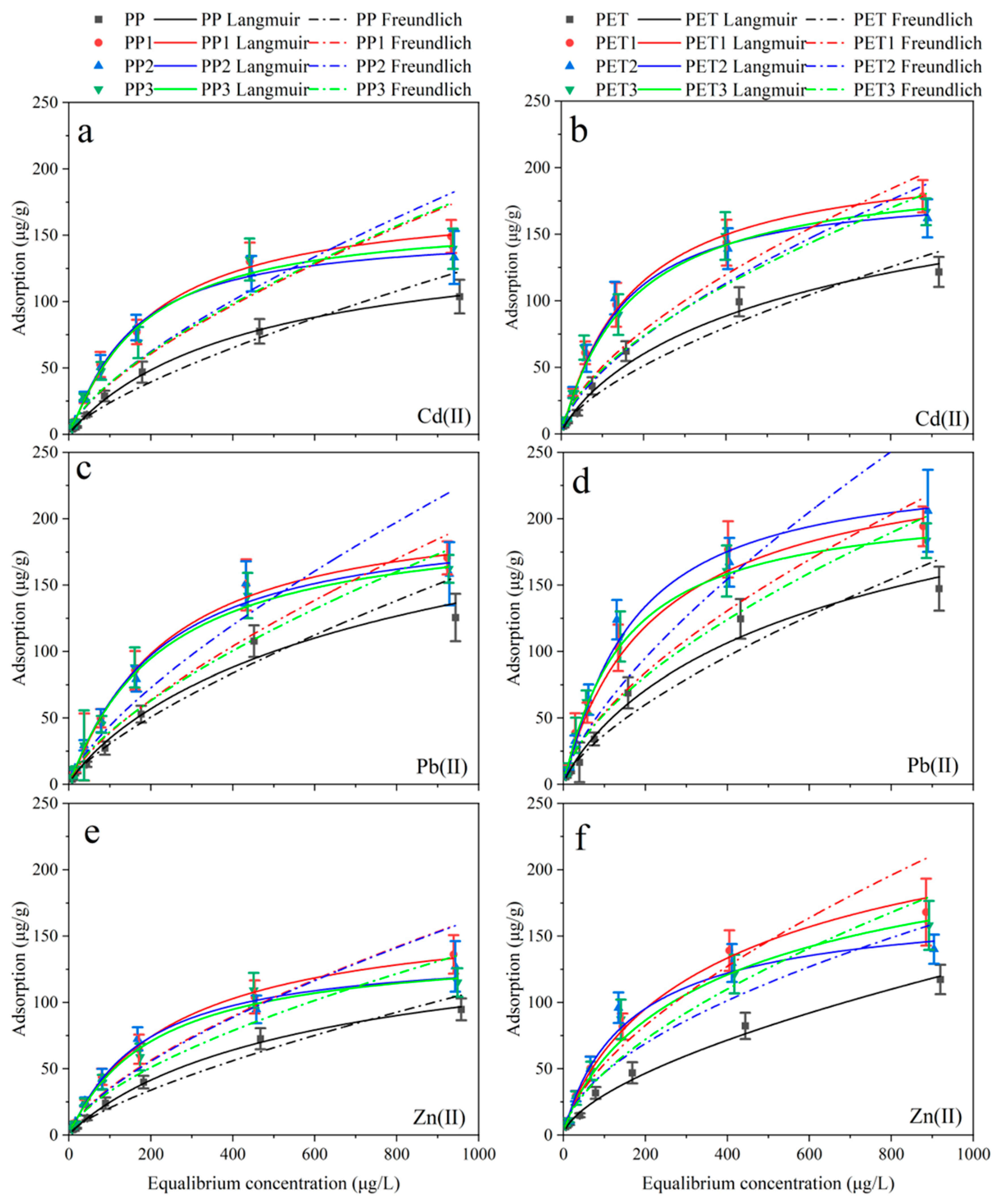
| Water | Plastic | Langumuir Isotherm | Freundlich Isotherm | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zn | Cd | Pb | Zn | Cd | Pb | ||||||||||||||
| Qm (μg/g) | KL (L/μg) | R2 | Qm (μg/g) | KL (L/μg) | R2 | Qm (μg/g) | KL (L/μg) | R2 | KF (L/μg) | 1/n | R2 | KF (L/μg) | 1/n | R2 | KF (L/μg) | 1/n | R2 | ||
| Virgin | PP | 141.70 | 2.02 × 10−3 | 0.98 | 152.91 | 2.12 × 10−3 | 0.98 | 176.35 | 2.41 × 10−3 | 0.96 | 0.63 | 0.74 | 0.95 | 0.71 | 0.75 | 0.94 | 0.87 | 0.75 | 0.95 |
| Virgin | PET | 151.07 | 2.64 × 10−3 | 0.92 | 169.99 | 2.63 × 10−3 | 0.97 | 216.51 | 2.41 × 10−3 | 0.98 | 1.21 | 0.67 | 0.98 | 1.14 | 0.69 | 0.94 | 1.07 | 0.74 | 0.96 |
| Xuanwu Lake | PP1 | 177.91 | 3.03 × 10−3 | 0.99 | 214.75 | 2.62 × 10−3 | 0.99 | 256.21 | 2.20 × 10−3 | 0.99 | 1.28 | 0.69 | 0.94 | 1.24 | 0.71 | 0.92 | 1.13 | 0.74 | 0.94 |
| PET1 | 225.26 | 3.00 × 10−3 | 0.98 | 247.04 | 2.75 × 10−3 | 0.98 | 277.38 | 2.20 × 10−3 | 0.99 | 1.67 | 0.70 | 0.95 | 1.67 | 0.69 | 0.93 | 1.62 | 0.70 | 0.94 | |
| Donghu Lake | PP2 | 162.69 | 3.29 × 10−3 | 0.99 | 202.13 | 2.80 × 10−3 | 0.98 | 250.90 | 2.38 × 10−3 | 0.99 | 1.21 | 0.70 | 0.92 | 1.19 | 0.73 | 0.89 | 1.17 | 0.76 | 0.91 |
| PET2 | 183.91 | 3.92 × 10−3 | 0.98 | 232.94 | 2.76 × 10−3 | 0.97 | 334.38 | 2.38 × 10−3 | 0.97 | 2.11 | 0.63 | 0.91 | 1.50 | 0.70 | 0.89 | 1.30 | 0.77 | 0.90 | |
| Qinhuai River | PP3 | 153.06 | 3.86 × 10−3 | 0.99 | 200.67 | 2.89 × 10−3 | 0.98 | 226.81 | 2.70 × 10−3 | 0.99 | 1.42 | 0.66 | 0.91 | 1.31 | 0.71 | 0.89 | 1.34 | 0.70 | 0.94 |
| PET3 | 203.84 | 3.20 × 10−3 | 0.97 | 228.35 | 2.97 × 10−3 | 0.98 | 248.10 | 2.70 × 10−3 | 0.99 | 1.63 | 0.68 | 0.94 | 1.76 | 0.67 | 0.93 | 1.80 | 0.68 | 0.92 | |
| Water | Plastic | Pseudo-First-Order | Pseudo-Second-Order | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zn | Cd | Pb | Zn | Cd | Pb | ||||||||||||||
| Qe (μg/g) | K1 (1/h) | R2 | Qe (μg/g) | K1 (1/h) | R2 | Qe (μg/g) | K1 (1/h) | R2 | Qe (μg/g) | K2 (g/μg h) | R2 | Qe (μg/g) | K2 (g/μg h) | R2 | Qe (μg/g) | K2 (g/μg h) | R2 | ||
| PP | 109.86 | 0.11 | 0.98 | 116.89 | 0.10 | 0.98 | 130.18 | 0.11 | 0.96 | 149.81 | 2.52 × 10−4 | 0.96 | 164.07 | 4.62 × 10−4 | 0.96 | 180.05 | 4.82 × 10−4 | 0.95 | |
| PET | 119.81 | 0.11 | 0.98 | 129.33 | 0.11 | 0.98 | 158.63 | 0.11 | 0.98 | 167.32 | 5.03 × 10−4 | 0.98 | 176.21 | 4.79 × 10−4 | 0.97 | 221.50 | 3.85 × 10−4 | 0.96 | |
| Xuanwu Lake | PP1 | 122.38 | 0.27 | 0.99 | 115.42 | 0.30 | 0.99 | 168.94 | 0.26 | 0.99 | 160.49 | 1.38 × 10−3 | 0.99 | 156.15 | 1.50 × 10−3 | 0.99 | 209.92 | 1.05 × 10−3 | 0.94 |
| PET1 | 114.30 | 0.25 | 0.98 | 127.62 | 0.48 | 0.98 | 175.63 | 0.21 | 0.99 | 185.05 | 1.12 × 10−3 | 0.99 | 163.06 | 2.52 × 10−3 | 0.99 | 223.86 | 8.13 × 10−4 | 0.94 | |
| Donghu Lake | PP2 | 122.80 | 0.27 | 0.99 | 102.90 | 0.33 | 0.98 | 143.56 | 0.26 | 0.99 | 158.04 | 1.54 × 10−3 | 0.99 | 135.63 | 2.02 × 10−3 | 0.99 | 180.73 | 1.21 × 10−3 | 0.91 |
| PET2 | 119.58 | 0.32 | 0.98 | 125.02 | 0.49 | 0.98 | 158.59 | 0.24 | 0.97 | 155.68 | 1.68 × 10−3 | 0.97 | 161.82 | 2.57 × 10−3 | 0.99 | 252.06 | 7.88 × 10−4 | 0.90 | |
| Qinhuai Lake | PP3 | 111.67 | 0.32 | 0.99 | 138.59 | 0.26 | 0.97 | 153.25 | 0.26 | 0.99 | 135.79 | 2.13 × 10−3 | 0.99 | 173.42 | 1.35 × 10−3 | 0.95 | 192.75 | 1.21 × 10−3 | 0.94 |
| PET3 | 134.23 | 0.30 | 0.99 | 155.75 | 0.31 | 0.98 | 183.96 | 0.24 | 0.99 | 170.44 | 1.45 × 10−3 | 0.96 | 178.76 | 1.84 × 10−3 | 0.98 | 213.79 | 9.50 × 10−4 | 0.92 | |
| Trace Metals | Waters | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Xuanwu Lake | Donghu Lake | Qinhuai River | |||||||
| PP | PET | PP1 | PET1 | PP2 | PET2 | PP3 | PET3 | ||
Zn(Ⅱ) | kt1 | 27.51 | 29.68 | 52.93 | 52.48 | 52.57 | 59.72 | 49.33 | 64.43 |
| C | −15.64 | −15.19 | −21.83 | −19.76 | −21.20 | −24.69 | −18.32 | −27.6 | |
| R2 | 0.98 | 0.99 | 0.99 | 0.98 | 0.99 | 0.99 | 0.98 | 0.99 | |
| kt2 | 0.68 | 5.83 | 24.67 | 29.87 | 22.39 | 13.10 | 25.55 | 21.49 | |
| C | 92.71 | 79.07 | 32.05 | 30.91 | 42.78 | 62.41 | 23.32 | 51.90 | |
| R2 | −0.05 | 0.93 | 0.95 | 0.88 | 0.98 | 0.57 | 0.99 | 0.99 | |
| kt3 | 2.94 | 6.63 | 3.88 | 7.01 | 1.92 | 2.63 | |||
| C | 112.5 | 118.78 | 105.97 | 92.39 | 105.31 | 117.95 | |||
| R2 | 0.74 | 0.95 | 0.85 | 0.84 | 0.77 | 0.72 | |||
Cd(Ⅱ) | kt1 | 30.22 | 34.12 | 49.1 | 63.21 | 44.55 | 64.08 | 67.25 | 56.88 |
| C | −16.73 | −18.4 | −18.65 | −17.15 | −15.25 | −17.65 | −34.74 | −12.83 | |
| R2 | 0.99 | 0.99 | 0.99 | 0.97 | 0.98 | 0.98 | 0.99 | 0.96 | |
| kt2 | 3.80 | 3.80 | 24.88 | 20.25 | 28.06 | 22.09 | 21.39 | 22.47 | |
| C | 81.97 | 96.74 | 28.96 | 61.58 | 10.75 | 55.19 | 51.34 | 63.3 | |
| R2 | 0.85 | 0.83 | 0.98 | 0.99 | 0.98 | 0.96 | 0.92 | 0.91 | |
| kt3 | 5.67 | 7.71 | 6.31 | 5.27 | 4.03 | 7.24 | |||
| C | 99.88 | 104.27 | 96.3 | 117.91 | 116.52 | 120 | |||
| R2 | 0.87 | 0.99 | 0.82 | 0.86 | 0.96 | 0.99 | |||
Pb(Ⅱ) | kt1 | 35.94 | 46.76 | 78.16 | 71.96 | 57.52 | 88.54 | 75.94 | 75.25 |
| C | −19.78 | −25.62 | −35.53 | −32.99 | −23.33 | −41.00 | −35.99 | −34.86 | |
| R2 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.98 | 0.99 | 0.97 | |
| kt2 | 4.00 | 38.29 | 26.11 | 38.29 | 35.45 | 39.10 | 25.07 | 31.61 | |
| C | 95.43 | 108.55 | 64.71 | 30.47 | 17.51 | 46.42 | 49.52 | 37.57 | |
| R2 | 0.87 | 0.59 | 0.98 | 0.95 | 0.99 | 0.96 | 0.95 | 0.75 | |
| kt3 | 5.31 | 5.01 | 4.48 | 8.82 | 9.30 | 10.65 | |||
| C | 139.59 | 149.77 | 122.85 | 149.39 | 106.79 | 119.58 | |||
| R2 | 0.69 | 0.85 | 0.98 | 0.84 | 0.86 | 0.82 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Adyel, T.M.; Wang, Z.; Wu, J.; Liu, J.; Miao, L.; Hou, J. Effects of Biofilms on Trace Metal Adsorption on Plastics in Freshwater Systems. Int. J. Environ. Res. Public Health 2022, 19, 13752. https://doi.org/10.3390/ijerph192113752
Liu Z, Adyel TM, Wang Z, Wu J, Liu J, Miao L, Hou J. Effects of Biofilms on Trace Metal Adsorption on Plastics in Freshwater Systems. International Journal of Environmental Research and Public Health. 2022; 19(21):13752. https://doi.org/10.3390/ijerph192113752
Chicago/Turabian StyleLiu, Zhilin, Tanveer M. Adyel, Zhiyuan Wang, Jun Wu, Jianchao Liu, Lingzhan Miao, and Jun Hou. 2022. "Effects of Biofilms on Trace Metal Adsorption on Plastics in Freshwater Systems" International Journal of Environmental Research and Public Health 19, no. 21: 13752. https://doi.org/10.3390/ijerph192113752
APA StyleLiu, Z., Adyel, T. M., Wang, Z., Wu, J., Liu, J., Miao, L., & Hou, J. (2022). Effects of Biofilms on Trace Metal Adsorption on Plastics in Freshwater Systems. International Journal of Environmental Research and Public Health, 19(21), 13752. https://doi.org/10.3390/ijerph192113752










