Acute Exposure to Simulated Nocturnal Train Noise Leads to Impaired Sleep Quality and Endothelial Dysfunction in Young Healthy Men and Women: A Sex-Specific Analysis
Abstract
1. Introduction
2. Methods
2.1. ZuG Study
2.2. Examinations
2.3. Statistical Analysis
3. Results
3.1. Baseline/Screening Characteristics of the Study Sample
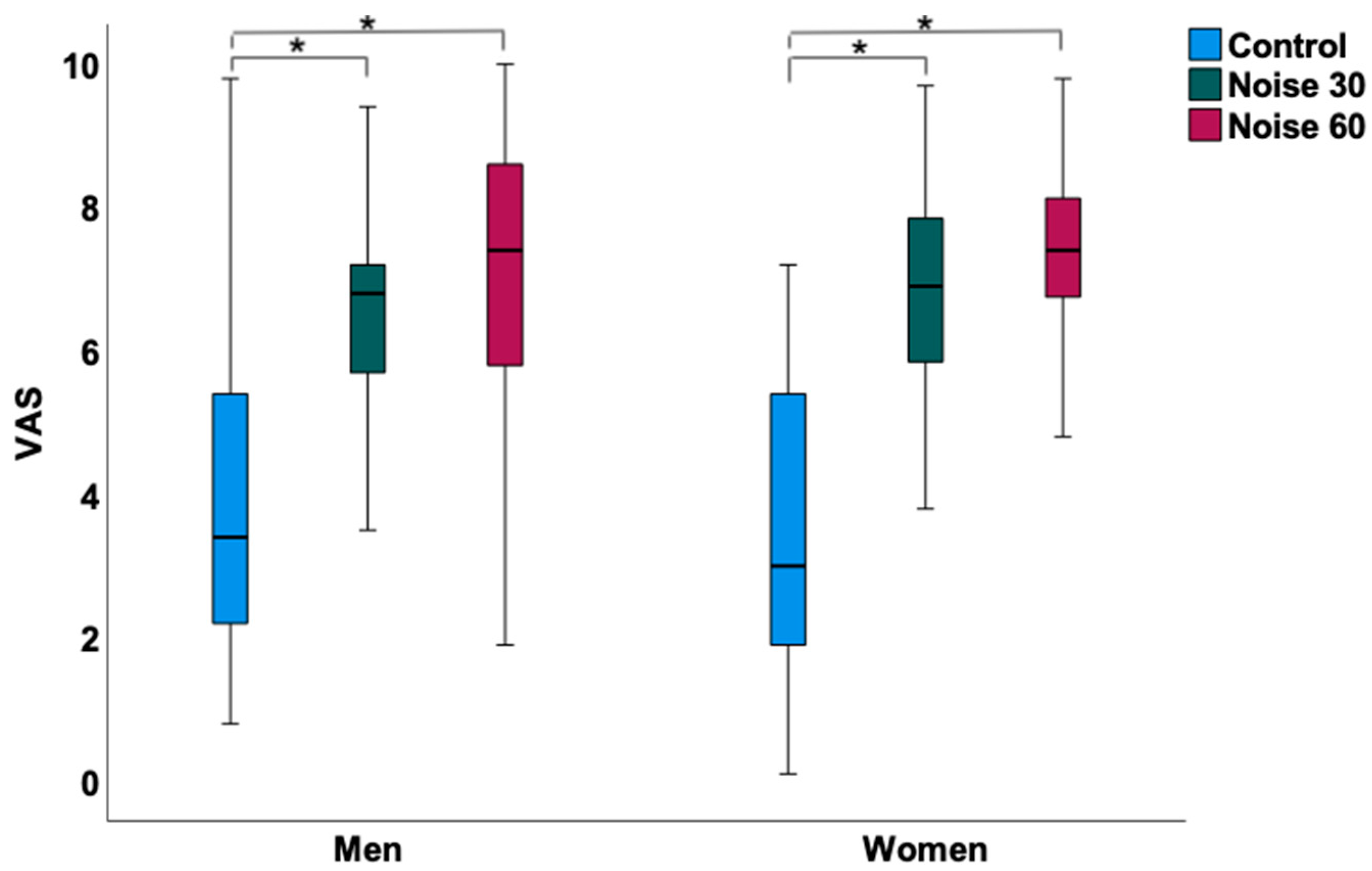
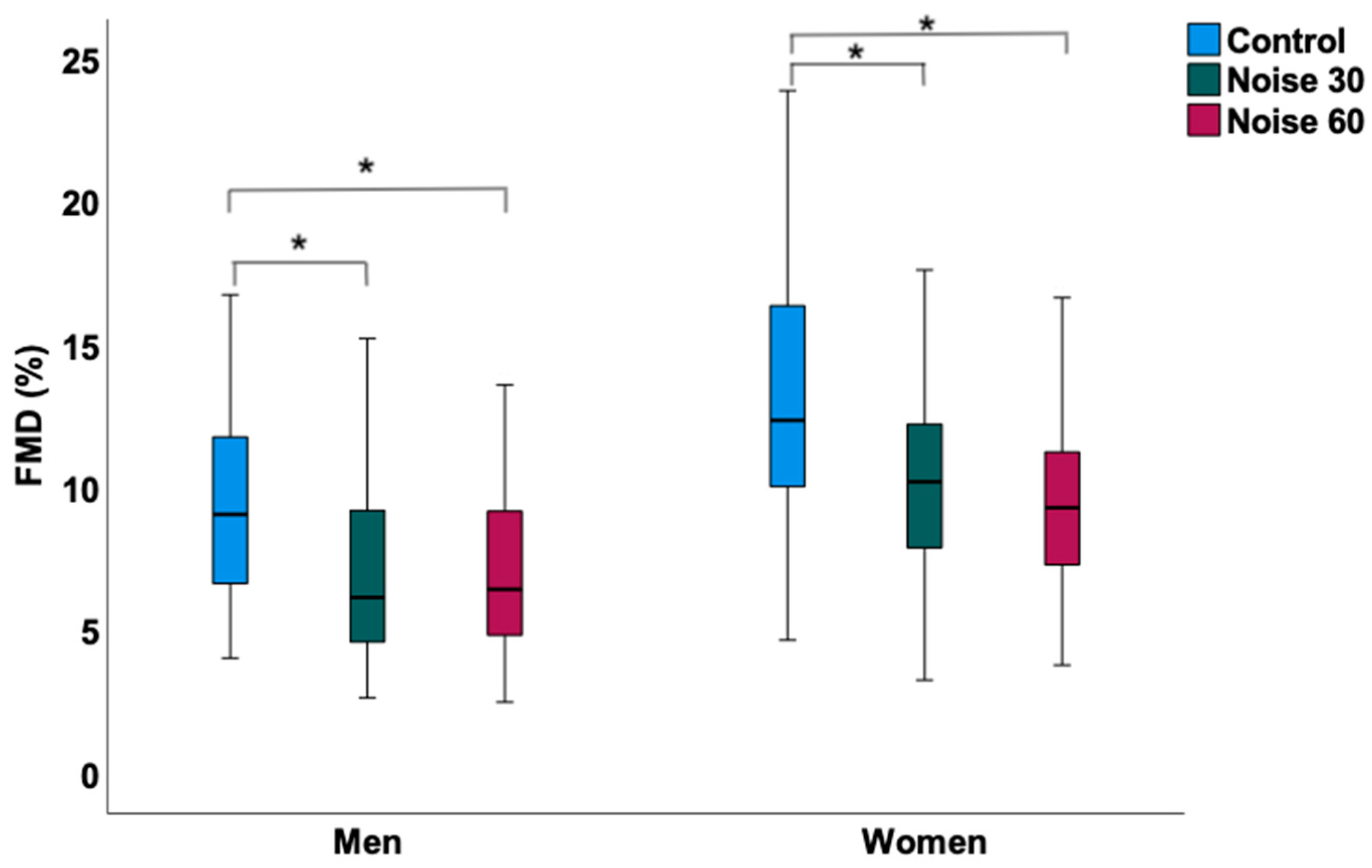
3.2. Effects of Nocturnal Train Noise on Sleep Quality, Hemodynamic, and Laboratory Parameters in Women
3.3. Effects of Nocturnal Train Noise on Sleep Quality, Hemodynamic, and Laboratory Parameters in Men
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hahad, O.; Kroller-Schon, S.; Daiber, A.; Munzel, T. The Cardiovascular Effects of Noise. Dtsch. Arztebl. Int. 2019, 116, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.P.; Basner, M.; Kroger, G.; Weck, S.; Schnorbus, B.; Muttray, A.; Sariyar, M.; Binder, H.; Gori, T.; Warnholtz, A.; et al. Effect of nighttime aircraft noise exposure on endothelial function and stress hormone release in healthy adults. Eur. Heart J. 2013, 34, 3508–3514. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.; Kolle, K.; Kreuder, K.; Schnorbus, B.; Wild, P.; Hechtner, M.; Binder, H.; Gori, T.; Munzel, T. Nighttime aircraft noise impairs endothelial function and increases blood pressure in patients with or at high risk for coronary artery disease. Clin. Res. Cardiol. 2015, 104, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Munzel, T.; Sinning, C.; Post, F.; Warnholtz, A.; Schulz, E. Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann. Med. 2008, 40, 180–196. [Google Scholar] [CrossRef]
- Hahad, O.; Arnold, N.; Prochaska, J.H.; Panova-Noeva, M.; Schulz, A.; Lackner, K.J.; Pfeiffer, N.; Schmidtmann, I.; Michal, M.; Beutel, M.; et al. Cigarette Smoking Is Related to Endothelial Dysfunction of Resistance, but Not Conduit Arteries in the General Population-Results From the Gutenberg Health Study. Front. Cardiovasc. Med. 2021, 8, 674622. [Google Scholar] [CrossRef]
- Herzog, J.; Schmidt, F.P.; Hahad, O.; Mahmoudpour, S.H.; Mangold, A.K.; Garcia Andreo, P.; Prochaska, J.; Koeck, T.; Wild, P.S.; Sorensen, M.; et al. Acute exposure to nocturnal train noise induces endothelial dysfunction and pro-thromboinflammatory changes of the plasma proteome in healthy subjects. Basic Res. Cardiol. 2019, 114, 46. [Google Scholar] [CrossRef]
- Beheshti, M.H.; Taban, E.; Samaei, S.E.; Faridan, M.; Khajehnasiri, F.; Khaveh, L.T.; Jebeli, M.B.; Mehri, A.; Tajpoor, A. The influence of personality traits and gender on noise annoyance in laboratory studies. Personal. Individ. Differ. 2019, 148, 95–100. [Google Scholar] [CrossRef]
- Roosli, M.; Mohler, E.; Frei, P.; Vienneau, D. Noise-related sleep disturbances: Does gender matter? Noise Health 2014, 16, 197–204. [Google Scholar] [CrossRef]
- Eriksson, C.; Bluhm, G.; Hilding, A.; Ostenson, C.G.; Pershagen, G. Aircraft noise and incidence of hypertension—Gender specific effects. Environ. Res. 2010, 110, 764–772. [Google Scholar] [CrossRef]
- Jarup, L.; Babisch, W.; Houthuijs, D.; Pershagen, G.; Katsouyanni, K.; Cadum, E.; Dudley, M.L.; Savigny, P.; Seiffert, I.; Swart, W.; et al. Hypertension and exposure to noise near airports: The HYENA study. Environ. Health Perspect. 2008, 116, 329–333. [Google Scholar] [CrossRef]
- Babisch, W. Road traffic noise and cardiovascular risk. Noise Health 2008, 10, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Koczorowski, M.; Bernard, N.; Mauny, F.; Chague, F.; Pujol, S.; Maza, M.; Cottin, Y.; Zeller, M.; Group, E.-M.S. Environmental noise exposure is associated with atherothrombotic risk. Sci. Rep. 2022, 12, 3151. [Google Scholar] [CrossRef] [PubMed]
- Zisberg, A.; Gur-Yaish, N.; Shochat, T. Contribution of routine to sleep quality in community elderly. Sleep 2010, 33, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59 (Suppl. 20, 22–33), quiz 34–57. [Google Scholar]
- Schmidt, F.P.; Herzog, J.; Schnorbus, B.; Ostad, M.A.; Lasetzki, L.; Hahad, O.; Schafers, G.; Gori, T.; Sorensen, M.; Daiber, A.; et al. The impact of aircraft noise on vascular and cardiac function in relation to noise event number: A randomized trial. Cardiovasc. Res. 2021, 117, 1382–1390. [Google Scholar] [CrossRef]
- Ostad, M.A.; Eggeling, S.; Tschentscher, P.; Schwedhelm, E.; Boger, R.; Wenzel, P.; Meinertz, T.; Munzel, T.; Warnholtz, A. Flow-mediated dilation in patients with coronary artery disease is enhanced by high dose atorvastatin compared to combined low dose atorvastatin and ezetimibe: Results of the CEZAR study. Atherosclerosis 2009, 205, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Raitakari, O.T.; Adams, M.R.; McCredie, R.J.; Griffiths, K.A.; Stocker, R.; Celermajer, D.S. Oral vitamin C and endothelial function in smokers: Short-term improvement, but no sustained beneficial effect. J. Am. Coll. Cardiol. 2000, 35, 1616–1621. [Google Scholar] [CrossRef]
- Schutte, M.; Marks, A.; Wenning, E.; Griefahn, B. The development of the noise sensitivity questionnaire. Noise Health 2007, 9, 15–24. [Google Scholar] [CrossRef]
- WHO. Environmental Noise Guidelines for the European Region. Available online: https://www.who.int/europe/publications/i/item/9789289053563 (accessed on 6 August 2022).
- EEA. Germany Noise Fact Sheet 2021. Available online: https://www.eea.europa.eu/themes/human/noise/noise-fact-sheets/noise-country-fact-sheets-2021/germany (accessed on 4 October 2022).
- Babisch, W. The Noise/Stress Concept, Risk Assessment and Research Needs. Noise Health 2002, 4, 1–11. [Google Scholar]
- Thacher, J.D.; Poulsen, A.H.; Raaschou-Nielsen, O.; Hvidtfeldt, U.A.; Brandt, J.; Christensen, J.H.; Khan, J.; Levin, G.; Munzel, T.; Sorensen, M. Exposure to transportation noise and risk for cardiovascular disease in a nationwide cohort study from Denmark. Environ. Res. 2022, 211, 113106. [Google Scholar] [CrossRef]
- Thacher, J.D.; Poulsen, A.H.; Hvidtfeldt, U.A.; Raaschou-Nielsen, O.; Ketzel, M.; Jensen, S.S.; Brandt, J.; Valencia, V.H.; Munzel, T.; Sorensen, M. Long-term exposure to transportation noise and risk for atrial fibrillation: A Danish nationwide cohort study. Environ. Res. 2022, 207, 112167. [Google Scholar] [CrossRef] [PubMed]
- Vienneau, D.; Saucy, A.; Schaffer, B.; Fluckiger, B.; Tangermann, L.; Stafoggia, M.; Wunderli, J.M.; Roosli, M.; SNC Study Group. Transportation noise exposure and cardiovascular mortality: 15-years of follow-up in a nationwide prospective cohort in Switzerland. Environ. Int. 2022, 158, 106974. [Google Scholar] [CrossRef] [PubMed]
- Roswall, N.; Pyko, A.; Ogren, M.; Oudin, A.; Rosengren, A.; Lager, A.; Poulsen, A.H.; Eriksson, C.; Segersson, D.; Rizzuto, D.; et al. Long-Term Exposure to Transportation Noise and Risk of Incident Stroke: A Pooled Study of Nine Scandinavian Cohorts. Environ. Health Perspect. 2021, 129, 107002. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, M.; Poulsen, A.H.; Hvidtfeldt, U.A.; Munzel, T.; Thacher, J.D.; Ketzel, M.; Brandt, J.; Christensen, J.H.; Levin, G.; Raaschou-Nielsen, O. Transportation noise and risk of stroke: A nationwide prospective cohort study covering Denmark. Int. J. Epidemiol. 2021, 50, 1147–1156. [Google Scholar] [CrossRef]
- Yankoty, L.I.; Gamache, P.; Plante, C.; Goudreau, S.; Blais, C.; Perron, S.; Fournier, M.; Ragettli, M.S.; Fallah-Shorshani, M.; Hatzopoulou, M.; et al. Long horizontal line term residential exposure to environmental/transportation noise and the incidence of myocardial infarction. Int. J. Hyg. Environ. Health 2021, 232, 113666. [Google Scholar] [CrossRef]
- Selander, J.; Bluhm, G.; Theorell, T.; Pershagen, G.; Babisch, W.; Seiffert, I.; Houthuijs, D.; Breugelmans, O.; Vigna-Taglianti, F.; Antoniotti, M.C.; et al. Saliva cortisol and exposure to aircraft noise in six European countries. Environ. Health Perspect. 2009, 117, 1713–1717. [Google Scholar] [CrossRef]
- Baudin, C.; Lefevre, M.; Selander, J.; Babisch, W.; Cadum, E.; Carlier, M.C.; Champelovier, P.; Dimakopoulou, K.; Huithuijs, D.; Lambert, J.; et al. Saliva cortisol in relation to aircraft noise exposure: Pooled-analysis results from seven European countries. Environ. Health 2019, 18, 102. [Google Scholar] [CrossRef]
- Turner, E.; Dishy, V.; Chung, C.P.; Harris, P.; Pierces, R.; Asanuma, Y.; Oeser, A.; Gebretsadik, T.; Shintani, A.; Raggi, P.; et al. Endothelial function in systemic lupus erythematosus: Relationship to disease activity, cardiovascular risk factors, corticosteroid therapy, and coronary calcification. Vasc. Health Risk Manag. 2005, 1, 357–360. [Google Scholar] [CrossRef]
- Bigert, C.; Bluhm, G.; Theorell, T. Saliva cortisol--a new approach in noise research to study stress effects. Int. J. Hyg. Environ. Health 2005, 208, 227–230. [Google Scholar] [CrossRef]
- Kajantie, E.; Phillips, D.I. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology 2006, 31, 151–178. [Google Scholar] [CrossRef]
- Rompel, S.; Schneider, A.; Peters, A.; Kraus, U.; On Behalf Of The Inger Study, G. Sex/Gender-Differences in the Health Effects of Environmental Noise Exposure on Hypertension and Ischemic Heart Disease-A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 9856. [Google Scholar] [CrossRef] [PubMed]

| Women (n = 35) | Men (n = 35) | p Value | |
|---|---|---|---|
| Age (years) | 24.31 ± 4.44 | 27.17 ± 6.19 | 0.030 |
| Weight (kg) | 60.34 ± 6.82 | 76.66 ± 10.42 | <0.001 |
| BMI (kg/m2) | 21.23 ± 2.18 | 23.42 ± 2.54 | <0.001 |
| NoiSeQ total (0–3) | 1.18 ± 0.36 | 1.35 ± 0.38 | 0.072 |
| NoiSeQ Sleep dimension (0–3) | 0.99 ± 0.51 | 1.23 ± 0.65 | 0.101 |
| PSQI (0–21) | 5.23 ± 1.63 | 5.34 ± 2.15 | 0.803 |
| Attitude towards train noise questionnaire (0–64) | 31.60 ± 5.51 | 31.03 ± 7.05 | 0.709 |
| CRP (mg/L) | 1.65 ± 2.56 | 0.98 ± 1.77 | 0.148 |
| HbA1c (%) | 5.05 ± 0.21 | 5.05 ± 0.38 | 0.908 |
| LDL (mg/dL) | 92.63 ± 27.0 | 100.69 ± 24.74 | 0.197 |
| HDL (mg/dL) | 67.86 ± 13.70 | 55.83 ± 7.62 | <0.001 |
| Total cholesterol (mg/dL) | 177.46 ± 32.08 | 177.37 ± 28.79 | 0.991 |
| Triglycerides (mg/L) | 84.89 ± 38.95 | 104.40 ± 45.89 | 0.059 |
| Creatinine (mg/dL) | 0.79 ± 0.16 | 0.93 ± 0.12 | <0.001 |
| Control | Noise 30 | Noise 60 | p Value | |
|---|---|---|---|---|
| Peak (dB(A)) | 66.44 ± 8.17 | 75.13 ± 3.09 | 73.80 ± 2.78 | <0.001 |
| LAeq (dB(A)) | 32.78 ± 3.91 | 52.58 ± 2.53 | 54.18 ± 2.36 | <0.001 |
| Sleep quality (VAS 0–10 cm) | 3.43 ± 2.02 | 6.72 ± 1.9 | 7.26 ± 1.5 | <0.001 |
| Hemodynamic parameters | ||||
| FMD (%) | 12.90 ± 4.76 | 10.38 ± 3.81 | 9.85 ± 3.90 | <0.001 |
| FMD relative change after vitamin C (%) | 1.49 ± 1.13 | 2.59 ± 1.45 | 2.50 ± 1.43 | 0.032 |
| HR mean (bpm) | 62.2 ± 8.9 | 61.6 ± 8.3 | 62.2 ± 9.2 | 0.713 |
| HR maximum (bpm) | 108.1 ± 16.9 | 107.6 ± 15.6 | 109.9 ± 12.9 | 0.667 |
| HR acceleration index | 156.6 ± 114.5 | 229.0 ± 187.9 | 189.7 ± 151.8 | 0.092 |
| BP systolic mean (mmHg) | 108.8 ± 10.8 | 110.8 ± 10.0 | 106.6 ± 12.9 | 0.103 |
| BP diastolic mean (mmHg) | 69.55 ± 10.8 | 70.31 ± 11.1 | 69.7 ± 10.5 | 0.913 |
| BP rise index | 28.8 ± 37.7 | 33.7 ± 32.8 | 28.9 ± 31.0 | 0.915 |
| PTT mean (m/s) | 325.2 ± 19.7 | 325.7 ± 21.5 | 326.5 ± 21.6 | 0.744 |
| PTT maximum (m/s) | 362.8 ± 58.2 | 375.9 ± 23.2 | 374.3 ± 22.2 | 0.263 |
| PTT minimum (m/s) | 270.4 ± 24.1 | 267.4 ± 25.8 | 264.8 ± 22.2 | 0.634 |
| Laboratory parameters | ||||
| C-reactive protein (mg/L) | 3.20 ± 10.81 | 3.09 ± 11.1 | 1.30 ± 2.10 | 0.914 |
| Neutrophils (%) | 51.8 ± 9.6 | 52.3 ± 11.4 | 52.6 ± 9.8 | 0.867 |
| Cortisol (μg/L) | 17.92 ± 5.84 | 17.75 ± 6.5 | 17.03 ± 5.18 | 0.330 |
| Glucose (mg/dL) | 84.2 ± 4.9 | 83.1 ± 5.5 | 84.7 ± 4.9 | 0.104 |
| Adrenaline (pg/mL) | 28.0 ± 22.01 | 23.3 ± 17.5 | 24.0 ± 16.7 | 0.855 |
| Dopamine (pg/mL) | 11.63 ± 12.3 | 11.47 ± 9.4 | 11.47 ± 9.9 | 0.567 |
| 8-isoprostane (pg/mL) | 39.4 ± 18.86 | 42.10 ± 23.9 | 39.2 ± 18.6 | 0.520 |
| Control | Noise 30 | Noise 60 | p Value | |
|---|---|---|---|---|
| Peak (dB(A)) | 62.65 ± 8.56 | 74.58 ± 3.94 | 74.79 ± 4.61 | <0.001 |
| LAeq (dB(A)) | 33.81 ± 5.13 | 51.47 ± 2.76 | 54.69 ± 2.81 | <0.001 |
| Sleep quality (VAS 0–10 cm) | 3.78 ± 2.12 | 6.52 ± 1.71 | 7.12 ± 1.92 | <0.001 |
| Hemodynamic parameters | ||||
| FMD (%) | 9.56 ± 3.99 | 7.04 ± 3.08 | 7.09 ± 3.01 | <0.001 |
| FMD relative change after vitamin C (%) | 1.44 ± 1.08 | 1.65 ± 0.69 | 1.62 ± 1.15 | 0.768 |
| HR mean (bpm) | 56.9 ± 6.2 | 55.9 ± 7.1 | 56.5 ± 6.6 | 0.474 |
| HR maximum (bpm) | 101.2 ± 10.1 | 105.4 ± 18.0 | 104.7 ± 11.9 | 0.212 |
| HR acceleration index | 153.7 ± 169.9 | 128.0 ± 150.2 | 147.6 ± 140.1 | 0.114 |
| BP systolic mean (mmHg) | 121.5 ± 13.7 | 122.7 ± 13.9 | 121.3 ± 10.7 | 0.853 |
| BP diastolic mean (mmHg) | 76.13 ± 10.4 | 77.77 ± 8.2 | 75.57 ± 9.0 | 0.509 |
| BP rise index | 32.8 ± 41.6 | 28.8 ± 30.6 | 45.9 ± 52.9 | 0.717 |
| PTT mean (m/s) | 341.9 ± 14.8 | 339.0 ± 26.3 | 338.7 ± 15.9 | 0.516 |
| PTT maximum (m/s) | 383.4 ± 15.4 | 383.91 ± 13.5 | 376.3 ± 60.4 | 0.480 |
| PTT minimum (m/s) | 292.5 ± 23.7 | 280.6 ± 27.4 | 280.7 ± 28.2 | 0.052 |
| Laboratory parameters | ||||
| C-reactive protein (mg/L) | 0.77 ± 1.03 | 0.84 ± 1.18 | 0.96 ± 1.67 | 0.720 |
| Neutrophils (%) | 52.1 ± 7.9 | 52.7 ± 5.8 | 53.0 ± 6.8 | 0.716 |
| Cortisol (μg/L) | 13.0 ± 2.53 | 13.35 ± 2.6 | 13.27 ± 2.36 | 0.749 |
| Glucose (mg/dL) | 89.4 ± 6.3 | 90.0 ± 5.8 | 91.3 ± 5.9 | 0.158 |
| Adrenaline (pg/mL) | 26.03 ± 21.65 | 22.7 ± 24.3 | 25.1 ± 21.5 | 0.024 |
| Dopamine (pg/mL) | 8.0 ± 8.3 | 9.26 ± 8.9 | 8.17 ± 9.0 | 0.421 |
| 8-isoprostane (pg/mL) | 38.8 ± 21.41 | 38.90 ± 20.8 | 41.1 ± 22.3 | 0.358 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hahad, O.; Herzog, J.; Röösli, M.; Schmidt, F.P.; Daiber, A.; Münzel, T. Acute Exposure to Simulated Nocturnal Train Noise Leads to Impaired Sleep Quality and Endothelial Dysfunction in Young Healthy Men and Women: A Sex-Specific Analysis. Int. J. Environ. Res. Public Health 2022, 19, 13844. https://doi.org/10.3390/ijerph192113844
Hahad O, Herzog J, Röösli M, Schmidt FP, Daiber A, Münzel T. Acute Exposure to Simulated Nocturnal Train Noise Leads to Impaired Sleep Quality and Endothelial Dysfunction in Young Healthy Men and Women: A Sex-Specific Analysis. International Journal of Environmental Research and Public Health. 2022; 19(21):13844. https://doi.org/10.3390/ijerph192113844
Chicago/Turabian StyleHahad, Omar, Johannes Herzog, Martin Röösli, Frank P. Schmidt, Andreas Daiber, and Thomas Münzel. 2022. "Acute Exposure to Simulated Nocturnal Train Noise Leads to Impaired Sleep Quality and Endothelial Dysfunction in Young Healthy Men and Women: A Sex-Specific Analysis" International Journal of Environmental Research and Public Health 19, no. 21: 13844. https://doi.org/10.3390/ijerph192113844
APA StyleHahad, O., Herzog, J., Röösli, M., Schmidt, F. P., Daiber, A., & Münzel, T. (2022). Acute Exposure to Simulated Nocturnal Train Noise Leads to Impaired Sleep Quality and Endothelial Dysfunction in Young Healthy Men and Women: A Sex-Specific Analysis. International Journal of Environmental Research and Public Health, 19(21), 13844. https://doi.org/10.3390/ijerph192113844











