Potential Adsorption Affinity of Estrogens on LDPE and PET Microplastics Exposed to Wastewater Treatment Plant Effluents
Abstract
1. Introduction
2. Materials and Methods
2.1. Microplastics
2.2. Microplastic Exposure in Wastewater Treatment Plant
2.3. Experimental Validation for Quality Control
2.4. Analytical Procedures
2.5. Statistical Analysis of Data
3. Results
3.1. Estrogen Concentrations Detected on LDPE and PET Microplastic Pellets
3.2. Elimination Efficiency of Estrogens under Influent and Effluent Treatment Conditions
3.3. Analysis of Virgin MPs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiseo, I. Plastic Waste Worldwide—Statistics & Facts; Statista: Hamburg, Germany, 2022. [Google Scholar]
- Ono, S.; Sirisena, A.H.H.T.; Visvanathan, C. Circularity in Plastic Waste Management: A Cross-Country Comparison between Japan and Sri Lanka; Research Square: Durham, NC, USA, 2022. [Google Scholar]
- Yu, Y.; Mo, W.Y.; Luukkonen, T. Adsorption behaviour and interaction of organic micropollutants with nano and microplastics—A review. Sci. Total Environ. 2021, 797, 149140. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.H.; Liang, Y.; Kim, M.; Byun, J.; Choi, H. Microplastics with adsorbed contaminants: Mechanisms and Treatment. Environ. Chall. 2021, 3, 100042. [Google Scholar] [CrossRef]
- Kumar, R.; Verma, A.; Shome, A.; Sinha, R.; Sinha, S.; Jha, P.K.; Kumar, R.; Kumar, P.; Shubham; Das, S.; et al. Impacts of Plastic Pollution on Ecosystem Services, Sustainable Development Goals, and Need to Focus on Circular Economy and Policy Interventions. Sustainability 2021, 13, 9963. [Google Scholar] [CrossRef]
- Chen, C.; Chen, L.; Li, Y.; Fu, W.; Shi, X.; Duan, J.; Zhang, W. Impacts of microplastics on organotins’ photodegradation in aquatic environments. Environ. Pollut. 2020, 267, 115686. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Emenike, P.; Araoye, O.; Academe, S.; Unokiwedi, P.; Omole, D. The effects of microplastics in oceans and marine environment on public health—A mini-review. IOP Conf. Ser. Earth Environ. Sci. 2022, 993, 012019. [Google Scholar] [CrossRef]
- Mai, L.; He, H.; Bao, L.-J.; Liu, L.-Y.; Zeng, E.Y. Plastics are an insignificant carrier of riverine organic pollutants to the coastal oceans. Environ. Sci. Technol. 2020, 54, 15852–15860. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Hamidian, A.H.; Ozumchelouei, E.J.; Feizi, F.; Wu, C.; Zhang, Y.; Yang, M. A review on the characteristics of microplastics in wastewater treatment plants: A source for toxic chemicals. J. Clean. Prod. 2021, 295, 126480. [Google Scholar]
- Gallo, F.; Fossi, C.; Weber, R.; Santillo, D.; Sousa, J.; Ingram, I.; Nadal, A.; Romano, D. Marine litter plastics and microplastics and their toxic chemicals components: The need for urgent preventive measures. Environ. Sci. Eur. 2018, 30, 13. [Google Scholar] [CrossRef]
- Galloway, T.S.; Cole, M.; Lewis, C. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. 2017, 1, 0116. [Google Scholar] [CrossRef] [PubMed]
- Mato, Y.; Isobe, T.; Takada, H.; Kanehiro, H.; Ohtake, C.; Kaminuma, T. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ. Sci. Technol. 2001, 35, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Ogata, Y.; Takada, H.; Mizukawa, K.; Hirai, H.; Iwasa, S.; Endo, S.; Mato, Y.; Saha, M.; Okuda, K.; Nakashima, A. International Pellet Watch: Global monitoring of persistent organic pollutants (POPs) in coastal waters. 1. Initial phase data on PCBs, DDTs, and HCHs. Mar. Pollut. Bull. 2009, 58, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Shim, W.J.; Kwon, J.-H. Sorption capacity of plastic debris for hydrophobic organic chemicals. Sci. Total Environ. 2014, 470, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.R.; Gold, H.S. Polypropylene as an adsorbent for trace organics in water. Anal. Chem. 1984, 56, 1436–1440. [Google Scholar] [CrossRef]
- McDougall, L.; Thomson, L.; Brand, S.; Wagstaff, A.; Lawton, L.A.; Petrie, B. Adsorption of a diverse range of pharmaceuticals to polyethylene microplastics in wastewater and their desorption in environmental matrices. Sci. Total Environ. 2022, 808, 152071. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic contamination in an urban area: A case study in Greater Paris. Environ. Chem. 2015, 12, 592–599. [Google Scholar] [CrossRef]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef]
- Leslie, H.; Brandsma, S.; Van Velzen, M.; Vethaak, A. Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 2017, 101, 133–142. [Google Scholar] [CrossRef]
- Mason, S.A.; Garneau, D.; Sutton, R.; Chu, Y.; Ehmann, K.; Barnes, J.; Fink, P.; Papazissimos, D.; Rogers, D.L. Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environ. Pollut. 2016, 218, 1045–1054. [Google Scholar] [CrossRef]
- Mintenig, S.M.; Int-Veen, I.; Löder, M.G.; Primpke, S.; Gerdts, G. Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. 2017, 108, 365–372. [Google Scholar] [CrossRef]
- Magnusson, K.; Norén, F. Screening of Microplastic Particles in and Down-Stream a Wastewater Treatment Plant; Swedish Environmental Protection Agency: Stockholm, Sweden, 2014; p. 22.
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef] [PubMed]
- Talvitie, J.; Heinonen, M.; Pääkkönen, J.-P.; Vahtera, E.; Mikola, A.; Setälä, O.; Vahala, R. Do wastewater treatment plants act as a potential point source of microplastics? Preliminary study in the coastal Gulf of Finland, Baltic Sea. Water Sci. Technol. 2015, 72, 1495–1504. [Google Scholar] [PubMed]
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to microplastic pollution–Removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res. 2017, 123, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Sutton, R.; Mason, S.; Stanek, S.; Willis-Norton, E.; Wren, I.; Box, C. Microplastic contamination in the San Francisco Bay, California, USA. Mar. Pollut. Bull. 2016, 109, 230–235. [Google Scholar] [CrossRef]
- Vermaire, J.C.; Pomeroy, C.; Herczegh, S.M.; Haggart, O.; Murphy, M. Microplastic abundance and distribution in the open water and sediment of the Ottawa River, Canada, and its tributaries. Facets 2017, 2, 301–314. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 2017, 112, 93–99. [Google Scholar]
- Yang, X.-D.; Gong, B.; Chen, W.; Qian, C.; Du, M.; Yu, H.-Q. In-situ quantitative monitoring the organic contaminants uptake onto suspended microplastics in aquatic environments. Water Res. 2022, 215, 118235. [Google Scholar]
- Wright, S.L.; Ulke, J.; Font, A.; Chan, K.L.A.; Kelly, F.J. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ. Int. 2020, 136, 105411. [Google Scholar] [CrossRef]
- Roch, S.; Friedrich, C.; Brinker, A. Uptake routes of microplastics in fishes: Practical and theoretical approaches to test existing theories. Sci. Rep. 2020, 10, 3896. [Google Scholar] [CrossRef]
- Ory, N.; Sobral, P.; Ferreira, J.; Thiel, M. Amberstripe scad Decapterus muroadsi (Carangidae) fish ingest blue microplastics resembling their copepod prey along the coast of Rapa Nui (Easter Island) in the South Pacific subtropical gyre. Sci. Total Environ. 2017, 586, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Tahir, A.; Williams, S.L.; Baxa, D.V.; Lam, R.; Miller, J.T.; Teh, F.-C.; Werorilangi, S.; Teh, S.J. Anthropogenic debris in seafood: Plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Sci. Rep. 2015, 5, 14340. [Google Scholar] [CrossRef] [PubMed]
- Collard, F.; Gilbert, B.; Compère, P.; Eppe, G.; Das, K.; Jauniaux, T.; Parmentier, E. Microplastics in livers of European anchovies (Engraulis encrasicolus, L.). Environ. Pollut. 2017, 229, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Soltani, N.; Keshavarzi, B.; Moore, F.; Turner, A.; Hassanaghaei, M. Microplastics in different tissues of fish and prawn from the Musa Estuary, Persian Gulf. Chemosphere 2018, 205, 80–87. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Lopes, C.; Oliveira, P.; Bessa, F.; Otero, V.; Henriques, B.; Raimundo, J.; Caetano, M.; Vale, C.; Guilhermino, L. Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci. Total Environ. 2020, 717, 134625. [Google Scholar] [CrossRef]
- Wang, W.; Ge, J.; Yu, X. Bioavailability and toxicity of microplastics to fish species: A review. Ecotoxicol. Environ. Saf. 2020, 189, 109913. [Google Scholar] [CrossRef]
- Hjelmeland, K.; Pedersen, B.; Nilssen, E. Trypsin content in intestines of herring larvae, Clupea harengus, ingesting inert polystyrene spheres or live crustacea prey. Mar. Biol. 1988, 98, 331–335. [Google Scholar] [CrossRef]
- Vethaak, A.D.; Leslie, H.A. Plastic debris is a human health issue. Environ. Sci. Technol. 2016, 50, 6825–6826. [Google Scholar] [CrossRef]
- Gray, A.; Weinstein, J. Size- and shape-dependent effects of microplastic particles on adult daggerblade grass shrimp (Palaemonetes pugio). Environ. Toxicol. Chem. 2017, 36, 3074–3080. [Google Scholar] [CrossRef]
- Endo, S.; Takizawa, R.; Okuda, K.; Takada, H.; Chiba, K.; Kanehiro, H.; Ogi, H.; Yamashita, R.; Date, T. Concentration of polychlorinated biphenyls (PCBs) in beached resin pellets: Variability among individual particles and regional differences. Mar. Pollut. Bull. 2005, 50, 1103–1114. [Google Scholar] [CrossRef]
- Hüffer, T.; Weniger, A.-K.; Hofmann, T. Sorption of organic compounds by aged polystyrene microplastic particles. Environ. Pollut. 2018, 236, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Liu, F.; Brookes, P.C.; Xu, J. Microplastics play a minor role in tetracycline sorption in the presence of dissolved organic matter. Environ. Pollut. 2018, 240, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Fok, L.; Cheung, P.K.; Tang, G.; Li, W.C. Size distribution of stranded small plastic debris on the coast of Guangdong, South China. Environ. Pollut. 2017, 220, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Abayomi, O.A.; Range, P.; Al-Ghouti, M.A.; Obbard, J.P.; Almeer, S.H.; Ben-Hamadou, R. Microplastics in coastal environments of the Arabian Gulf. Mar. Pollut. Bull. 2017, 124, 181–188. [Google Scholar] [CrossRef]
- Ruiz-Compean, P.; Ellis, J.; Cúrdia, J.; Payumo, R.; Langner, U.; Jones, B.; Carvalho, S. Baseline evaluation of sediment contamination in the shallow coastal areas of Saudi Arabian Red Sea. Mar. Pollut. Bull. 2017, 123, 205–218. [Google Scholar] [CrossRef]
- Baalkhuyur, F.; Bin Dohaish, A.J.; Elhalwagy, M.; Alikunhi, N.; AlSuwailem, A.; Røstad, A.; Coker, D.; Berumen, M.; Duarte, C. Microplastic in the gastrointestinal tract of fishes along the Saudi Arabian Red Sea coast. Mar. Pollut. Bull. 2018, 131, 407–415. [Google Scholar] [CrossRef]
- Aliabad, M.K.; Nassiri, M.; Kor, K. Microplastics in the surface seawaters of Chabahar Bay, Gulf of Oman (Makran coasts). Mar. Pollut. Bull. 2019, 143, 125–133. [Google Scholar] [CrossRef]
- Engler, R.E. The complex interaction between marine debris and toxic chemicals in the ocean. Environ. Sci. Technol. 2012, 46, 12302–12315. [Google Scholar] [CrossRef]
- Backhaus, T.; Wagner, M. Microplastics in the environment: Much ado about nothing? A debate. Glob. Chall. 2020, 4, 1900022. [Google Scholar] [CrossRef]
- Scholz, S.; Klüver, N. Effects of endocrine disrupters on sexual, gonadal development in fish. Sex. Dev. 2009, 3, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Senior, A.M.; Nakagawa, S. A comparative analysis of chemically induced sex reversal in teleosts: Challenging conventional suppositions. Fish Fish. 2013, 14, 60–76. [Google Scholar] [CrossRef]
- Katti, P.A.; Goundadkar, B.B. Waves of follicle development, growth and degeneration in adult ovary of zebrafish (Danio rerio) on chronic exposure to environmental estrogens in laboratory. Reprod. Toxicol. 2022, 110, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kernen, L.; Phan, A.; Bo, J.; Herzog, E.L.; Huynh, J.; Segner, H.; Baumann, L. Estrogens as immunotoxicants: 17α-ethinylestradiol exposure retards thymus development in zebrafish (Danio Rerio). Aquat. Toxicol. 2022, 242, 106025. [Google Scholar] [CrossRef]
- Roy, B.; Basak, R.; Rai, U. Impact of xenoestrogens on sex differentiation and reproduction in teleosts. Aquac. Fish. 2022, 7, 562–571. [Google Scholar] [CrossRef]
- Saeed, T.; Al-Jandal, N.; Abusam, A.; Taqi, H.; Al-Khabbaz, A.; Zafar, J. Sources and levels of endocrine disrupting compounds (EDCs) in Kuwait’s coastal areas. Mar. Pollut. Bull. 2017, 118, 407–412. [Google Scholar] [CrossRef]
- Abusam, A. Fate of Estrogens in Kuwaiti Municipal Wastewater Treatment Plants. In Proceedings of the 14th Gulf Water Conference: Water in the GCC… Towards Economic Efficiency and Financial Sustainability, Manama, Bahrain, 13–15 February 2022; Volume 13, p. 15. [Google Scholar]
- Abusam, A.; Saeed, T.; Al-Jandal, N. Removal of Estrogens in Kuwaiti Municipal Wastewater Treatment Plants. J. Environ. Treat. Tech. 2021, 9, 642–646. [Google Scholar]
- Al-Jandal, N.; Saeed, T.; Azad, I.; Al-Subiai, S.; Al-Zekri, W.; Hussain, S.; Al-Hasan, E. Impact of endocrine disrupting compounds in sewage impacted coastal area on seabream. Ecotoxicol. Environ. Saf. 2018, 150, 280–288. [Google Scholar] [CrossRef]
- Snyder, S.A.; Keith, T.L.; Verbrugge, D.A.; Snyder, E.M.; Gross, T.S.; Kannan, K.; Giesy, J.P. Analytical methods for detection of selected estrogenic compounds in aqueous mixtures. Environ. Sci. Technol. 1999, 33, 2814–2820. [Google Scholar] [CrossRef]
- Aerni, H.-R.; Kobler, B.; Rutishauser, B.V.; Wettstein, F.E.; Fischer, R.; Giger, W.; Hungerbühler, A.; Marazuela, M.D.; Peter, A.; Schönenberger, R. Combined biological and chemical assessment of estrogenic activities in wastewater treatment plant effluents. Anal. Bioanal. Chem. 2004, 378, 688–696. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.-H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions—A review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef] [PubMed]
- Mohagheghian, A.; Nabizadeh, R.; Mesdghinia, A.; Rastkari, N.; Mahvi, A.H.; Alimohammadi, M.; Yunesian, M.; Ahmadkhaniha, R.; Nazmara, S. Distribution of estrogenic steroids in municipal wastewater treatment plants in Tehran, Iran. J. Environ. Health Sci. Eng. 2014, 12, 97. [Google Scholar] [CrossRef] [PubMed]
- Cortes, L.G.; Marinov, D.; Sanseverino, I.; Cuenca, A.N.; Niegowska, M.; Rodriguez, E.P.; Lettieri, T. Selection of substances for the 3rd Watch List under the Water Framework Directive. Off. Eur. Union Luxemb. 2020. [Google Scholar] [CrossRef]
- Verla, A.W.; Enyoh, C.E.; Verla, E.N.; Nwarnorh, K.O. Microplastic–toxic chemical interaction: A review study on quantified levels, mechanism and implication. SN Appl. Sci. 2019, 1, 1400. [Google Scholar] [CrossRef]
- Fisner, M.; Majer, A.; Taniguchi, S.; Bícego, M.; Turra, A.; Gorman, D. Colour spectrum and resin-type determine the concentration and composition of Polycyclic Aromatic Hydrocarbons (PAHs) in plastic pellets. Mar. Pollut. Bull. 2017, 122, 323–330. [Google Scholar] [CrossRef]
- Frias, J.P.; Antunes, J.C.; Sobral, P. Local marine litter survey—A case study in Alcobaça municipality, Portugal. Rev. Gestão Costeira Integr. J. Integr. Coast. Zone Manag. 2013, 13, 169–179. [Google Scholar]
- Llorca, M.; Schirinzi, G.; Martínez, M.; Barceló, D.; Farré, M. Adsorption of perfluoroalkyl substances on microplastics under environmental conditions. Environ. Pollut. 2018, 235, 680–691. [Google Scholar] [CrossRef]
- Ma, Z.F.; Ibrahim, Y.S.; Lee, Y.Y. Microplastic Pollution and Health and Relevance to the Malaysia’s Roadmap to Zero Single-Use Plastics 2018-2030. Malays. J. Med. Sci. 2020, 27, 1–6. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of antibiotics on microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef]
- Wang, F.; Shih, K.M.; Li, X.Y. The partition behavior of perfluorooctanesulfonate (PFOS) and perfluorooctanesulfonamide (FOSA) on microplastics. Chemosphere 2015, 119, 841–847. [Google Scholar] [CrossRef]
- Amelia, T.S.M.; Khalik, W.M.A.W.M.; Ong, M.C.; Shao, Y.T.; Pan, H.-J.; Bhubalan, K. Marine microplastics as vectors of major ocean pollutants and its hazards to the marine ecosystem and humans. Prog. Earth Planet. Sci. 2021, 8, 12. [Google Scholar] [CrossRef]
- Pascall, M.A.; Zabik, M.E.; Zabik, M.J.; Hernandez, R.J. Uptake of polychlorinated biphenyls (PCBs) from an aqueous medium by polyethylene, polyvinyl chloride, and polystyrene films. J. Agric. Food Chem. 2005, 53, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2027–2045. [Google Scholar] [CrossRef] [PubMed]
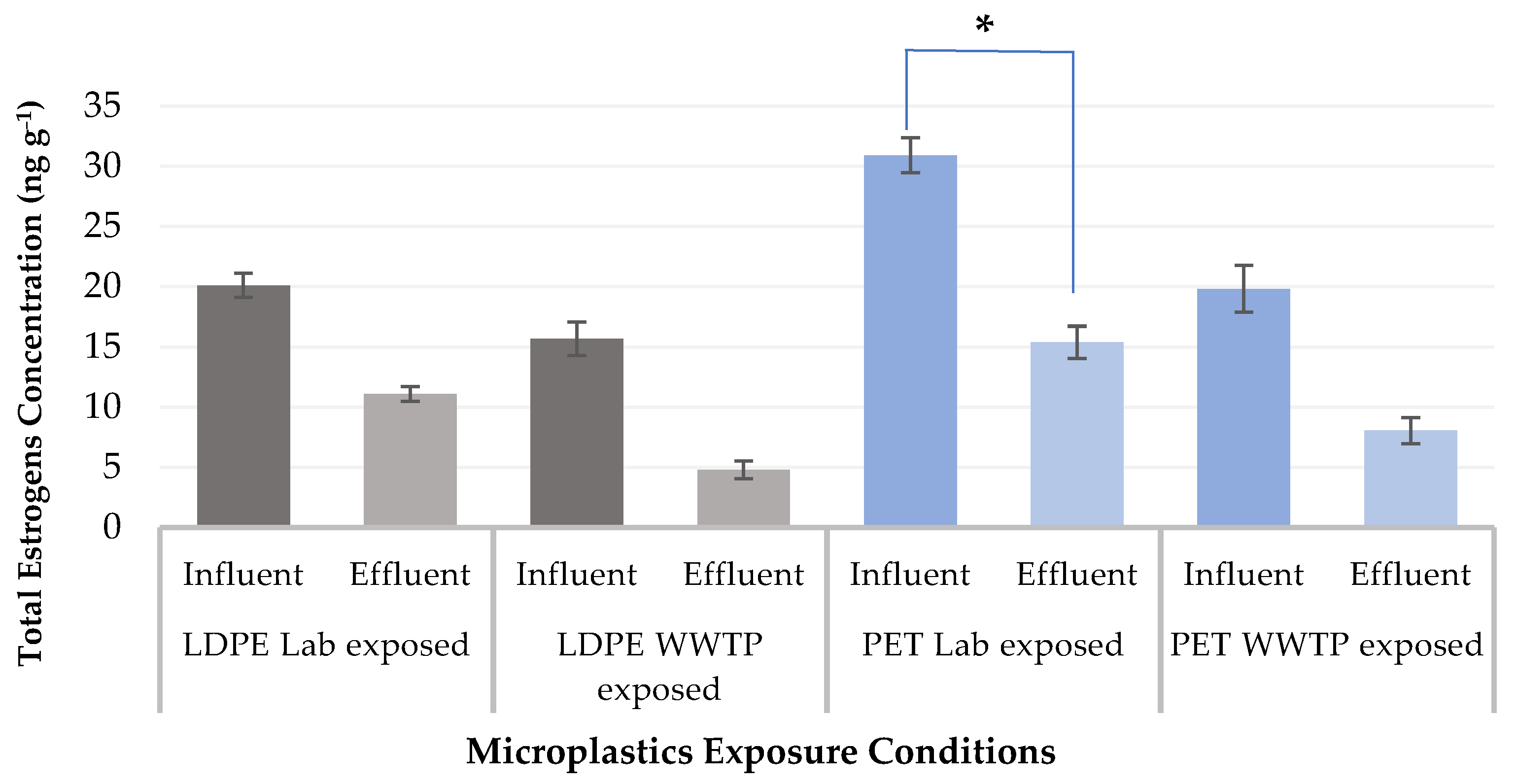
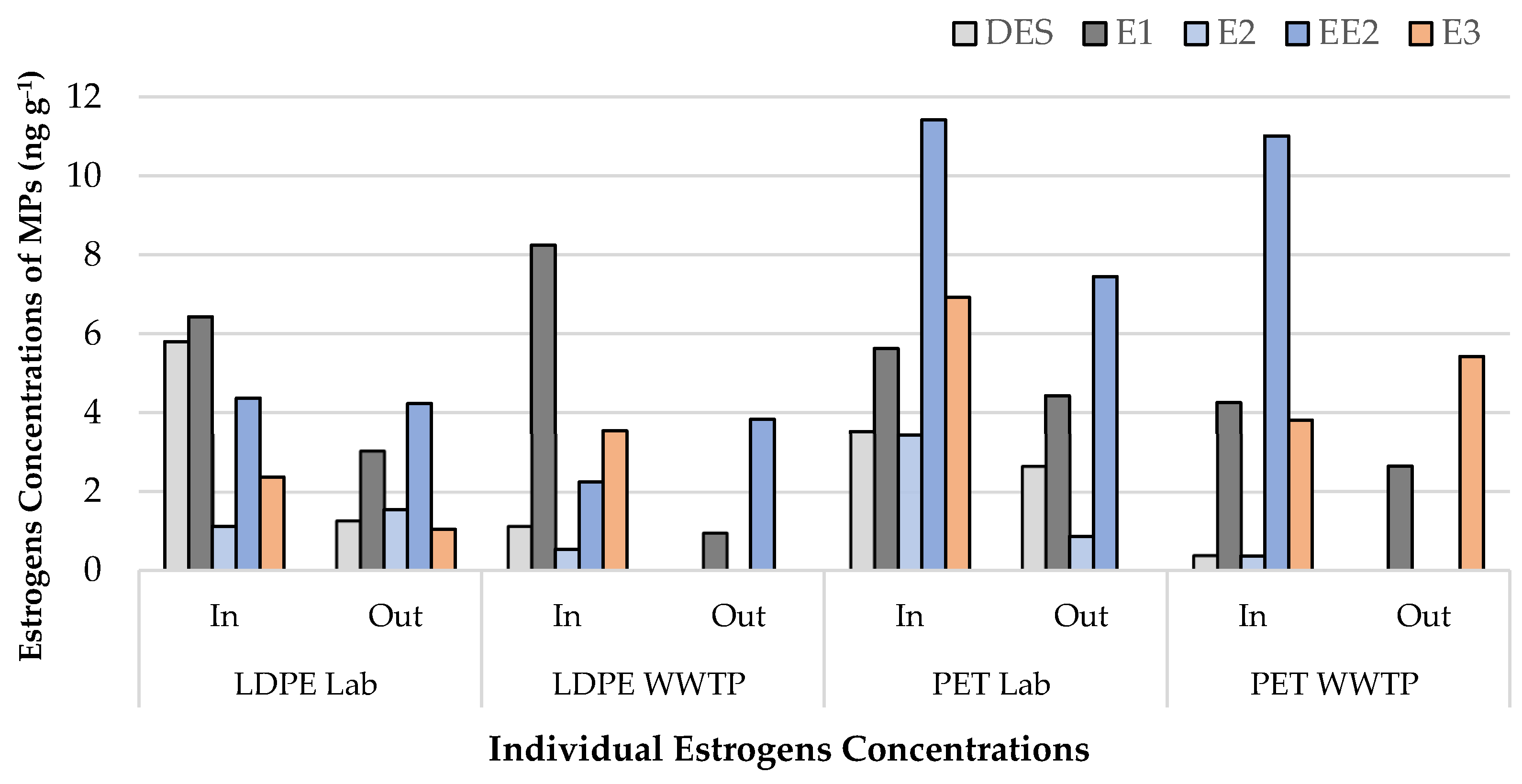
| Estrogens | LDPE Lab In | LDPE Lab Out | p- Value | LDPE WWTP In | LDPE WWTP Out | p- Value | PET Lab In | PET Lab Out | p- Value | PET WWTP In | PET WWTP Out | p- Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DES | 5.80 | 1.27 | 0.13 | 1.13 | 0.00 | 0.22 | 3.52 | 2.65 | 0.04 * | 0.39 | 0.00 | 0.37 |
| E1 | 6.43 | 3.04 | 8.24 | 0.96 | 5.62 | 4.43 | 4.25 | 2.64 | ||||
| E2 | 1.12 | 1.54 | 0.54 | 0.00 | 3.43 | 0.86 | 0.36 | 0.00 | ||||
| EE2 | 4.37 | 4.23 | 2.24 | 3.83 | 11.42 | 7.45 | 11.01 | 0.00 | ||||
| E3 | 2.37 | 1.04 | 3.54 | 0.00 | 6.92 | 0.00 | 3.81 | 5.42 | ||||
| Σ Estrogens | 20.09 | 11.13 | 15.68 | 4.79 | 30.91 | 15.38 | 19.82 | 8.06 |
| Microplastic Virgin Pellets | DES | E1 | E2 | EE2 | E3 |
|---|---|---|---|---|---|
| PET Sample 1 | ND | ND | ND | ND | ND |
| PET Sample 2 | ND | ND | ND | ND | ND |
| LDPE Sample 1 | ND | ND | ND | ND | ND |
| LDPE Sample 2 | ND | ND | ND | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Jandal, N.; AlKhubaizi, A.; Saeed, T.; Hajeyah, M. Potential Adsorption Affinity of Estrogens on LDPE and PET Microplastics Exposed to Wastewater Treatment Plant Effluents. Int. J. Environ. Res. Public Health 2022, 19, 16027. https://doi.org/10.3390/ijerph192316027
Al-Jandal N, AlKhubaizi A, Saeed T, Hajeyah M. Potential Adsorption Affinity of Estrogens on LDPE and PET Microplastics Exposed to Wastewater Treatment Plant Effluents. International Journal of Environmental Research and Public Health. 2022; 19(23):16027. https://doi.org/10.3390/ijerph192316027
Chicago/Turabian StyleAl-Jandal, Noura, Abdulaziz AlKhubaizi, Talat Saeed, and Mariam Hajeyah. 2022. "Potential Adsorption Affinity of Estrogens on LDPE and PET Microplastics Exposed to Wastewater Treatment Plant Effluents" International Journal of Environmental Research and Public Health 19, no. 23: 16027. https://doi.org/10.3390/ijerph192316027
APA StyleAl-Jandal, N., AlKhubaizi, A., Saeed, T., & Hajeyah, M. (2022). Potential Adsorption Affinity of Estrogens on LDPE and PET Microplastics Exposed to Wastewater Treatment Plant Effluents. International Journal of Environmental Research and Public Health, 19(23), 16027. https://doi.org/10.3390/ijerph192316027






