Current Progress, Challenges and Perspectives in the Microalgal-Bacterial Aerobic Granular Sludge Process: A Review
Abstract
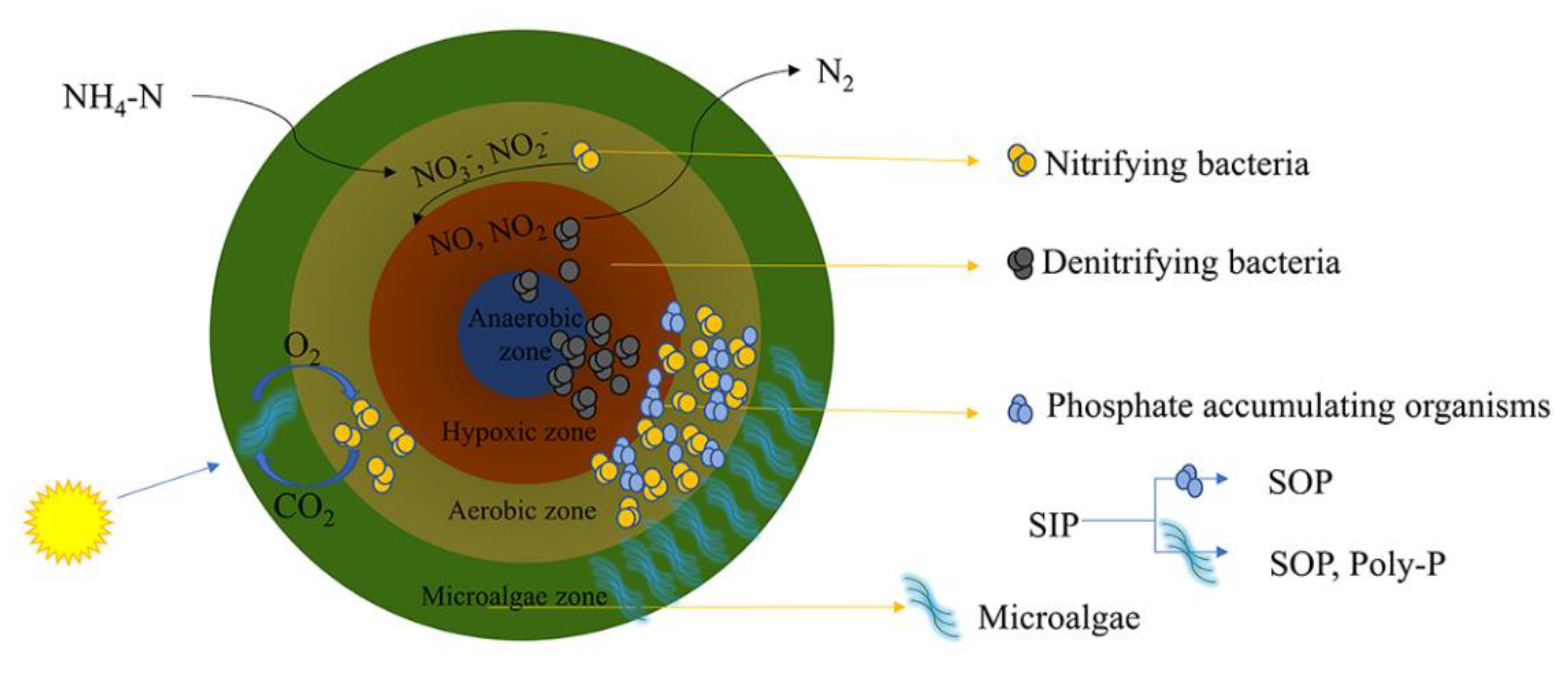
:1. Introduction
2. MBGS Culture and Operating Parameters
2.1. Lighting
2.2. Shear Force
2.3. Hydraulic Retention Time (HRT)
2.4. Inoculum Concentration
2.5. Organic Load
2.6. Algal and Bacterial Species
3. MBGS Application in Different Wastewaters
| Wastewater Type | Reactor Type | Initial Characteristics | Volume | Operating Parameters | Removal Efficiency | References |
|---|---|---|---|---|---|---|
| Municipal Wastewater (Synthetic) | glass container | NH4-N 99.4 mg/L; TP 13.2 mg/L.; CH3COONa 3H2O 552.8 mg/L | 60 mL | The number of cycles is eight. The first three cycles were 8 h, and the last five cycles were 6 h. | Organic matter 92.69%; NH4-N 96.84%; TP 87.16%. | [26] |
| Municipal Wastewater (Synthetic) | glass container | KH2PO4 13.2–21.9 mg/L | 60 mL | Six h cycle, light-dark ratio 2:4 h; PPFD illumination is 200 ± 10 μmol/m2/s. | Phosphorus 86% | [26] |
| Simulated wastewater | glass anaerobic bottle | NaAc 113.1 mg/L; NH4Cl 30.3 mg/L; K2HPO4 50 mg/L; | 50 mL | HRT is 12 h. | (COD), NH4+-N and PO43−-P could reach 59.9 ± 6.8%, 78.1 ± 7.9% and 61.5 ± 4.5% on daytime; at night were 47.6 ± 8.0%, 56.5 ± 17.9% and 74.2 ± 7.6% | [51] |
| Municipal Wastewater (Synthetic) | SBR | COD 320 mg/L; NH4-N 35 mg/L; TP 9 mg/L. | 6 L | Eight h cycle, water inflow for 3 min, anaerobic for 120 min, aerobic for 210 min, anoxic for 114–142 min, sedimentation for 30 min, and discharge for 3 min. VRT is 50%. | COD 95%; NH4-N 97–99%; TP about 93–96%. | [13] |
| aquaculture wastewater | glass container | COD 280.91 mg/L; NH4-N 11.44 mg/L; (NO3-N) (NaNO3) 16.61 mg/L; NO2-N 9.86 mg/L; PO4-P 2.83 mg/L | 40 mL | Eight h cycle, 36 cycles in total; light intensity: 200 μmol/m2/s. | COD 64.8%; NH4-N 84.9%; NO3-N 70.8%; NO2-N 50.0%; P 84.2% | [53] |
| Domestic sewage (synthetic) | series reactor | COD 300 mg/L; H4-N 100 mg/L; PO4-P 10 mg/L | 1 L | Aeration (0.5 cm/s) and no aeration 60 min: 30 min alternately; HRT is 6 h. | COD 96%; NH4-N 99%; TP 50% | [57] |
| Low Carbon Wastewater (Synthetic) | SBR | PO4-P 10 mg/L | 0.92 L | Light to dark ratio 12:12 h; HRT is 7.5 h; volume exchange ratio is 53%; 4 h/cycle, 2 min of water inflow, 60 min of no aeration, 173 min of aeration, 2 min of precipitation and 3 min of water effluent. | COD/N = 1, COD 82.5%; TP 98%. COD/N = 2, COD 90.6%; TP 98%. | [58] |
| Eutrophic Brackish Water (2%, 4%) | SBR | NH4-N 50 mg/L; PO4-P 10 mg/L | 2 L | One hundred and eighty µmol m−2 s−1 light; HRT is 24 h; VRT is 50%; Ventilation flow 3 L/min; 12 h/cycle, feeding 2 min, no aeration 2 min, aeration 706 min, precipitation 5 min, decantation 3 min, idling 2 min. | 2% salinity wastewater, TN and TP 98%; 4% salinity wastewater, TIN 17%. | [59] |
| Salty wastewater (1–3%) | CFR | COD 600 mg/L; NH4-N 50 mg/L; PO4-P 10 mg/L | 20 L | Three hundred µmol m−2 s−1 light for 12 h; HRT is 9.5 h Influent flow rate 35 mL/min; The sludge return area is about 4.5 L/min; The aeration zone is about 13.5 L/min. | COD 91.5%; TP 41.8–49.2%; TIN 45.6–55.6%. | [52] |
4. Biofuels and Bioproducts
4.1. Biofuels
| Microalgae | Algae Type | Biofuel | Productivity of Biofuel | References |
|---|---|---|---|---|
| Arthrospira maxima | Green | Hydrogen, Biodiesel | 40–69% | [79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100] |
| Chlamydomonas reinhardtii | Green | Hydrogen | 2.5 mL/h/11.73 g/L | [100,101,102,103,104] |
| Chlorella | Green | Biodiesel | [105,106] | |
| Chlorella biomass | Green | Ethanol | 22.6 g/L | [107] |
| Chlorella minutissima | Green | Methanol | [108] | |
| Chlorella protothecoides | Green | Biodiesel | 15.5 g/L | [109,110] |
| Chlorella regularis | Green | Ethanol | [111] | |
| Chlorella vulgaris | Green | Ethanol | [112] | |
| Chlorococcum humicola | Green | Ethanol | 72 g/L or 10 g/L | [113] |
| Chlorococcum infusionum | Green | Ethanol | 0.26 g ethanol/g algae | [114] |
| Dunaliella sp. | Green | Ethanol | 11.0 mg/g | [115] |
| Haematococcus pluvialis | Red | Biodiesel | 420 CJ/ha/yr | [116,117] |
| Neochlorosis oleabundans | Green | Biodiesel | 56.0 g/g | [86] |
| Platymonas subcordiformis | Green | Hydrogen | [118] | |
| Scenedesmus obliquus | Green | Methanol, Hydrogen | [107,119,120] | |
| Spirogyra | Green | Ethanol | [121] | |
| Spirulina platensis | Green | Hydrogen | 1.8 umol/mg | [122] |
| S. platensis UTEX 1926 | Blue-Green | Methane | 0.40 m3/kg | [123] |
| Spirulina Leb 18 | Blue-Green | Methane | 0.79 g/L | [124] |
4.2. Phosphorus
4.3. N-Acyl Homoserine Lactone (ALE)
4.4. Adsorbents
4.5. Reduction of the Greenhouse Gas Production
5. Challenging Prospects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- De Bruin, L.; De Kreuk, M.; Van Der Roest, H.; Uijterlinde, C.; van Loosdrecht, M. Aerobic granular sludge technology: An alternative to activated sludge? Water Sci. Technol. 2004, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Xie, C.; Alhassan, S.I.; Huang, S.; Chen, R.; Xiang, S.; Wang, Z.; Huang, L. Oxygen reduction reaction in the field of water environment for application of nanomaterials. Nanomaterials 2020, 10, 1719. [Google Scholar] [CrossRef] [PubMed]
- Mishima, K.; Nakamura, M. Self-immobilization of aerobic activated sludge–a pilot study of the aerobic upflow sludge blanket process in municipal sewage treatment. Water Sci. Technol. 1991, 23, 981–990. [Google Scholar] [CrossRef]
- Hamza, R.; Rabii, A.; Ezzahraoui, F.-Z.; Morgan, G.; Iorhemen, O.T. A review of the state of development of aerobic granular sludge technology over the last 20 years: Full-scale applications and resource recovery. Case Stud. Chem. Environ. Eng. 2021, 5, 100173. [Google Scholar] [CrossRef]
- He, Q.; Xie, Z.; Fu, Z.; Wang, M.; Xu, P.; Yu, J.; Ma, J.; Gao, S.; Chen, L.; Zhang, W. Interaction and removal of oxytetracycline with aerobic granular sludge. Bioresour. Technol. 2021, 320, 124358. [Google Scholar] [CrossRef]
- Lin, H.; Ma, R.; Hu, Y.; Lin, J.; Sun, S.; Jiang, J.; Li, T.; Liao, Q.; Luo, J. Reviewing bottlenecks in aerobic granular sludge technology: Slow granulation and low granular stability. Environ. Pollut. 2020, 263, 114638. [Google Scholar] [CrossRef]
- Hou, Y.; Gan, C.; Chen, R.; Chen, Y.; Yuan, S.; Chen, Y. Structural Characteristics of Aerobic Granular Sludge and Factors That Influence Its Stability: A Mini Review. Water 2021, 13, 2726. [Google Scholar] [CrossRef]
- Luo, Y.; Su, R.; Yao, H.; Zhang, A.; Xiang, S.; Huang, L. Degradation of trimethoprim by sulfate radical-based advanced oxidation processes: Kinetics, mechanisms, and effects of natural water matrices. Environ. Sci. Pollut. Res. 2021, 28, 62572–62582. [Google Scholar] [CrossRef]
- Su, R.; Zhang, H.; Chen, F.; Wang, Z.; Huang, L. Applications of single atom catalysts for environmental management. Int. J. Environ. Res. Public Health 2022, 19, 11155. [Google Scholar] [CrossRef]
- Wang, S.; Ji, B.; Zhang, M.; Ma, Y.; Gu, J.; Liu, Y. Defensive responses of microalgal-bacterial granules to tetracycline in municipal wastewater treatment. Bioresour. Technol. 2020, 312, 123605. [Google Scholar] [CrossRef]
- Zhang, M.; Ji, B.; Liu, Y. Microalgal-bacterial granular sludge process: A game changer of future municipal wastewater treatment? Sci. Total Environ. 2021, 752, 141957. [Google Scholar] [CrossRef]
- Brockmann, D.; Gérand, Y.; Park, C.; Milferstedt, K.; Hélias, A.; Hamelin, J. Wastewater treatment using oxygenic photogranule-based process has lower environmental impact than conventional activated sludge process. Bioresour. Technol. 2021, 319, 124204. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Zhang, X.; Shi, Y.; Cui, B.; Fan, J.; Ji, B.; Yuan, J. Microalgal-bacterial granular sludge process outperformed aerobic granular sludge process in municipal wastewater treatment with less carbon dioxide emissions. Environ. Sci. Pollut. Res. 2021, 28, 13616–13623. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Chai, L.; Tang, C.; Li, B.; Yang, Z. Comparison of the degradation of molecular and ionic ibuprofen in a UV/H2O2 system. Water Sci. Technol. 2018, 77, 2174–2183. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, X.; Liu, S.; Lei, Z.; Shimizu, K.; Zhang, Z.; Adachi, Y.; Lee, D.-J. Rapid establishment and stable performance of a new algal-bacterial granule system from conventional bacterial aerobic granular sludge and preliminary analysis of mechanisms involved. J. Water Process. Eng. 2020, 34, 101073. [Google Scholar] [CrossRef]
- Su, R.; Dai, X.; Wang, H.; Wang, Z.; Li, Z.; Chen, Y.; Luo, Y.; Ouyang, D. Metronidazole degradation by UV and UV/H2O2 advanced oxidation processes: Kinetics, mechanisms, and effects of natural water matrices. Int. J. Environ. Res. Public Health 2022, 19, 12354. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ho, S.-H.; Cheng, C.-L.; Guo, W.-Q.; Nagarajan, D.; Ren, N.-Q.; Lee, D.-J.; Chang, J.-S. Perspectives on the feasibility of using microalgae for industrial wastewater treatment. Bioresour. Technol. 2016, 222, 485–497. [Google Scholar] [CrossRef]
- Liu, J.; Pemberton, B.; Lewis, J.; Scales, P.J.; Martin, G.J. Wastewater treatment using filamentous algae–a review. Bioresour. Technol. 2020, 298, 122556. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, W.; Yen, H.-W.; Ho, S.-H.; Lo, Y.-C.; Cheng, C.-L.; Ren, N.; Chang, J.-S. Cultivation of Chlorella vulgaris JSC-6 with swine wastewater for simultaneous nutrient/COD removal and carbohydrate production. Bioresour. Technol. 2015, 198, 619–625. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Ficara, E.; Aboulkas, A.; Barakat, A.; Carrère, H. New opportunities for agricultural digestate valorization: Current situation and perspectives. Energy Environ. Sci. 2015, 8, 2600–2621. [Google Scholar] [CrossRef]
- Van Den Berg, T.E.; Chukhutsina, V.U.; Van Amerongen, H.; Croce, R.; Van Oort, B. Light acclimation of the colonial green alga Botryococcus braunii strain Showa. Plant. Physiol. 2019, 179, 1132–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udovič, G.; Žutinic, P.; Kralj Borojević, K.; Plenković-Moraj, A. Co-occurrence of functional groups in phytoplankton assemblages dominated by diatoms, chrysophytes and dinoflagellates. Fundam. Appl. Limnol. 2015, 187, 101–111. [Google Scholar] [CrossRef]
- Kang, C.D.; An, J.Y.; Park, T.H.; Sim, S.J. Astaxanthin biosynthesis from simultaneous N and P uptake by the green alga Haematococcus pluvialis in primary-treated wastewater. Biochem. Eng. J. 2006, 31, 234–238. [Google Scholar] [CrossRef]
- Du, X.; Tao, Y.; Li, H.; Liu, Y.; Feng, K. Synergistic methane production from the anaerobic co-digestion of Spirulina platensis with food waste and sewage sludge at high solid concentrations. Renew. Energ. 2019, 142, 55–61. [Google Scholar] [CrossRef]
- Zhuang, L.-L.; Yu, D.; Zhang, J.; Liu, F.-f.; Wu, Y.-H.; Zhang, T.-Y.; Dao, G.-H.; Hu, H.-Y. The characteristics and influencing factors of the attached microalgae cultivation: A review. Renew. Sustain. Energy Rev. 2018, 94, 1110–1119. [Google Scholar] [CrossRef]
- Ji, B.; Zhang, M.; Gu, J.; Ma, Y.; Liu, Y. A self-sustaining synergetic microalgal-bacterial granular sludge process towards energy-efficient and environmentally sustainable municipal wastewater treatment. Water Res. 2020, 179, 115884. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Chen, Z.; Guo, D.; Liu, Y. Investigations on the pyrolysis of microalgal-bacterial granular sludge: Products, kinetics, and potential mechanisms. Bioresour. Technol. 2021, 349, 126328. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chang, H.; Zhang, C.; Xie, Y.; Ho, S.-H. Emerging biological wastewater treatment using microalgal-bacterial granules: A review. Bioresour. Technol. 2022, 351, 127089. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Kumar, P.S.; Varjani, S.; Jeevanantham, S.; Yaashikaa, P.; Thamarai, P.; Abirami, B.; George, C.S. A review on algal-bacterial symbiotic system for effective treatment of wastewater. Chemosphere 2021, 271, 129540. [Google Scholar] [CrossRef] [PubMed]
- Abouhend, A.S.; Milferstedt, K.; Hamelin, J.; Ansari, A.A.; Butler, C.; Carbajal-González, B.I.; Park, C. Growth progression of oxygenic photogranules and its impact on bioactivity for aeration-free wastewater treatment. Environ. Sci. Technol. 2019, 54, 486–496. [Google Scholar] [CrossRef]
- Quijano, G.; Arcila, J.S.; Buitrón, G. Microalgal-bacterial aggregates: Applications and perspectives for wastewater treatment. Biotechnol. Adv. 2017, 35, 772–781. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, Y.; Lens, P.N.; Zhang, Z.; Shi, W.; Cui, F.; Tay, J.H. Effect of light intensity on the characteristics of algal-bacterial granular sludge and the role of N-acyl-homoserine lactone in the granulation. Sci. Total Environ. 2019, 659, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Milferstedt, K.; Kuo-Dahab, W.C.; Butler, C.S.; Hamelin, J.; Abouhend, A.S.; Stauch-White, K.; McNair, A.; Watt, C.; Carbajal-Gonzalez, B.I.; Dolan, S.; et al. The importance of filamentous cyanobacteria in the development of oxygenic photogranules. Sci. Rep. 2017, 7, 17944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, B. Towards environment-sustainable wastewater treatment and reclamation by the non-aerated microalgal-bacterial granular sludge process: Recent advances and future directions. Sci. Total Environ. 2022, 806, 150707. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Li, B.; Zhang, C.; Zhang, Z.; Lei, Z.; Lu, B.; Zhou, B. Effect of algae growth on aerobic granulation and nutrients removal from synthetic wastewater by using sequencing batch reactors. Bioresour. Technol. 2015, 179, 187–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trebuch, L.M.; Oyserman, B.O.; Janssen, M.; Wijffels, R.H.; Vet, L.E.M.; Fernandes, T.V. Impact of hydraulic retention time on community assembly and function of photogranules for wastewater treatment. Water Res. 2020, 173, 115506. [Google Scholar] [CrossRef]
- Ruiz-Martinez, A.; Garcia, N.M.; Romero, I.; Seco, A.; Ferrer, J. Microalgae cultivation in wastewater: Nutrient removal from anaerobic membrane bioreactor effluent. Bioresour. Technol. 2012, 126, 247–253. [Google Scholar] [CrossRef]
- Solovchenko, A.; Verschoor, A.M.; Jablonowski, N.D.; Nedbal, L. Phosphorus from wastewater to crops: An alternative path involving microalgae. Biotechnol. Adv. 2016, 34, 550–564. [Google Scholar] [CrossRef]
- Kube, M.; Jefferson, B.; Fan, L.; Roddick, F. The impact of wastewater characteristics, algal species selection and immobilisation on simultaneous nitrogen and phosphorus removal. Algal Res. 2018, 31, 478–488. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Mennerich, A.; Urban, B. Municipal wastewater treatment and biomass accumulation with a wastewater-born and settleable algal-bacterial culture. Water Res. 2011, 45, 3351–3358. [Google Scholar] [CrossRef]
- Doncaster, C.P.; Jackson, A.; Watson, R.A. Manipulated into giving: When parasitism drives apparent or incidental altruism. Proc. R. Soc. B Biol. Sci. 2013, 280, 20130108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, D.-H.; Ramanan, R.; Heo, J.; Lee, J.; Kim, B.-H.; Oh, H.-M.; Kim, H.-S. Enhancing microalgal biomass productivity by engineering a microalgal–bacterial community. Bioresour. Technol. 2015, 175, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Vyverman, W. Differences in nutrient uptake capacity of the benthic filamentous algae Cladophora sp., Klebsormidium sp. and Pseudanabaena sp. under varying N/P conditions. Bioresour. Technol. 2015, 179, 234–242. [Google Scholar] [CrossRef]
- Su, R.; Yang, X.-H.; Hu, M.; Wang, Q.-A.; Li, J.-H. Annulation cascades of N-Allyl-N-((2-bromoaryl)ethynyl)amides involving C–H functionalization. Org. Lett. 2019, 21, 2786–2789. [Google Scholar] [CrossRef] [PubMed]
- Hancock, L.; Goff, L.; Lane, C. Red algae lose key mitochondrial genes in response to becoming parasitic. Genome Biol. Evol. 2010, 2, 897–910. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, Z.; Su, J.; Tian, Y.; Ning, X.; Hong, H.; Zheng, T. Lysis of a red-tide causing alga, Alexandrium tamarense, caused by bacteria from its phycosphere. Biol. Control. 2010, 52, 123–130. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, W.; Fu, Z.; Cheng, Y.; Min, M.; Liu, Y.; Zhang, Y.; Chen, P.; Ruan, R. Effect of wastewater-borne bacteria on algal growth and nutrients removal in wastewater-based algae cultivation system. Bioresour. Technol. 2014, 167, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.L.; Garbayo, I.; Cuaresma, M.; Montero, Z.; González-del-Valle, M.; Vílchez, C. Impact of microalgae-bacteria interactions on the production of algal biomass and associated compounds. Mar. Drugs 2016, 14, 100. [Google Scholar] [CrossRef] [Green Version]
- Su, R.; Li, Y.; Min, M.-Y.; Ouyang, X.-H.; Song, R.-J.; Li, J.-H. Copper-catalyzed oxidative intermolecular 1,2-alkylarylation of styrenes with ethers and indoles. Chem. Commun. 2018, 54, 13511–13514. [Google Scholar] [CrossRef]
- Yang, J.; Shi, W.; Fang, F.; Guo, J.; Lu, L.; Xiao, Y.; Jiang, X. Exploring the feasibility of sewage treatment by algal–bacterial consortia. Crit. Rev. Biotechnol. 2020, 40, 169–179. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, L.; Ji, B.; Hou, H.; Ma, Y. Microalgal-bacterial granular sludge process in non-aerated municipal wastewater treatment under natural day-night conditions: Performance and microbial community. Water 2021, 13, 1479. [Google Scholar] [CrossRef]
- Meng, F.; Huang, W.; Liu, D.; Zhao, Y.; Huang, W.; Lei, Z.; Zhang, Z. Application of aerobic granules-continuous flow reactor for saline wastewater treatment: Granular stability, lipid production and symbiotic relationship between bacteria and algae. Bioresour. Technol. 2020, 295, 122291. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Ji, B.; Abu Hasan, H.; Fan, J.; Guo, S.; Wang, J.; Yuan, J. Microalgal–bacterial granular sludge process for non-aerated aquaculture wastewater treatment. Bioprocess. Biosyst. Eng. 2021, 44, 1733–1739. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, S.; Yang, X.; Lei, Z.; Shimizu, K.; Zhang, Z.; Lee, D.-J.; Adachi, Y. Stability and performance of algal-bacterial granular sludge in shaking photo-sequencing batch reactors with special focus on phosphorus accumulation. Bioresour. Technol. 2019, 280, 497–501. [Google Scholar] [CrossRef]
- Huang, W.; Liu, D.; Huang, W.; Cai, W.; Zhang, Z.; Lei, Z. Achieving partial nitrification and high lipid production in an algal-bacterial granule system when treating low COD/NH4-N wastewater. Chemosphere 2020, 248, 126106. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Hu, P.; Li, Z.; Kang, Q.; Chen, H.; Zhang, J.; Zhu, S. Effect of high ammonia on granular stability and phosphorus recovery of algal-bacterial granules in treatment of synthetic biogas slurry. Heliyon 2022, 8, e09844–e09844. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.S.M.; Cai, W.; Zhao, Z.; Zhang, Z.; Shimizu, K.; Lei, Z.; Lee, D.-J. Stability of algal-bacterial granules in continuous-flow reactors to treat varying strength domestic wastewater. Bioresour. Technol. 2017, 244, 225–233. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, X.; Cai, W.; Lei, Z.; Shimizu, K.; Zhang, Z.; Utsumi, M.; Lee, D.-J. Response of algal-bacterial granular system to low carbon wastewater: Focus on granular stability, nutrients removal and accumulation. Bioresour. Technol. 2018, 268, 221–229. [Google Scholar] [CrossRef]
- Meng, F.; Liu, Y.; Zhang, P.; Liu, D.; Huang, W. Advanced Treatment of Salty Eutrophication Water Using Algal-Bacterial Granular Sludge: With Focus on Nitrogen Removal, Phosphorus Removal, and Lipid Accumulation. BioResources 2019, 14, 9518–9530. [Google Scholar] [CrossRef]
- Wang, X.; Hong, Y. Microalgae biofilm and bacteria symbiosis in nutrient removal and carbon fixation from wastewater: A review. Curr. Pollut. Rep. 2022, 8, 128–146. [Google Scholar] [CrossRef]
- Duan, X.; Chen, Y.; Yan, Y.; Feng, L.; Chen, Y.; Zhou, Q. New method for algae comprehensive utilization: Algae-derived biochar enhances algae anaerobic fermentation for short-chain fatty acids production. Bioresour. Technol. 2019, 289, 121637. [Google Scholar] [CrossRef]
- Li, W.; Li, W.; Liu, H. The resource utilization of algae-Preparing coal slurry with algae. Fuel 2010, 89, 965–970. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Wang, Q.; Li, Z.; Yuan, T.; Lei, Z.; Zhang, Z.; Shimizu, K.; Lee, D.-J. A comparative study on simultaneous recovery of phosphorus and alginate-like exopolymers from bacterial and algal-bacterial aerobic granular sludges: Effects of organic loading rate. Bioresour. Technol. 2022, 357, 127343. [Google Scholar] [CrossRef]
- Zhang, B.; Li, W.; Guo, Y.; Zhang, Z.; Shi, W.; Cui, F.; Lens, P.N.; Tay, J.H. Microalgal-bacterial consortia: From interspecies interactions to biotechnological applications. Renew. Sustain. Energy Rev. 2020, 118, 109563. [Google Scholar] [CrossRef]
- Wang, C.; Chen, L.; Rakesh, B.; Qin, Y.; Lv, R. Technologies for extracting lipids from oleaginous microorganisms for biodiesel production. Front. Energy 2012, 6, 266–274. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Inda, C.S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A comprehensive review of physicochemical properties, production process, performance and emissions characteristics of 2nd generation biodiesel feedstock: Jatropha curcas. Fuel 2021, 285, 119110. [Google Scholar] [CrossRef]
- Su, R.; Wang, Y.; Huang, S.; Chen, R.; Wang, J. Application for ecological restoration of contaminated soil: Phytoremediation. Int. J. Environ. Res. Public Health 2022, 19, 13124. [Google Scholar] [CrossRef]
- Verma, S.; Kuila, A. Involvement of green technology in microalgal biodiesel production. Rev. Environ. Health 2020, 35, 173–188. [Google Scholar] [CrossRef]
- Luo, Y.; Su, R.; Yang, H. Efficient copper(I)-catalyzed oxidative intermolecular 1,2-estersulfenylation of styrenes with peroxyesters and disulfides. Org. Biomol. Chem. 2020, 18, 5045–5049. [Google Scholar] [CrossRef]
- Maity, J.P.; Bundschuh, J.; Chen, C.-Y.; Bhattacharya, P. Microalgae for third generation biofuel production, mitigation of greenhouse gas emissions and wastewater treatment: Present and future perspectives–A mini review. Energy 2014, 78, 104–113. [Google Scholar] [CrossRef]
- Meng, F.; Xi, L.; Liu, D.; Huang, W.; Lei, Z.; Zhang, Z.; Huang, W. Effects of light intensity on oxygen distribution, lipid production and biological community of algal-bacterial granules in photo-sequencing batch reactors. Bioresour. Technol. 2019, 272, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zeng, Z.; Bee, M.; Gibson, V.; Wei, L.; Huang, X.; Liu, C. Characteristics and performance of aerobic algae-bacteria granular consortia in a photo-sequencing batch reactor. J. Hazard. Mater. 2018, 349, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Huo, H.; Meng, F. Partial nitrification algal-bacterial granule system cultivation: Performance, lipid production and biological community. Water Air. Soil. Poll. 2020, 231, 236. [Google Scholar] [CrossRef]
- Yaakob, M.A.; Mohamed, R.M.S.R.; Al-Gheethi, A.; Aswathnarayana Gokare, R.; Ambati, R.R. Influence of nitrogen and phosphorus on microalgal growth, biomass, lipid, and fatty acid production: An overview. Cells 2021, 10, 393. [Google Scholar] [CrossRef] [PubMed]
- Szwaja, S.; Debowski, M.; Zielinski, M.; Kisielewska, M.; Stanczyk-Mazanek, E.; Sikorska, M. Influence of a light source on microalgae growth and subsequent anaerobic digestion of harvested biomass. Biomass Bioenergy 2016, 91, 243–249. [Google Scholar] [CrossRef]
- Grima, E.M.; Belarbi, E.-H.; Fernández, F.A.; Medina, A.R.; Chisti, Y. Recovery of microalgal biomass and metabolites: Process options and economics. Biotechnol. Adv. 2003, 20, 491–515. [Google Scholar] [CrossRef]
- Matos, C.T.; Santos, M.; Nobre, B.P.; Gouveia, L. Nannochloropsis sp. biomass recovery by Electro-Coagulation for biodiesel and pigment production. Bioresour. Technol. 2013, 134, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Pacheco, R.; Ferreira, A.F.; Pinto, T.; Nobre, B.P.; Loureiro, D.; Moura, P.; Gouveia, L.; Silva, C.M. The production of pigments & hydrogen through a Spirogyra sp. biorefinery. Energ. Convers. Manag. 2015, 89, 789–797. [Google Scholar]
- Baunillo, K.E.; Tan, R.S.; Barros, H.R.; Luque, R. Investigations on microalgal oil production from Arthrospira platensis: Towards more sustainable biodiesel production. RSC Adv. 2012, 2, 11267–11272. [Google Scholar] [CrossRef]
- Xiaodong, D.; Yajun, L.; Xiaowen, F. Microalgae: A promising feedstock for biodiesel. Afr. J. Microbiol. Res. 2009, 3, 1008–1014. [Google Scholar]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Sawayama, S.; Minowa, T.; Yokoyama, S.-Y. Possibility of renewable energy production and CO2 mitigation by thermochemical liquefaction of microalgae. Biomass Bioenergy 1999, 17, 33–39. [Google Scholar] [CrossRef]
- Dote, Y.; Sawayama, S.; Inoue, S.; Minowa, T.; Yokoyama, S.-y. Recovery of liquid fuel from hydrocarbon-rich microalgae by thermochemical liquefaction. Fuel 1994, 73, 1855–1857. [Google Scholar] [CrossRef]
- Rodolfi, L.; Chini Zittelli, G.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef]
- Natrah, F.; Yusoff, F.; Shariff, M.; Abas, F.; Mariana, N. Screening of Malaysian indigenous microalgae for antioxidant properties and nutritional value. J. Appl. Phycol. 2007, 19, 711–718. [Google Scholar] [CrossRef]
- Gouveia, L.; Oliveira, A.C. Microalgae as a raw material for biofuels production. J. Ind. Microbiol. Biotechnol. 2009, 36, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Wu, Q. High yield bio-oil production from fast pyrolysis by metabolic controlling of Chlorella protothecoides. J. Biotechnol. 2004, 110, 85–93. [Google Scholar] [CrossRef]
- Peng, W.; Wu, Q.; Tu, P.; Zhao, N. Pyrolytic characteristics of microalgae as renewable energy source determined by thermogravimetric analysis. Bioresour. Technol. 2001, 80, 1–7. [Google Scholar] [CrossRef]
- Scragg, A.; Illman, A.; Carden, A.; Shales, S. Growth of microalgae with increased calorific values in a tubular bioreactor. Biomass Bioenergy 2002, 23, 67–73. [Google Scholar] [CrossRef]
- Nigam, S.; Rai, M.P.; Sharma, R. Effect of nitrogen on growth and lipid content of Chlorella pyrenoidosa. Am. J. Biochem. Biotechnol. 2011, 7, 124–129. [Google Scholar] [CrossRef]
- Gouveia, L.; Marques, A.E.; Da Silva, T.L.; Reis, A. Neochloris oleabundans UTEX# 1185: A suitable renewable lipid source for biofuel production. J. Ind. Microbiol. Biotechnol. 2009, 36, 821–826. [Google Scholar] [PubMed]
- Pai, T.-Y.; Lai, W.-J. Analyzing algae growth and oil production in a batch reactor under high nitrogen and phosphorus conditions. Int. J. Appl. Sci. Eng. 2011, 9, 161–168. [Google Scholar]
- Weldy, C.S.; Huesemann, M. Lipid production by Dunaliella salina in batch culture: Effects of nitrogen limitation and light intensity. J. Undergrad. Res. 2007, 7, 115–122. [Google Scholar]
- Minowa, T.; Yokoyama, S.-y.; Kishimoto, M.; Okakura, T. Oil production from algal cells of Dunaliella tertiolecta by direct thermochemical liquefaction. Fuel 1995, 74, 1735–1738. [Google Scholar] [CrossRef]
- Becker, E.W. Microalgae: Biotechnology and Microbiology; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Damiani, M.C.; Popovich, C.A.; Constenla, D.; Leonardi, P.I. Lipid analysis in Haematococcus pluvialis to assess its potential use as a biodiesel feedstock. Bioresour. Technol. 2010, 101, 3801–3807. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.-Y.; Kao, C.-Y.; Tsai, M.-T.; Ong, S.-C.; Chen, C.-H.; Lin, C.-S. Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour. Technol. 2009, 100, 833–838. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef] [Green Version]
- Ananyev, G.; Carrieri, D.; Dismukes, G.C. Optimization of metabolic capacity and flux through environmental cues to maximize hydrogen production by the cyanobacterium “Arthrospira (Spirulina) maxima”. Appl. Environ. Microb. 2008, 74, 6102–6113. [Google Scholar] [CrossRef] [Green Version]
- Ghirardi, M.L.; Zhang, L.; Lee, J.W.; Flynn, T.; Seibert, M.; Greenbaum, E.; Melis, A. Microalgae: A green source of renewable H2. Trends. Biotechnol. 2000, 18, 506–511. [Google Scholar] [CrossRef]
- Choi, S.P.; Nguyen, M.T.; Sim, S.J. Enzymatic pretreatment of Chlamydomonas reinhardtii biomass for ethanol production. Bioresour. Technol. 2010, 101, 5330–5336. [Google Scholar] [CrossRef]
- Saleem, M.; Chakrabarti, M.H.; Raman, A.A.A.; Daud, W.M.A.W.; Mustafa, A. Hydrogen production by Chlamydomonas reinhardtii in a two-stage process with and without illumination at alkaline pH. Int. J. Hydrogen Energy 2012, 37, 4930–4934. [Google Scholar] [CrossRef]
- Hemschemeier, A.; Melis, A.; Happe, T. Analytical approaches to photobiological hydrogen production in unicellular green algae. Photosynth Res. 2009, 102, 523–540. [Google Scholar] [CrossRef] [Green Version]
- Burgess, S.J.; Tamburic, B.; Zemichael, F.; Hellgardt, K.; Nixon, P.J. Solar-driven hydrogen production in green algae. Adv. Appl. Microbiol. 2011, 75, 71–110. [Google Scholar] [PubMed]
- Huntley, M.E.; Redalje, D.G. CO2 mitigation and renewable oil from photosynthetic microbes: A new appraisal. Mitig. Adapt. Strateg. Glob. Chang. 2007, 12, 573–608. [Google Scholar] [CrossRef]
- Seefeldt, L. Utah group plans to make biodiesel from algae. Ind. Bioprocess. 2007, 29, 5–6. [Google Scholar]
- Zhou, N.; Zhang, Y.; Wu, X.; Gong, X.; Wang, Q. Hydrolysis of Chlorella biomass for fermentable sugars in the presence of HCl and MgCl2. Bioresour. Technol. 2011, 102, 10158–10161. [Google Scholar] [CrossRef]
- Kotzabasis, K.; Hatziathanasiou, A.; Bengoa-Ruigomez, M.; Kentouri, M.; Divanach, P. Methanol as alternative carbon source for quicker efficient production of the microalgae Chlorella minutissima: Role of the concentration and frequence of administration. In Progress in Industrial Microbiology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 35, pp. 357–362. [Google Scholar]
- Chen, Y.-H.; Walker, T.H. Biomass and lipid production of heterotrophic microalgae Chlorella protothecoides by using biodiesel-derived crude glycerol. Biotechnol. Lett. 2011, 33, 1973–1983. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Wu, Q. Large-scale biodiesel production from microalga Chlorella protothecoides through heterotrophic cultivation in bioreactors. Biotechnol. Bioeng. 2007, 98, 764–771. [Google Scholar] [CrossRef]
- Endo, H.; Hosoya, H.; Koibuchi, T. Growth yields of chlorella regularis in dark-heterotrophic continuous cultures using acetate: Studies on Chlorella regularis, heterotrophic fast-growing strain (III). J. Ferment. Technol. 1977, 55, 369–379. [Google Scholar]
- Hirano, A.; Ueda, R.; Hirayama, S.; Ogushi, Y. CO2 fixation and ethanol production with microalgal photosynthesis and intracellular anaerobic fermentation. Energy 1997, 22, 137–142. [Google Scholar] [CrossRef]
- Harun, R.; Danquah, M.K. Influence of acid pre-treatment on microalgal biomass for bioethanol production. Process. Biochem. 2011, 46, 304–309. [Google Scholar] [CrossRef]
- Harun, R.; Jason, W.; Cherrington, T.; Danquah, M.K. Exploring alkaline pre-treatment of microalgal biomass for bioethanol production. Appl. Energ. 2011, 88, 3464–3467. [Google Scholar] [CrossRef]
- Shirai, F.; Kunii, K.; Sato, C.; Teramoto, Y.; Mizuki, E.; Murao, S.; Nakayama, S. Cultivation of microalgae in the solution from the desalting process of soy sauce waste treatment and utilization of the algal biomass for ethanol fermentation. World J. Microbiol. Biotechnol. 1998, 14, 839–842. [Google Scholar] [CrossRef]
- Li, Y.; Horsman, M.; Wu, N.; Lan, C.Q.; Dubois-Calero, N. Articles: Biocatalysts and bioreactor design, biofuels from microalgae. Biotechnol. Prog. 2008, 24, 815–820. [Google Scholar]
- Li, Q.; Du, W.; Liu, D. Perspectives of microbial oils for biodiesel production. Appl. Microbiol. Biot. 2008, 80, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Deng, M.; Yu, X.; Zhang, W. Two-stage photo-biological production of hydrogen by marine green alga Platymonas subcordiformis. Biochem. Eng. J. 2004, 19, 69–73. [Google Scholar] [CrossRef]
- Schulz, R.; Schnackenberg, J.; Stangier, K.; Wünschiers, R.; Zinn, T.; Senger, H. Light-dependent hydrogen production of the green alga Scenedesmus obliquus. In BioHydrogen; Springer: Berlin/Heidelberg, Germany, 1998; pp. 243–251. [Google Scholar]
- Papazi, A.; Andronis, E.; Ioannidis, N.E.; Chaniotakis, N.; Kotzabasis, K. High yields of hydrogen production induced by meta-substituted dichlorophenols biodegradation from the green alga Scenedesmus obliquus. PLoS ONE 2012, 7, e49037. [Google Scholar] [CrossRef] [PubMed]
- Sulfahri, M.S.; Sunarto, E.; Irvansyah, M.Y.; Utami, R.S.; Mangkoedihardjo, S. Ethanol production from algae Spirogyra with fermentation by Zymomonas mobilis and Saccharomyces cerevisiae. J. Basic. Appl. Sci. Res. 2011, 1, 589–593. [Google Scholar]
- Aoyama, K.; Uemura, I.; Miyake, J.; Asada, Y. Fermentative metabolism to produce hydrogen gas and organic compounds in a cyanobacterium. Spirulina Platensis. J. Ferment. Bioeng. 1997, 83, 17–20. [Google Scholar] [CrossRef]
- Converti, A.; Oliveira, R.; Torres, B.; Lodi, A.; Zilli, M. Biogas production and valorization by means of a two-step biological process. Bioresour. Technol. 2009, 100, 5771–5776. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.A.V.; Santana, F.B.; da Rosa Andrade, M.; Lima, M.B.; Franck, D.T. Microalga biomass and biomethane production in the south of Brazil. J. Biotechnol. 2008, 136, S430. [Google Scholar] [CrossRef]
- Münch, E.V.; Barr, K. Controlled struvite crystallisation for removing phosphorus from anaerobic digester sidestreams. Water Res. 2001, 35, 151–159. [Google Scholar] [CrossRef]
- Su, R.; Ou, Q.; Wang, H.; Luo, Y.; Dai, X.; Wang, Y.; Chen, Y.; Shi, L. Comparison of phytoremediation potential of nerium indicum with inorganic modifier calcium carbonate and organic modifier mushroom residue to lead-zinc tailings. In Int. J. Environ. Res. Public Health 2022, 19, 10353. [Google Scholar] [CrossRef]
- He, L.; Su, R.; Chen, Y.; Zeng, P.; Du, L.; Cai, B.; Zhang, A.; Zhu, H. Integration of manganese accumulation, subcellular distribution, chemical forms, and physiological responses to understand manganese tolerance in macleaya cordata. Environ. Sci. Pollut. R 2022, 29, 39017–39026. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, Z.; Yu, Y.; Shimizu, K.; Zhang, Z.; Lei, Z.; Lee, D.-J. Enhanced biosorption of Cr(VI) from synthetic wastewater using algal-bacterial aerobic granular sludge: Batch experiments, kinetics and mechanisms. Sep. Purif. Technol. 2020, 251, 117323. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Z.; Van Nguyen, B.; Hirayama, S.; Tian, C.; Lei, Z.; Shimizu, K.; Zhang, Z. Cr(VI) bioremediation by active algal-bacterial aerobic granular sludge: Importance of microbial viability, contribution of microalgae and fractionation of loaded Cr. J. Hazard. Mater. 2021, 418, 126342. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Z.; Zhang, G.; Hirayama, S.; Bach Van, N.; Lei, Z.; Shimizu, K.; Zhang, Z. Insight into Cr(VI) biosorption onto algal-bacterial granular sludge: Cr(VI) bioreduction and its intracellular accumulation in addition to the effects of environmental factors. J. Hazard. Mater. 2021, 414, 125479. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.V.; Yang, X.; Hirayama, S.; Wang, J.; Zhao, Z.; Lei, Z.; Shimizu, K.; Zhang, Z.; Le, S.X. Effect of Salinity on Cr(VI) Bioremediation by Algal-Bacterial Aerobic Granular Sludge Treating Synthetic Wastewater. Processes 2021, 9, 1400. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Ahuja, V.; Chandel, N.; Mehariya, S.; Kumar, P.; Vanayak, V.; Saratale, G.D.; Raj, T.; Kim, S.-H.; Yang, Y.-H. An overview on microalgal-bacterial granular consortia for resource recovery and wastewater treatment. Bioresour. Technol. 2022, 351, 127028. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Fan, S.; Liu, Y. A continuous-flow non-aerated microalgal-bacterial granular sludge process for aquaculture wastewater treatment under natural day-night conditions. Bioresour. Technol. 2022, 350, 126914. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Lei, Z. Wastewater treatment using microalgal-bacterial aggregate process at zero-aeration scenario: Most recent research focuses and perspectives. Bioresour. Technol. Rep. 2022, 17, 100943. [Google Scholar] [CrossRef]
- Rajitha, K.; Sarvajith, M.; Venugopalan, V.; Nancharaiah, Y. Development and performance of halophilic microalgae-colonized aerobic granular sludge for treating seawater-based wastewater. Bioresour. Technol. Rep. 2020, 11, 100432. [Google Scholar] [CrossRef]
- Wang, J.; Lei, Z.; Wei, Y.; Wang, Q.; Tian, C.; Shimizu, K.; Zhang, Z.; Adachi, Y.; Lee, D.-J. Behavior of algal-bacterial granular sludge in a novel closed photo-sequencing batch reactor under no external O-2 supply. Bioresour. Technol. 2020, 318, 124190. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Liu, C. CO2 improves the microalgal-bacterial granular sludge towards carbon-negative wastewater treatment. Water Res. 2022, 208, 117865. [Google Scholar] [CrossRef] [PubMed]
- Safitri, A.S.; Hamelin, J.; Kommedal, R.; Milferstedt, K. Engineered methanotrophic syntrophy in photogranule communities removes dissolved methane. Water Res. X 2021, 12, 100106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lei, Z.; Liu, Y. Microalgal-bacterial granular sludge for municipal wastewater treatment: From concept to practice. Bioresour. Technol. 2022, 354, 127201. [Google Scholar] [CrossRef]
- Gikonyo, J.G.; Ansari, A.A.; Abouhend, A.S.; Tobiason, J.E.; Park, C. Hydrodynamic granulation of oxygenic photogranules. Environ. Sci. -Water Res. Technol. 2021, 7, 427–440. [Google Scholar] [CrossRef]
- Zhang, B.; Lens, P.N.L.; Shi, W.; Zhang, R.; Zhang, Z.; Guo, Y.; Bao, X.; Cui, F. The attachment potential and N-acyl-homoserine lactone-based quorum sensing in aerobic granular sludge and algal-bacterial granular sludge. Appl. Microbiol. Biot. 2018, 102, 5343–5353. [Google Scholar] [CrossRef]
- Weber, W.; Daoud-El Baba, M.; Fussenegger, M. Synthetic ecosystems based on airborne inter-and intrakingdom communication. Proc. Natl. Acad. Sci. USA 2007, 104, 10435–10440. [Google Scholar] [CrossRef] [Green Version]
- Schenk, P.M.; Thomas-Hall, S.R.; Stephens, E.; Marx, U.C.; Mussgnug, J.H.; Posten, C.; Kruse, O.; Hankamer, B. Second generation biofuels: High-efficiency microalgae for biodiesel production. Bioenergy Res. 2008, 1, 20–43. [Google Scholar] [CrossRef]
- Liu, L.; Fan, H.; Liu, Y.; Liu, C.; Huang, X. Development of algae-bacteria granular consortia in photo-sequencing batch reactor. Bioresour. Technol. 2017, 232, 64–71. [Google Scholar] [CrossRef]
- Jiang, L.; Li, Y.; Pei, H. Algal–bacterial consortia for bioproduct generation and wastewater treatment. Renew. Sustain. Energy Rev. 2021, 149, 111395. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, W.; Bai, L.; Chen, R.; Zeng, W.; Yan, Z.; Li, G.; Liang, H. Aeration-induced CO2 stripping, instead of high dissolved oxygen, have a negative impact on algae–bacteria symbiosis (ABS) system stability and wastewater treatment efficiency. Chem. Eng. J. 2020, 382, 122957. [Google Scholar] [CrossRef]
- Tang, C.-C.; Zuo, W.; Tian, Y.; Sun, N.; Wang, Z.-W.; Zhang, J. Effect of aeration rate on performance and stability of algal-bacterial symbiosis system to treat domestic wastewater in sequencing batch reactors. Bioresour. Technol. 2016, 222, 156–164. [Google Scholar] [CrossRef]
- Sutapa, B.M.; Dhruti, A.; Gopa, R.B. Pharmacological, pharmaceutical, cosmetic and diagnostic applications of sulfated polysaccharides from marine algae and bacteria. Afr. J. Pharm. Pharmacol. 2017, 11, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Majee, S.B.; Avlani, D.; Ghosh, P.; Biswas, G.R. Absorption, distribution, metabolism and elimination (ADME) and toxicity profile of marine sulfated polysaccharides used in bionanotechnology. Afr. J. Pharm. Pharmacol. 2018, 12, 1–10. [Google Scholar]
- Luo, L.; Lin, X.; Zeng, F.; Luo, S.; Chen, Z.; Tian, G. Performance of a novel photobioreactor for nutrient removal from piggery biogas slurry: Operation parameters, microbial diversity and nutrient recovery potential. Bioresour. Technol. 2019, 272, 421–432. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Q.; Chen, H.; Fu, Z.; Fu, X.; Wang, J.; Liang, Y.; Yin, H.; Yang, J.; Jiang, J.; Yang, X.; et al. Current Progress, Challenges and Perspectives in the Microalgal-Bacterial Aerobic Granular Sludge Process: A Review. Int. J. Environ. Res. Public Health 2022, 19, 13950. https://doi.org/10.3390/ijerph192113950
Jiang Q, Chen H, Fu Z, Fu X, Wang J, Liang Y, Yin H, Yang J, Jiang J, Yang X, et al. Current Progress, Challenges and Perspectives in the Microalgal-Bacterial Aerobic Granular Sludge Process: A Review. International Journal of Environmental Research and Public Health. 2022; 19(21):13950. https://doi.org/10.3390/ijerph192113950
Chicago/Turabian StyleJiang, Qianrong, Honglei Chen, Zeding Fu, Xiaohua Fu, Jiacheng Wang, Yingqi Liang, Hailong Yin, Junbo Yang, Jie Jiang, Xinxin Yang, and et al. 2022. "Current Progress, Challenges and Perspectives in the Microalgal-Bacterial Aerobic Granular Sludge Process: A Review" International Journal of Environmental Research and Public Health 19, no. 21: 13950. https://doi.org/10.3390/ijerph192113950





