Impact of Intermittent Fasting on Metabolic Syndrome and Periodontal Disease—A Suggested Preventive Strategy to Reduce the Public Health Burden
Abstract
1. Introduction
2. Search Strategy and Selection Criteria
3. Review Analysis
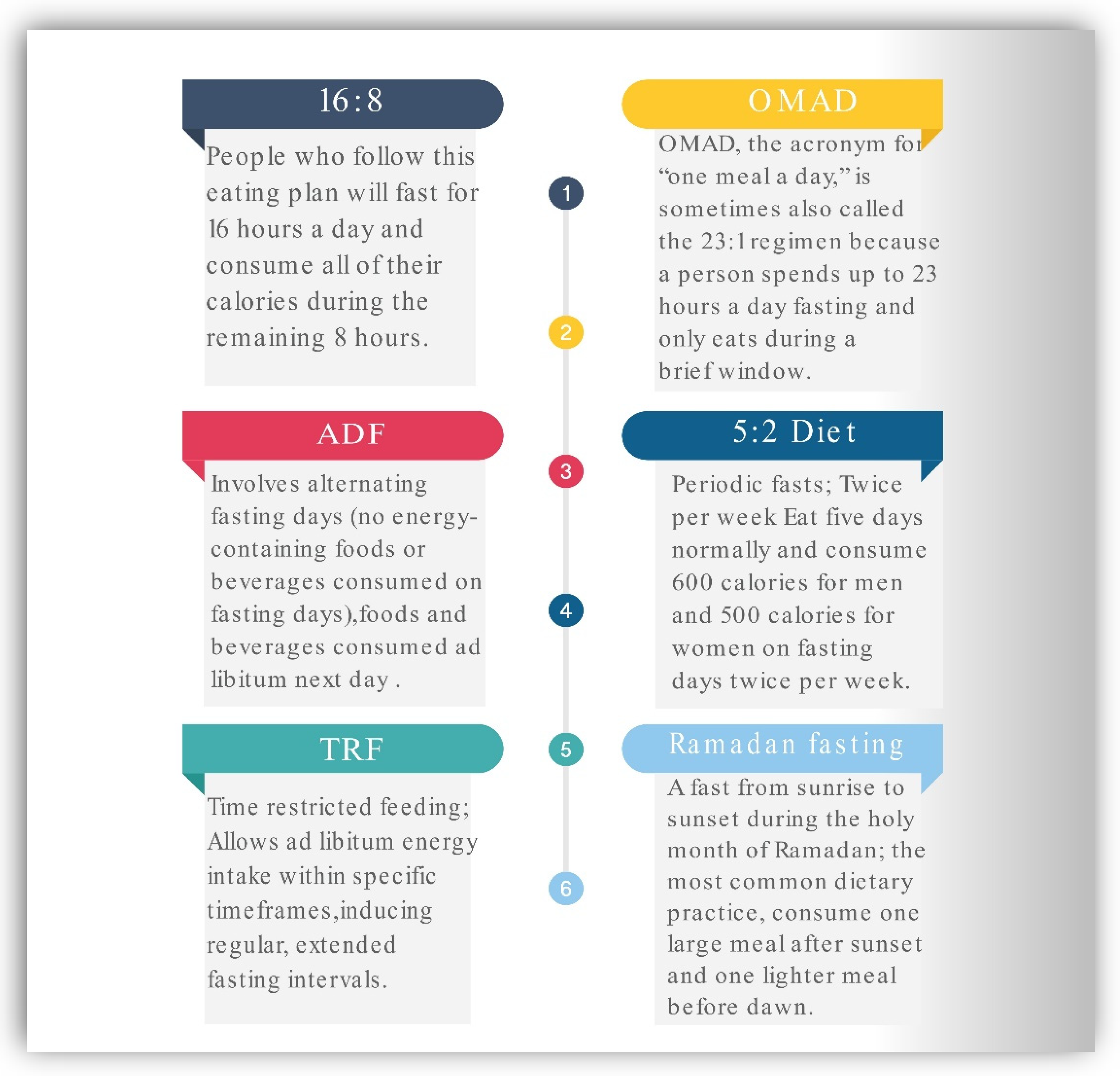
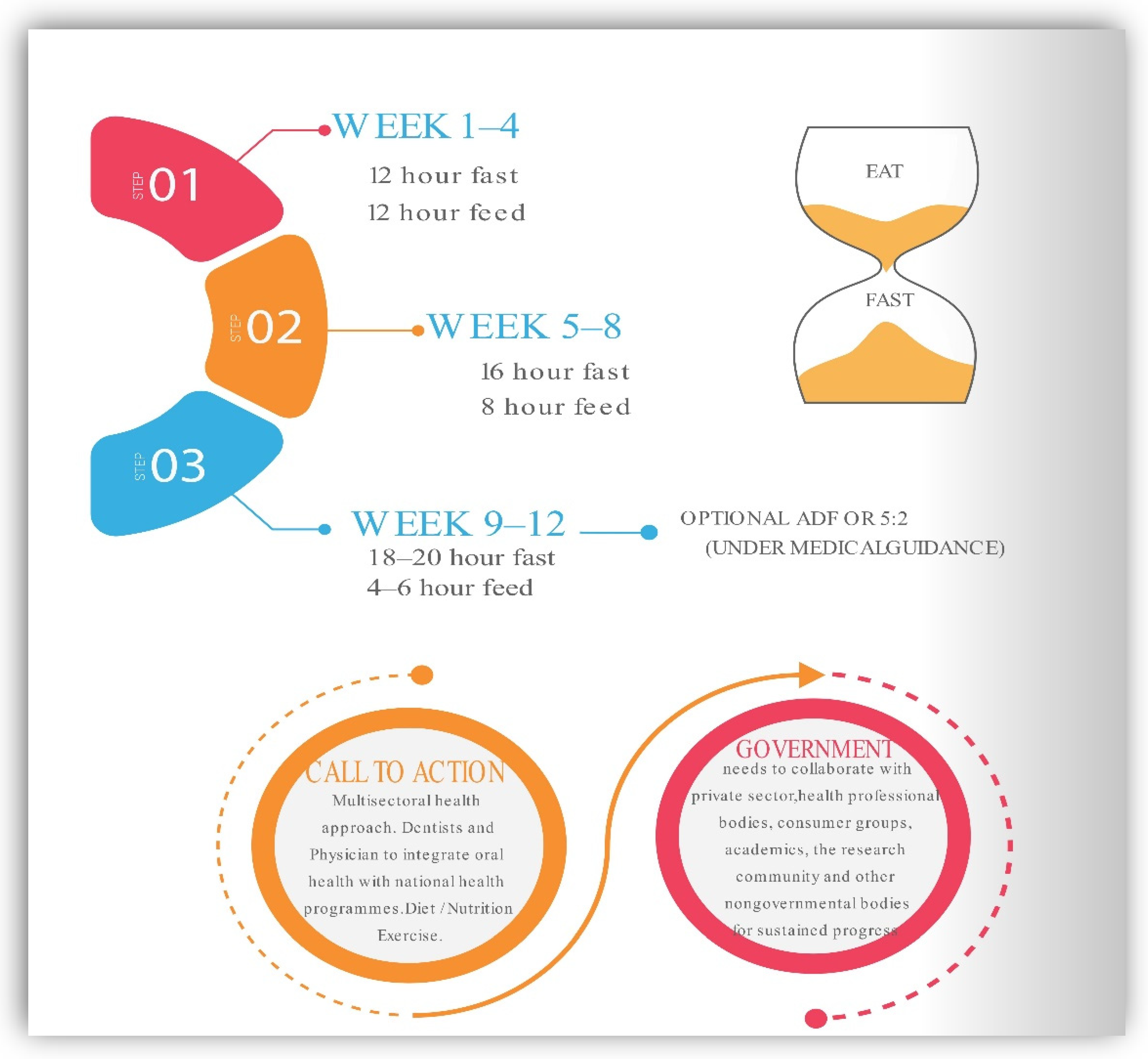
Most Common Plans of Intermittent Fasting
4. Cellular and Molecular Level Interactions during IF and Calorie Restriction (CR)
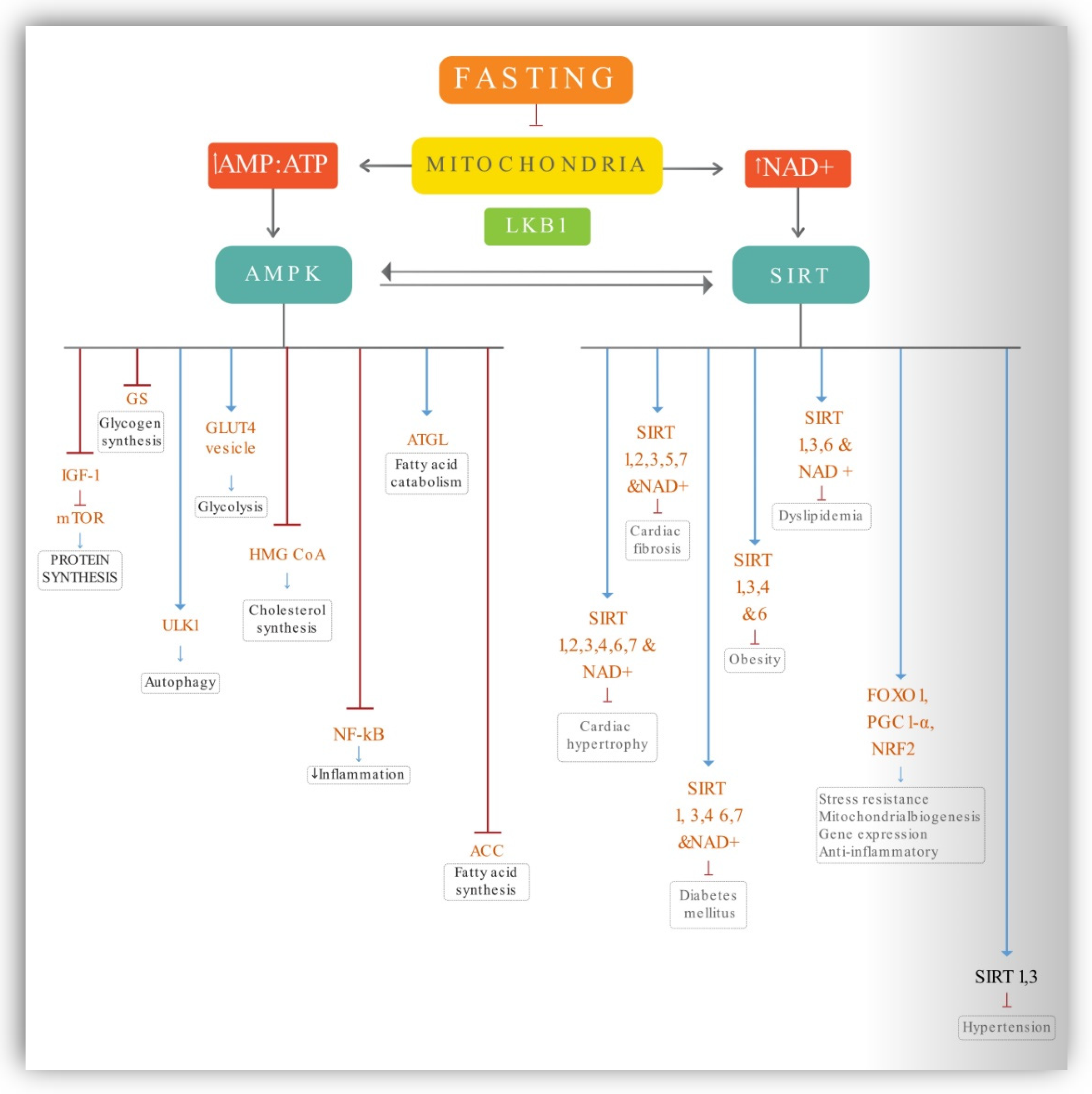
4.1. AMPK Activation
4.2. Sirtuins
4.3. Nrf2, FOXO, PGC-1α
5. Impact of IF on PD and Mets
5.1. Periodontal Diseases
5.2. Activation and Regulation of Periodontal Inflammation by AMPK Pathway and Role of Sirtuins
5.3. Impact of Intermittent Fasting and Calorie Restriction on Periodontal Inflammation Diseases
5.4. Evidence of Correlation between MetS and PD
5.5. Inflammation and Immune Mediation between PD and MetS
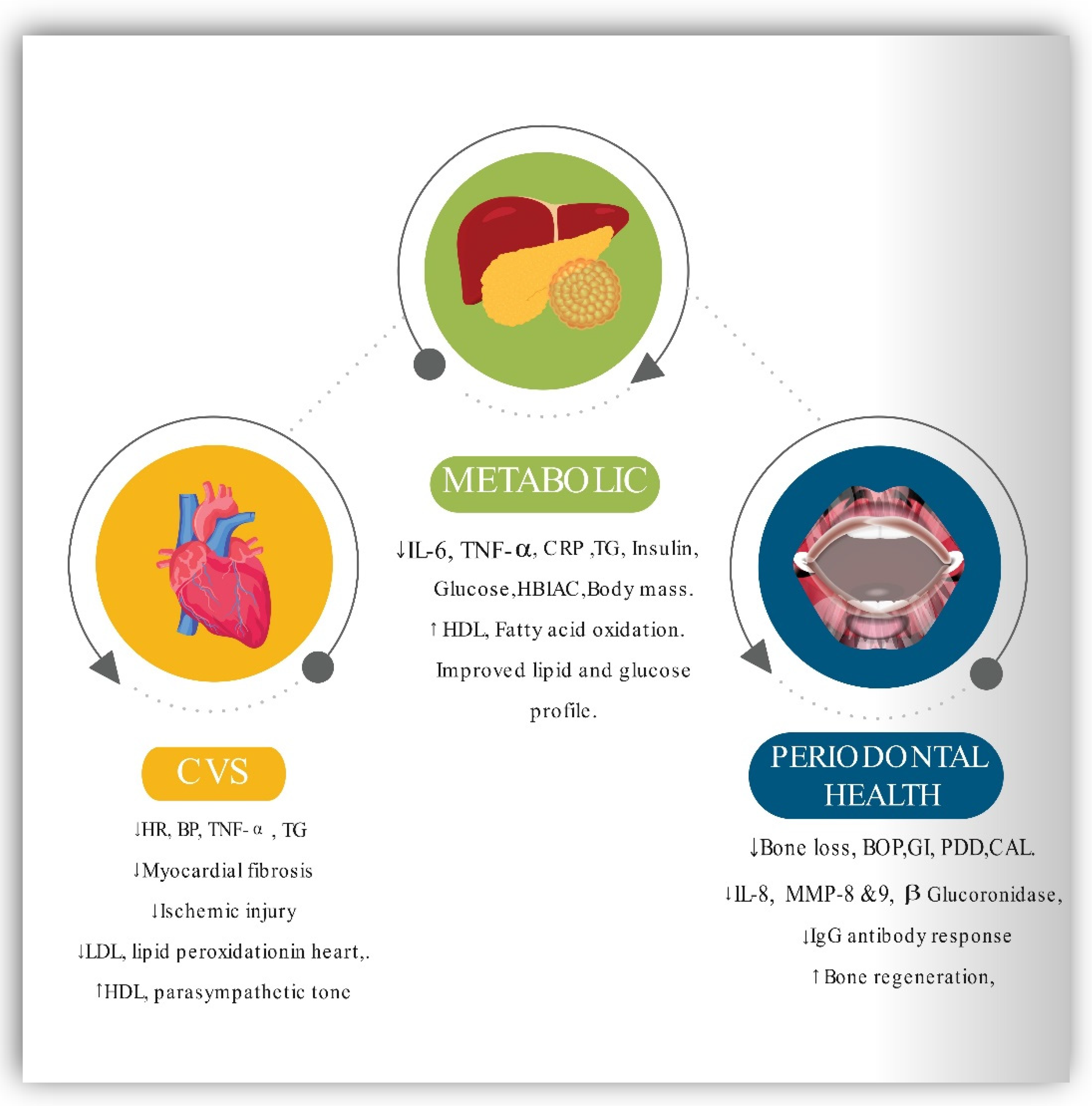
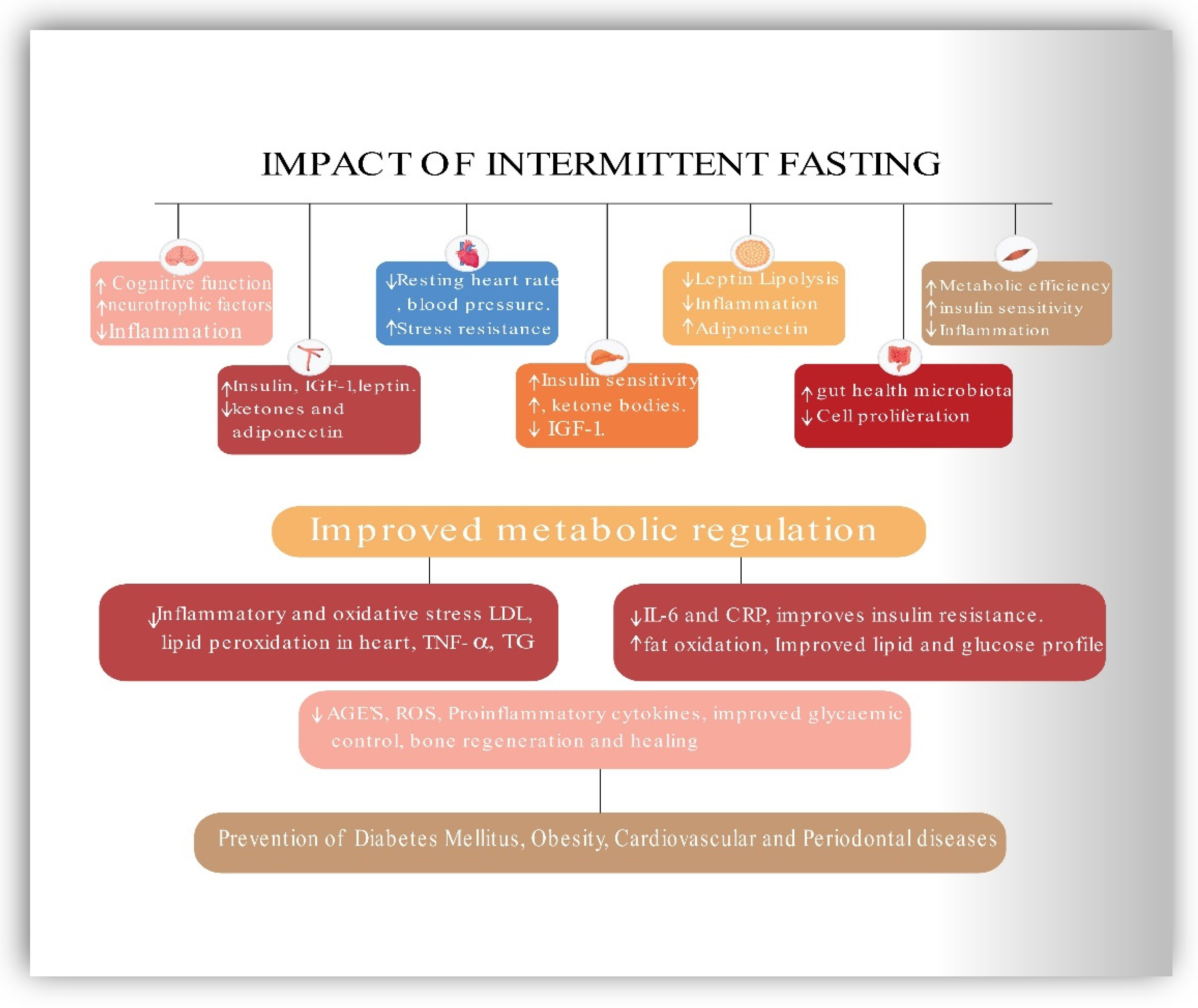
5.6. Impact of Intermittent Fasting on MetS
6. Contribution, Significance, and Implications of the Current Review
7. Current Research Gaps and Limitations
8. Future Directions
9. Recommendations for Intermittent Fasting in Practice
10. Conclusions
Funding
Conflicts of Interest
Abbreviations
References
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C.; et al. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Bovolini, A.; Garcia, J.; Andrade, M.A.; Duarte, J.A. Metabolic Syndrome Pathophysiology and Predisposing Factors. Int. J. Sports Med. 2021, 42, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Global Nutrition Report. 2020. Available online: https://globalnutritionreport.org/reports/2020-global-nutrition-report/ (accessed on 17 July 2020).
- Bulló, M.; García-Lorda, P.; Megias, I.; Salas-Salvadó, J. Systemic Inflammation, Adipose Tissue Tumor Necrosis Factor, and Leptin Expression. Obes. Res. 2003, 11, 525–531. [Google Scholar] [CrossRef]
- Taylor, G.W. Periodontal Treatment and Its Effects on Glycemic Control: A Review of the Evidence. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1999, 87, 311–316. [Google Scholar] [CrossRef]
- Emrich, L.J.; Shlossman, M.; Genco, R.J. Periodontal Disease in Non-Insulin-Dependent Diabetes Mellitus. J. Periodontol. 1991, 62, 123–131. [Google Scholar] [CrossRef]
- Pischon, N.; Heng, N.; Bernimoulin, J.-P.; Kleber, B.-M.; Willich, S.N.; Pischon, T. Obesity, Inflammation, and Periodontal Disease. J. Dent. Res. 2007, 86, 400–409. [Google Scholar] [CrossRef]
- Baumgartner, S.; Imfeld, T.; Schicht, O.; Rath, C.; Persson, R.; Persson, G.R. The Impact of the Stone Age Diet on Gingival Conditions in the Absence of Oral Hygiene. J. Periodontol. 2009, 80, 759–768. [Google Scholar] [CrossRef]
- El Makaky, Y.; Beltagy, T.; El Makakey, A. The Effects of an Anti-Inflammatory Diet on Gingival Health in Children (Randomized Controlled Trial). Egypt. Dent. J. 2019, 65, 1995–2002. [Google Scholar] [CrossRef][Green Version]
- Woelber, J.P.; Bremer, K.; Vach, K.; König, D.; Hellwig, E.; Ratka-Krüger, P.; Al-Ahmad, A.; Tennert, C. An Oral Health Optimized Diet Can Reduce Gingival and Periodontal Inflammation in Humans—A Randomized Controlled Pilot Study. BMC Oral Health 2017, 17, 28. [Google Scholar] [CrossRef]
- Iacopino, A.M.; Cutler, C.W. Pathophysiological Relationships between Periodontitis and Systemic Disease: Recent Concepts Involving Serum Lipids. J. Periodontol. 2000, 71, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Amar, S. Periodontal Disease and Systemic Conditions: A Bidirectional Relationship. Odontology 2006, 94, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-H.; Chasman, D.I.; Buring, J.E.; Rose, L.; Ridker, P.M. Cardiovascular Risks Associated with Incident and Prevalent Periodontal Disease. J. Clin. Periodontol. 2015, 42, 21–28. [Google Scholar] [CrossRef]
- Makkar, H.; Reynolds, M.A.; Wadhawan, A.; Dagdag, A.; Merchant, A.T.; Postolache, T.T. Periodontal, Metabolic, and Cardiovascular Disease: Exploring the Role of Inflammation and Mental Health. Pteridines 2018, 29, 124–163. [Google Scholar] [CrossRef] [PubMed]
- Desvarieux, M.; Demmer, R.T.; Jacobs, D.R.; Rundek, T.; Boden-Albala, B.; Sacco, R.L.; Papapanou, P.N. Periodontal Bacteria and Hypertension: The Oral Infections and Vascular Disease Epidemiology Study (INVEST). J. Hypertens. 2010, 28, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Arcaro, G.; Cretti, A.; Balzano, S.; Lechi, A.; Muggeo, M.; Bonora, E.; Bonadonna, R.C. Insulin Causes Endothelial Dysfunction in Humans: Sites and Mechanisms. Circulation 2002, 105, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Montagnani, M.; Koh, K.K.; Quon, M.J. Reciprocal Relationships between Insulin Resistance and Endothelial Dysfunction: Molecular and Pathophysiological Mechanisms. Circulation 2006, 113, 1888–1904. [Google Scholar] [CrossRef]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular Mechanisms and Clinical Applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef]
- Grajower, M.M.; Horne, B.D. Clinical Management of Intermittent Fasting in Patients with Diabetes Mellitus. Nutrients 2019, 11, 873. [Google Scholar] [CrossRef]
- Varady, K.A.; Bhutani, S.; Church, E.C.; Klempel, M.C. Short-Term Modified Alternate-Day Fasting: A Novel Dietary Strategy for Weight Loss and Cardioprotection in Obese Adults. Am. J. Clin. Nutr. 2009, 90, 1138–1143. [Google Scholar] [CrossRef]
- Templeman, I.; Thompson, D.; Gonzalez, J.; Walhin, J.-P.; Reeves, S.; Rogers, P.J.; Brunstrom, J.M.; Karagounis, L.G.; Tsintzas, K.; Betts, J.A. Intermittent Fasting, Energy Balance and Associated Health Outcomes in Adults: Study Protocol for a Randomised Controlled Trial. Trials 2018, 19, 86. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of Metabolism and Mitochondrial Homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.E.; Sinclair, D.A. Sirtuins and NAD+ in the Development and Treatment of Metabolic and Cardiovascular Diseases. Circ. Res. 2018, 123, 868–885. [Google Scholar] [CrossRef] [PubMed]
- De Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef]
- DOGAN, S.; ROGOZINA, O.P.; LOKSHIN, A.E.; GRANDE, J.P.; CLEARY, M.P. Effects of Chronic vs. Intermittent Calorie Restriction on Mammary Tumor Incidence and Serum Adiponectin and Leptin Levels in MMTV-TGF-α Mice at Different Ages. Oncol. Lett. 2010, 1, 167–176. [Google Scholar] [CrossRef]
- Di Francesco, A.; Di Germanio, C.; Bernier, M.; de Cabo, R. A Time to Fast. Science 2018, 362, 770–775. [Google Scholar] [CrossRef]
- Mattson, M.P.; Moehl, K.; Ghena, N.; Schmaedick, M.; Cheng, A. Intermittent Metabolic Switching, Neuroplasticity and Brain Health. Nat. Rev. Neurosci. 2018, 19, 63–80. [Google Scholar] [CrossRef]
- Panda, S. Circadian Physiology of Metabolism. Science 2016, 354, 1008–1015. [Google Scholar] [CrossRef]
- Patterson, R.E.; Sears, D.D. Metabolic Effects of Intermittent Fasting. Annu. Rev. Nutr. 2017, 37, 371–393. [Google Scholar] [CrossRef]
- Salminen, A.; Hyttinen, J.M.T.; Kaarniranta, K. AMP-Activated Protein Kinase Inhibits NF-ΚB Signaling and Inflammation: Impact on Healthspan and Lifespan. J. Mol. Med. 2011, 89, 667–676. [Google Scholar] [CrossRef]
- Long, Y.C.; Zierath, J.R. AMP-Activated Protein Kinase Signaling in Metabolic Regulation. J. Clin. Investig. 2006, 116, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMPK and Autophagy Get Connected. EMBO J. 2011, 30, 634–635. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, Z.E.; Pickering, J.; Eskiw, C.H. Better Living through Chemistry: Caloric Restriction (CR) and CR Mimetics Alter Genome Function to Promote Increased Health and Lifespan. Front. Genet. 2016, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Margină, D.; Ungurianu, A.; Purdel, C.; Tsoukalas, D.; Sarandi, E.; Thanasoula, M.; Tekos, F.; Mesnage, R.; Kouretas, D.; Tsatsakis, A. Chronic Inflammation in the Context of Everyday Life: Dietary Changes as Mitigating Factors. Int. J. Environ. Res. Public Health 2020, 17, 4135. [Google Scholar] [CrossRef]
- Zhu, Y.; Yan, Y.; Gius, D.R.; Vassilopoulos, A. Metabolic Regulation of Sirtuins upon Fasting and the Implication for Cancer. Curr. Opin. Oncol. 2013, 25, 630–636. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, X.; Ren, Y.; Li, J. Analysis of the Correlation between Periodontal Disease and Metabolic Syndrome among Coal Mine Workers: A Clinical Study. Medicine 2020, 99, e21566. [Google Scholar] [CrossRef]
- Lettieri-Barbato, D.; Minopoli, G.; Caggiano, R.; Izzo, R.; Santillo, M.; Aquilano, K.; Faraonio, R. Fasting Drives Nrf2-Related Antioxidant Response in Skeletal Muscle. Int. J. Mol. Sci. 2020, 21, 7780. [Google Scholar] [CrossRef]
- Peng, S.; Li, W.; Hou, N.; Huang, N. A Review of FoxO1-Regulated Metabolic Diseases and Related Drug Discoveries. Cells 2020, 9, 184. [Google Scholar] [CrossRef]
- Gross, D.N.; van den Heuvel, A.P.J.; Birnbaum, M.J. The Role of FoxO in the Regulation of Metabolism. Oncogene 2008, 27, 2320–2336. [Google Scholar] [CrossRef]
- Rius-Pérez, S.; Torres-Cuevas, I.; Millán, I.; Ortega, Á.L.; Pérez, S. PGC-1α, Inflammation, and Oxidative Stress: An Integrative View in Metabolism. Oxid. Med. Cell. Longev. 2020, 2020, 1452696. [Google Scholar] [CrossRef]
- Hasturk, H.; Kantarci, A. Activation and Resolution of Periodontal Inflammation and Its Systemic Impact. Periodontol. 2000 2015, 69, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Sorsa, T.; Ingman, T.; Suomalainen, K.; Haapasalo, M.; Konttinen, Y.T.; Lindy, O.; Saari, H.; Uitto, V.J. Identification of Proteases from Periodontopathogenic Bacteria as Activators of Latent Human Neutrophil and Fibroblast-Type Interstitial Collagenases. Infect. Immun. 1992, 60, 4491–4495. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Matthews, J.B. The Role of Reactive Oxygen and Antioxidant Species in Periodontal Tissue Destruction. Periodontol. 2000 2007, 43, 160–232. [Google Scholar] [CrossRef]
- Nagpal, R.; Yamashiro, Y.; Izumi, Y. The Two-Way Association of Periodontal Infection with Systemic Disorders: An Overview. Mediat. Inflamm. 2015, 2015, 793898. [Google Scholar] [CrossRef]
- Konkel, J.E.; O’Boyle, C.; Krishnan, S. Distal Consequences of Oral Inflammation. Front. Immunol. 2019, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; White, S.; Bartold, M. Periodontal Disease as a Risk Factor for Rheumatoid Arthritis: A Systematic Review. JBI Libr. Syst. Rev. 2012, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pitiphat, W.; Joshipura, K.J.; Gillman, M.W.; Williams, P.L.; Douglass, C.W.; Rich-Edwards, J.W. Maternal Periodontitis and Adverse Pregnancy Outcomes. Commun. Dent. Oral Epidemiol. 2008, 36, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Dong, H.; Huang, N.; Fang, J. Oxidative Stress and Inflammation Regulation of Sirtuins: New Insights into Common Oral Diseases. Front. Physiol. 2022, 13, 953078. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Gao, J.; Li, T.; Gan, X.; Yu, H. Sirtuin 3 Deficiency Exacerbates Age-Related Periodontal Disease. J. Periodontal Res. 2021, 56, 1163–1173. [Google Scholar] [CrossRef]
- Huang, L.; Sun, H.; Song, F.; Cao, Z.; Jiang, X.; Zhang, L.; Li, Z.; Huang, C. SIRT6 Overexpression Inhibits Cementogenesis by Suppressing Glucose Transporter 1. J. Cell. Physiol. 2019, 234, 4005–4014. [Google Scholar] [CrossRef]
- der Velden, U.V.; Kuzmanova, D.; Chapple, I.L.C. Micronutritional Approaches to Periodontal Therapy. J. Clin. Periodontol. 2011, 38, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahrani, M.S.; Borawski, E.A.; Bissada, N.F. Periodontitis and Three Health-Enhancing Behaviors: Maintaining Normal Weight, Engaging in Recommended Level of Exercise, and Consuming a High-Quality Diet. J. Periodontol. 2005, 76, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S. Impact of Calorie Restriction and Intermittent Fasting on Periodontal Health. Periodontol. 2000 2021, 87, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Wulansari, L.; Kaboosaya, B.; Khan, M.; Takahashi, M.; Nakata, H.; Kuroda, S.; Aoki, K.; Kasugai, S. Beneficial Effects of Fasting Regimens on Periodontal Tissues in Experimental Periodontitis Mice Model. J. Int. Dent. Med. Res. 2018, 11, 362–369. [Google Scholar]
- Branch-Mays, G.L.; Dawson, D.R.; Gunsolley, J.C.; Reynolds, M.A.; Ebersole, J.L.; Novak, K.F.; Mattison, J.A.; Ingram, D.K.; Novak, M.J. The Effects of a Calorie-Reduced Diet on Periodontal Inflammation and Disease in a Non-Human Primate Model. J. Periodontol. 2008, 79, 1184–1191. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Steffen, M.J.; Reynolds, M.A.; Branch-Mays, G.L.; Dawson, D.R.; Novak, K.F.; Gunsolley, J.C.; Mattison, J.A.; Ingram, D.K.; Novak, M.J. Differential Gender Effects of a Reduced Calorie Diet on Systemic Inflammatory and Immune Parameters in Nonhuman Primates. J. Periodontal Res. 2008, 43, 500–507. [Google Scholar] [CrossRef]
- Reynolds, M.A.; Dawson, D.R.; Novak, K.F.; Ebersole, J.L.; Gunsolley, J.C.; Branch-Mays, G.L.; Holt, S.C.; Mattison, J.A.; Ingram, D.K.; Novak, M.J. Effects of Caloric Restriction on Inflammatory Periodontal Disease. Nutrition 2009, 25, 88–97. [Google Scholar] [CrossRef]
- Pappe, C.L.; Steckhan, N.; Hoedke, D.; Jepsen, S.; Rauch, G.; Keller, T.; Michalsen, A.; Dommisch, H. Prolonged Multimodal Fasting Modulates Periodontal Inflammation in Female Patients with Metabolic Syndrome: A Prospective Cohort Study. J. Clin. Periodontol. 2021, 48, 492–502. [Google Scholar] [CrossRef]
- Saito, T.; Shimazaki, Y.; Sakamoto, M. Obesity and Periodontitis. N. Engl. J. Med. 1998, 339, 482–483. [Google Scholar] [CrossRef]
- Saito, T.; Shimazaki, Y.; Koga, T.; Tsuzuki, M.; Ohshima, A. Relationship between Upper Body Obesity and Periodontitis. J. Dent. Res. 2001, 80, 1631–1636. [Google Scholar] [CrossRef]
- Chávarry, N.G.M.; Vettore, M.V.; Sansone, C.; Sheiham, A. The Relationship between Diabetes Mellitus and Destructive Periodontal Disease: A Meta-Analysis. Oral Health Prev. Dent. 2009, 7, 107–127. [Google Scholar] [PubMed]
- Khader, Y.S.; Dauod, A.S.; El-Qaderi, S.S.; Alkafajei, A.; Batayha, W.Q. Periodontal Status of Diabetics Compared with Nondiabetics: A Meta-Analysis. J. Diabetes Complic. 2006, 20, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.W.; Burt, B.A.; Becker, M.P.; Genco, R.J.; Shlossman, M. Glycemic Control and Alveolar Bone Loss Progression in Type 2 Diabetes. Ann. Periodontol. 1998, 3, 30–39. [Google Scholar] [CrossRef]
- Cutler, C.W.; Shinedling, E.A.; Nunn, M.; Jotwani, R.; Kim, B.O.; Nares, S.; Iacopino, A.M. Association between Periodontitis and Hyperlipidemia: Cause or Effect? J. Periodontol. 1999, 70, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, F.; Murayama, Y. Periodontal Inflammation and Insulin Resistance--Lessons from Obesity. J. Dent. Res. 2001, 80, 1690–1694. [Google Scholar] [CrossRef] [PubMed]
- Chaffee, B.W.; Weston, S.J. Association Between Chronic Periodontal Disease and Obesity: A Systematic Review and Meta-Analysis. J. Periodontol. 2010, 81, 1708–1724. [Google Scholar] [CrossRef]
- Marchetti, E.; Monaco, A.; Procaccini, L.; Mummolo, S.; Gatto, R.; Tetè, S.; Baldini, A.; Tecco, S.; Marzo, G. Periodontal Disease: The Influence of Metabolic Syndrome. Nutr. Metab. 2012, 9, 88. [Google Scholar] [CrossRef]
- Lee, K.-S.; Lee, S.G.; Kim, E.-K.; Jin, H.-J.; Im, S.-U.; Lee, H.-K.; Merchant, A.T.; Song, K.-B.; Choi, Y.-H. Metabolic Syndrome Parameters in Adolescents May Be Determinants for the Future Periodontal Diseases. J. Clin. Periodontol. 2015, 42, 105–112. [Google Scholar] [CrossRef]
- Shimazaki, Y.; Saito, T.; Yonemoto, K.; Kiyohara, Y.; Iida, M.; Yamashita, Y. Relationship of Metabolic Syndrome to Periodontal Disease in Japanese Women: The Hisayama Study. J. Dent. Res. 2007, 86, 271–275. [Google Scholar] [CrossRef]
- Nishimura, F.; Iwamoto, Y.; Mineshiba, J.; Shimizu, A.; Soga, Y.; Murayama, Y. Periodontal Disease and Diabetes Mellitus: The Role of Tumor Necrosis Factor-Alpha in a 2-Way Relationship. J. Periodontol. 2003, 74, 97–102. [Google Scholar] [CrossRef]
- Kaye, E.K.; Chen, N.; Cabral, H.J.; Vokonas, P.; Garcia, R.I. Metabolic Syndrome and Periodontal Disease Progression in Men. J. Dent. Res. 2016, 95, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Tegelberg, P.; Tervonen, T.; Knuuttila, M.; Jokelainen, J.; Keinänen-Kiukaanniemi, S.; Auvinen, J.; Ylöstalo, P. Long-Term Metabolic Syndrome Is Associated with Periodontal Pockets and Alveolar Bone Loss. J. Clin. Periodontol. 2019, 46, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Yamazaki, Y.; Mita, A.; Takada, K.; Seto, M.; Nishinoue, N.; Sasaki, Y.; Motohashi, M.; Maeno, M. A Cohort Study on the Association between Periodontal Disease and the Development of Metabolic Syndrome. J. Periodontol. 2010, 81, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, S.-I.; Yamada, S.-I.; Karasawa, I.; Sakurai, A.; Kurita, H. A Longitudinal Study on the Relationship between Dental Health and Metabolic Syndrome in Japan. J. Periodontol. 2019, 90, 728–746. [Google Scholar] [CrossRef]
- Bullon, P.; Morillo, J.M.; Ramirez-Tortosa, M.C.; Quiles, J.L.; Newman, H.N.; Battino, M. Metabolic Syndrome and Periodontitis: Is Oxidative Stress a Common Link? J. Dent. Res. 2009, 88, 503–518. [Google Scholar] [CrossRef]
- Adachi, N.; Kobayashi, Y. One-Year Follow-up Study on Associations between Dental Caries, Periodontitis, and Metabolic Syndrome. J. Oral Sci. 2020, 62, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, G.G.; Leite, F.R.M.; Peres, K.G.; Demarco, F.F.; Corrêa, M.B.; Peres, M.A. Metabolic Syndrome and Periodontitis: A Structural Equation Modeling Approach. J. Periodontol. 2019, 90, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Sato, M.; Minagawa, K.; Manz, M.C.; Yoshihara, A.; Miyazaki, H. Longitudinal Relationship between Metabolic Syndrome and Periodontal Disease among Japanese Adults Aged ≥ 70 Years: The Niigata Study. J. Periodontol. 2015, 86, 491–498. [Google Scholar] [CrossRef]
- Li, P.; He, L.; Sha, Y.-Q.; Luan, Q.-X. Relationship of Metabolic Syndrome to Chronic Periodontitis. J. Periodontol. 2009, 80, 541–549. [Google Scholar] [CrossRef]
- Borges, P.K.D.O.; Gimeno, S.G.A.; Tomita, N.E.; Ferreira, S.R. Prevalência e características associadas à síndrome metabólica em nipo-brasileiros com e sem doença periodontal. Cad. Saúde Pública 2007, 23, 657–668. [Google Scholar] [CrossRef]
- Khader, Y.; Khassawneh, B.; Obeidat, B.; Hammad, M.; El-Salem, K.; Bawadi, H.; Al-akour, N. Periodontal Status of Patients with Metabolic Syndrome Compared to Those without Metabolic Syndrome. J. Periodontol. 2008, 79, 2048–2053. [Google Scholar] [CrossRef] [PubMed]
- D’Aiuto, F.; Sabbah, W.; Netuveli, G.; Donos, N.; Hingorani, A.D.; Deanfield, J.; Tsakos, G. Association of the Metabolic Syndrome with Severe Periodontitis in a Large U.S. Population-Based Survey. J. Clin. Endocrinol. Metab. 2008, 93, 3989–3994. [Google Scholar] [CrossRef] [PubMed]
- Andriankaja, O.M.; Genco, R.J.; Dorn, J.; Dmochowski, J.; Hovey, K.; Falkner, K.L.; Trevisan, M. Periodontal Disease and Risk of Myocardial Infarction: The Role of Gender and Smoking. Eur. J. Epidemiol. 2007, 22, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Kushiyama, M.; Shimazaki, Y.; Yamashita, Y. Relationship between Metabolic Syndrome and Periodontal Disease in Japanese Adults. J. Periodontol. 2009, 80, 1610–1615. [Google Scholar] [CrossRef]
- Benguigui, C.; Bongard, V.; Ruidavets, J.-B.; Chamontin, B.; Sixou, M.; Ferrières, J.; Amar, J. Metabolic Syndrome, Insulin Resistance, and Periodontitis: A Cross-Sectional Study in a Middle-Aged French Population. J. Clin. Periodontol. 2010, 37, 601–608. [Google Scholar] [CrossRef]
- Timonen, P.; Niskanen, M.; Suominen-Taipale, L.; Jula, A.; Knuuttila, M.; Ylöstalo, P. Metabolic Syndrome, Periodontal Infection, and Dental Caries. J. Dent. Res. 2010, 89, 1068–1073. [Google Scholar] [CrossRef]
- Han, D.-H.; Lim, S.-Y.; Sun, B.-C.; Paek, D.; Kim, H.-D. The Association of Metabolic Syndrome with Periodontal Disease Is Confounded by Age and Smoking in a Korean Population: The Shiwha-Banwol Environmental Health Study. J. Clin. Periodontol. 2010, 37, 609–616. [Google Scholar] [CrossRef]
- Furuta, M.; Shimazaki, Y.; Takeshita, T.; Shibata, Y.; Akifusa, S.; Eshima, N.; Kiyohara, Y.; Ninomiya, T.; Hirakawa, Y.; Mukai, N.; et al. Gender Differences in the Association between Metabolic Syndrome and Periodontal Disease: The Hisayama Study. J. Clin. Periodontol. 2013, 40, 743–752. [Google Scholar] [CrossRef]
- Chen, L.-P.; Hsu, S.-P.; Peng, Y.-S.; Chiang, C.-K.; Hung, K.-Y. Periodontal Disease Is Associated with Metabolic Syndrome in Hemodialysis Patients. Nephrol. Dial. Transplant. 2011, 26, 4068–4073. [Google Scholar] [CrossRef][Green Version]
- Nesbitt, M.J.; Reynolds, M.A.; Shiau, H.; Choe, K.; Simonsick, E.M.; Ferrucci, L. Association of Periodontitis and Metabolic Syndrome in the Baltimore Longitudinal Study of Aging. Aging Clin. Exp. Res. 2010, 22, 238–242. [Google Scholar] [CrossRef][Green Version]
- Fukui, N.; Shimazaki, Y.; Shinagawa, T.; Yamashita, Y. Periodontal Status and Metabolic Syndrome in Middle-Aged Japanese. J. Periodontol. 2012, 83, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-E.; Ha, J.-E.; Paik, D.-I.; Jin, B.-H.; Bae, K.-H. The Relationship between Periodontitis and Metabolic Syndrome among a Korean Nationally Representative Sample of Adults. J. Clin. Periodontol. 2011, 38, 781–786. [Google Scholar] [CrossRef] [PubMed]
- LaMonte, M.J.; Williams, A.M.; Genco, R.J.; Andrews, C.A.; Hovey, K.M.; Millen, A.E.; Browne, R.W.; Trevisan, M.; Wactawski-Wende, J. Association between Metabolic Syndrome and Periodontal Disease Measures in Postmenopausal Women: The Buffalo OsteoPerio Study. J. Periodontol. 2014, 85, 1489–1501. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, K.; Iwasaki, M.; Ogawa, H.; Yoshihara, A.; Miyazaki, H. Relationship between Metabolic Syndrome and Periodontitis in 80-Year-Old Japanese Subjects. J. Periodontal Res. 2015, 50, 173–179. [Google Scholar] [CrossRef]
- Gomes-Filho, I.S.; das Mercês, M.C.; de Santana Passos-Soares, J.; Seixas da Cruz, S.; Teixeira Ladeia, A.M.; Trindade, S.C.; de Moraes Marcílio Cerqueira, E.; Freitas Coelho, J.M.; Marques Monteiro, F.M.; Barreto, M.L.; et al. Severity of Periodontitis and Metabolic Syndrome: Is There an Association? J. Periodontol. 2016, 87, 357–366. [Google Scholar] [CrossRef]
- Jaramillo, A.; Contreras, A.; Lafaurie, G.I.; Duque, A.; Ardila, C.M.; Duarte, S.; Osorio, L. Association of Metabolic Syndrome and Chronic Periodontitis in Colombians. Clin. Oral Investig. 2017, 21, 1537–1544. [Google Scholar] [CrossRef]
- Kim, O.S.; Shin, M.H.; Kweon, S.S.; Lee, Y.H.; Kim, O.J.; Kim, Y.J.; Chung, H.J. The Severity of Periodontitis and Metabolic Syndrome in Korean Population: The Dong-Gu Study. J. Periodontal Res. 2018, 53, 362–368. [Google Scholar] [CrossRef]
- Campos, J.R.; Costa, F.O.; Cota, L.O.M. Association between Periodontitis and Metabolic Syndrome: A Case-Control Study. J. Periodontol. 2020, 91, 784–791. [Google Scholar] [CrossRef]
- Sora, N.D.; Marlow, N.M.; Bandyopadhyay, D.; Leite, R.S.; Slate, E.H.; Fernandes, J.K. Metabolic Syndrome and Periodontitis in Gullah African Americans with Type 2 Diabetes Mellitus. J. Clin. Periodontol. 2013, 40, 599–606. [Google Scholar] [CrossRef]
- Thanakun, S.; Watanabe, H.; Thaweboon, S.; Izumi, Y. Comparison of Salivary and Plasma Adiponectin and Leptin in Patients with Metabolic Syndrome. Diabetol. Metab. Syndr. 2014, 6, 19. [Google Scholar] [CrossRef]
- Musskopf, M.L.; Daudt, L.D.; Weidlich, P.; Gerchman, F.; Gross, J.L.; Oppermann, R.V. Metabolic Syndrome as a Risk Indicator for Periodontal Disease and Tooth Loss. Clin. Oral Investig. 2017, 21, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Kikui, M.; Kokubo, Y.; Ono, T.; Kida, M.; Kosaka, T.; Yamamoto, M.; Watanabe, M.; Maeda, Y.; Miyamoto, Y. Relationship between Metabolic Syndrome Components and Periodontal Disease in a Japanese General Population: The Suita Study. J. Atheroscler. Thromb. 2017, 24, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Pham, T. The Association between Periodontal Disease Severity and Metabolic Syndrome in Vietnamese Patients. Int. J. Dent. Hyg. 2018, 16, 484–491. [Google Scholar] [CrossRef] [PubMed]
- López, N.J.; Quintero, A.; Casanova, P.A.; Ibieta, C.I.; Baelum, V.; López, R. Effects of Periodontal Therapy on Systemic Markers of Inflammation in Patients with Metabolic Syndrome: A Controlled Clinical Trial. J. Periodontol. 2012, 83, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Azevedo, I. Chronic Inflammation in Obesity and the Metabolic Syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.D.; Manson, J.E.; Rifai, N.; Buring, J.E.; Ridker, P.M. C-Reactive Protein, Interleukin 6, and Risk of Developing Type 2 Diabetes Mellitus. JAMA 2001, 286, 327–334. [Google Scholar] [CrossRef]
- Vozarova, B.; Weyer, C.; Hanson, K.; Tataranni, P.A.; Bogardus, C.; Pratley, R.E. Circulating Interleukin-6 in Relation to Adiposity, Insulin Action, and Insulin Secretion. Obes. Res. 2001, 9, 414–417. [Google Scholar] [CrossRef]
- Dandona, P.; Weinstock, R.; Thusu, K.; Abdel-Rahman, E.; Aljada, A.; Wadden, T. Tumor Necrosis Factor-Alpha in Sera of Obese Patients: Fall with Weight Loss. J. Clin. Endocrinol. Metab. 1998, 83, 2907–2910. [Google Scholar] [CrossRef]
- Kern, P.A.; Ranganathan, S.; Li, C.; Wood, L.; Ranganathan, G. Adipose Tissue Tumor Necrosis Factor and Interleukin-6 Expression in Human Obesity and Insulin Resistance. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E745–E751. [Google Scholar] [CrossRef]
- Dandona, P.; Aljada, A.; Bandyopadhyay, A. Inflammation: The Link between Insulin Resistance, Obesity and Diabetes. Trends Immunol. 2004, 25, 4–7. [Google Scholar] [CrossRef]
- Grace, C.S.; Goldrick, R.B. Fibrinolysis and Body Bulid. Interrelationships between Blood Fibrinolysis, Body Composition and Parameters of Lipid and Carbohydrate Metabolism. J. Atheroscler. Res. 1968, 8, 705–719. [Google Scholar] [CrossRef]
- Blood Fibrinolytic Activity in Diabetes Mellitus and Its Bearing on Ischaemic Heart Disease and Obesity. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2122789/ (accessed on 26 July 2021).
- Eggesbø, J.B.; Hjermann, I.; Høstmark, A.T.; Kierulf, P. LPS Induced Release of IL-1 Beta, IL-6, IL-8 and TNF-Alpha in EDTA or Heparin Anticoagulated Whole Blood from Persons with High or Low Levels of Serum HDL. Cytokine 1996, 8, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Yalda, B.; Collins, J.G.; Jones, B.H.; Smith, F.W.; Arnold, R.R.; Offenbacher, S. Inflammatory Mediator Response as a Potential Risk Marker for Periodontal Diseases in Insulin-Dependent Diabetes Mellitus Patients. J. Periodontol. 1997, 68, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Stashenko, P.; Fujiyoshi, P.; Obernesser, M.S.; Prostak, L.; Haffajee, A.D.; Socransky, S.S. Levels of Interleukin 1 Beta in Tissue from Sites of Active Periodontal Disease. J. Clin. Periodontol. 1991, 18, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Ciampolillo, A.; Guastamacchia, E.; Caragiulo, L.; Lollino, G.; De Robertis, O.; Lattanzi, V.; Giorgino, R. In Vitro Secretion of Interleukin-1 Beta and Interferon-Gamma by Peripheral Blood Lymphomononuclear Cells in Diabetic Patients. Diabetes Res. Clin. Pract. 1993, 21, 87–93. [Google Scholar] [CrossRef]
- Lalla, E.; Papapanou, P.N. Diabetes Mellitus and Periodontitis: A Tale of Two Common Interrelated Diseases. Nat. Rev. Endocrinol. 2011, 7, 738–748. [Google Scholar] [CrossRef]
- Kebschull, M.; Demmer, R.T.; Papapanou, P.N. “Gum Bug, Leave My Heart Alone!”—Epidemiologic and Mechanistic Evidence Linking Periodontal Infections and Atherosclerosis. J. Dent. Res. 2010, 89, 879–902. [Google Scholar] [CrossRef]
- Loos, B.G. Systemic Markers of Inflammation in Periodontitis. J. Periodontol. 2005, 76, 2106–2115. [Google Scholar] [CrossRef]
- Paraskevas, S.; Huizinga, J.D.; Loos, B.G. A Systematic Review and Meta-Analyses on C-Reactive Protein in Relation to Periodontitis. J. Clin. Periodontol. 2008, 35, 277–290. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Budavari, A.; Murray, D.; Spiegelman, B.M. Reduced Tyrosine Kinase Activity of the Insulin Receptor in Obesity-Diabetes. Central Role of Tumor Necrosis Factor-Alpha. J. Clin. Investig. 1994, 94, 1543–1549. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose Expression of Tumor Necrosis Factor-Alpha: Direct Role in Obesity-Linked Insulin Resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Pickup, J.C. Inflammation and Activated Innate Immunity in the Pathogenesis of Type 2 Diabetes. Diabetes Care 2004, 27, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and Insulin Resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- King, G.L. The Role of Inflammatory Cytokines in Diabetes and Its Complications. J. Periodontol. 2008, 79, 1527–1534. [Google Scholar] [CrossRef]
- Abbatecola, A.M.; Ferrucci, L.; Grella, R.; Bandinelli, S.; Bonafè, M.; Barbieri, M.; Corsi, A.M.; Lauretani, F.; Franceschi, C.; Paolisso, G. Diverse Effect of Inflammatory Markers on Insulin Resistance and Insulin-Resistance Syndrome in the Elderly. J. Am. Geriatr. Soc. 2004, 52, 399–404. [Google Scholar] [CrossRef]
- Serum Levels of Soluble Tumor Necrosis Factor-Alpha Receptor 2 Are Linked to Insulin Resistance and Glucose Intolerance in Children—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/15679072/ (accessed on 26 July 2021).
- Engebretson, S.; Chertog, R.; Nichols, A.; Hey-Hadavi, J.; Celenti, R.; Grbic, J. Plasma Levels of Tumour Necrosis Factor-Alpha in Patients with Chronic Periodontitis and Type 2 Diabetes. J. Clin. Periodontol. 2007, 34, 18–24. [Google Scholar] [CrossRef]
- Naguib, G.; Al-Mashat, H.; Desta, T.; Graves, D.T. Diabetes Prolongs the Inflammatory Response to a Bacterial Stimulus through Cytokine Dysregulation. J. Investig. Dermatol. 2004, 123, 87–92. [Google Scholar] [CrossRef]
- Takano, M.; Nishihara, R.; Sugano, N.; Matsumoto, K.; Yamada, Y.; Takane, M.; Fujisaki, Y.; Ito, K. The Effect of Systemic Anti-Tumor Necrosis Factor-Alpha Treatment on Porphyromonas Gingivalis Infection in Type 2 Diabetic Mice. Arch. Oral Biol. 2010, 55, 379–384. [Google Scholar] [CrossRef]
- Santos, V.R.; Lima, J.A.; Gonçalves, T.E.D.; Bastos, M.F.; Figueiredo, L.C.; Shibli, J.A.; Duarte, P.M. Receptor Activator of Nuclear Factor-Kappa B Ligand/Osteoprotegerin Ratio in Sites of Chronic Periodontitis of Subjects with Poorly and Well-Controlled Type 2 Diabetes. J. Periodontol. 2010, 81, 1455–1465. [Google Scholar] [CrossRef]
- Duarte, P.M.; Neto, J.B.C.; Casati, M.Z.; Sallum, E.A.; Nociti, F.H. Diabetes Modulates Gene Expression in the Gingival Tissues of Patients with Chronic Periodontitis. Oral Dis. 2007, 13, 594–599. [Google Scholar] [CrossRef]
- Mahamed, D.A.; Marleau, A.; Alnaeeli, M.; Singh, B.; Zhang, X.; Penninger, J.M.; Teng, Y.-T.A. G(−) Anaerobes–Reactive CD4+ T-Cells Trigger RANKL-Mediated Enhanced Alveolar Bone Loss in Diabetic NOD Mice. Diabetes 2005, 54, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Liu, R.; Desta, T.; Leone, C.; Gerstenfeld, L.C.; Graves, D.T. Diabetes Causes Decreased Osteoclastogenesis, Reduced Bone Formation, and Enhanced Apoptosis of Osteoblastic Cells in Bacteria Stimulated Bone Loss. Endocrinology 2004, 145, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Bal, H.S.; Desta, T.; Krothapalli, N.; Alyassi, M.; Luan, Q.; Graves, D.T. Diabetes Enhances Periodontal Bone Loss through Enhanced Resorption and Diminished Bone Formation. J. Dent. Res. 2006, 85, 510–514. [Google Scholar] [CrossRef]
- Liu, R.; Desta, T.; He, H.; Graves, D.T. Diabetes Alters the Response to Bacteria by Enhancing Fibroblast Apoptosis. Endocrinology 2004, 145, 2997–3003. [Google Scholar] [CrossRef] [PubMed]
- Lalla, E.; Lamster, I.B.; Schmidt, A.M. Enhanced Interaction of Advanced Glycation End Products with Their Cellular Receptor RAGE: Implications for the Pathogenesis of Accelerated Periodontal Disease in Diabetes. Ann. Periodontol. 1998, 3, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Lalla, E.; Lamster, I.B.; Feit, M.; Huang, L.; Spessot, A.; Qu, W.; Kislinger, T.; Lu, Y.; Stern, D.M.; Schmidt, A.M. Blockade of RAGE Suppresses Periodontitis-Associated Bone Loss in Diabetic Mice. J. Clin. Investig. 2000, 105, 1117–1124. [Google Scholar] [CrossRef]
- Genco, R.J.; Grossi, S.G.; Ho, A.; Nishimura, F.; Murayama, Y. A Proposed Model Linking Inflammation to Obesity, Diabetes, and Periodontal Infections. J. Periodontol. 2005, 76, 2075–2084. [Google Scholar] [CrossRef]
- Johnson, R.B.; Serio, F.G. Leptin within Healthy and Diseased Human Gingiva. J. Periodontol. 2001, 72, 1254–1257. [Google Scholar] [CrossRef]
- Karthikeyan, B.V.; Pradeep, A.R. Leptin Levels in Gingival Crevicular Fluid in Periodontal Health and Disease. J. Periodontal Res. 2007, 42, 300–304. [Google Scholar] [CrossRef]
- Karthikeyan, B.V.; Pradeep, A.R. Gingival Crevicular Fluid and Serum Leptin: Their Relationship to Periodontal Health and Disease. J. Clin. Periodontol. 2007, 34, 467–472. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Kukita, T.; Li, Y.-J.; Martinez Argueta, J.G.; Saito, T.; Hanazawa, S.; Yamashita, Y. Adiponectin Inhibits Osteoclast Formation Stimulated by Lipopolysaccharide from Actinobacillus Actinomycetemcomitans. FEMS Immunol. Med. Microbiol. 2007, 49, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, Y.; Nishimura, F.; Soga, Y.; Takeuchi, K.; Kurihara, M.; Takashiba, S.; Murayama, Y. Antimicrobial Periodontal Treatment Decreases Serum C-Reactive Protein, Tumor Necrosis Factor-Alpha, but Not Adiponectin Levels in Patients with Chronic Periodontitis. J. Periodontol. 2003, 74, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Furugen, R.; Hayashida, H.; Yamaguchi, N.; Yoshihara, A.; Ogawa, H.; Miyazaki, H.; Saito, T. The Relationship between Periodontal Condition and Serum Levels of Resistin and Adiponectin in Elderly Japanese. J. Periodontal Res. 2008, 43, 556–562. [Google Scholar] [CrossRef]
- Saito, T.; Yamaguchi, N.; Shimazaki, Y.; Hayashida, H.; Yonemoto, K.; Doi, Y.; Kiyohara, Y.; Iida, M.; Yamashita, Y. Serum Levels of Resistin and Adiponectin in Women with Periodontitis: The Hisayama Study. J. Dent. Res. 2008, 87, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Bokarewa, M.; Nagaev, I.; Dahlberg, L.; Smith, U.; Tarkowski, A. Resistin, an Adipokine with Potent Proinflammatory Properties. J. Immunol. 2005, 174, 5789–5795. [Google Scholar] [CrossRef] [PubMed]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The Hormone Resistin Links Obesity to Diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef]
- Aksungar, F.B.; Topkaya, A.E.; Akyildiz, M. Interleukin-6, C-Reactive Protein and Biochemical Parameters during Prolonged Intermittent Fasting. Ann. Nutr. Metab. 2007, 51, 88–95. [Google Scholar] [CrossRef]
- Faris, M.A.-I.E.; Kacimi, S.; Al-Kurd, R.A.; Fararjeh, M.A.; Bustanji, Y.K.; Mohammad, M.K.; Salem, M.L. Intermittent Fasting during Ramadan Attenuates Proinflammatory Cytokines and Immune Cells in Healthy Subjects. Nutr. Res. 2012, 32, 947–955. [Google Scholar] [CrossRef]
- Coppack, S.W. Pro-Inflammatory Cytokines and Adipose Tissue. Proc. Nutr. Soc. 2001, 60, 349–356. [Google Scholar] [CrossRef]
- Abd El-Kader, S.M.; Saiem Al-Dahr, M.H. Impact of Weight Loss on Oxidative Stress and Inflammatory Cytokines in Obese Type 2 Diabetic Patients. Afr. Health Sci. 2016, 16, 725–733. [Google Scholar] [CrossRef]
- Montagner, A.; Polizzi, A.; Fouché, E.; Ducheix, S.; Lippi, Y.; Lasserre, F.; Barquissau, V.; Régnier, M.; Lukowicz, C.; Benhamed, F.; et al. Liver PPARα Is Crucial for Whole-Body Fatty Acid Homeostasis and Is Protective against NAFLD. Gut 2016, 65, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Hong, N.; Kim, K.-W.; Cho, S.J.; Lee, M.; Lee, Y.-H.; Lee, Y.-H.; Kang, E.S.; Cha, B.-S.; Lee, B.-W. The Effectiveness of Intermittent Fasting to Reduce Body Mass Index and Glucose Metabolism: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 1645. [Google Scholar] [CrossRef]
- Most, J.; Tosti, V.; Redman, L.M.; Fontana, L. Calorie Restriction in Humans: An Update. Aging Res. Rev. 2017, 39, 36–45. [Google Scholar] [CrossRef]
- Zubrzycki, A.; Cierpka-Kmiec, K.; Kmiec, Z.; Wronska, A. The Role of Low-Calorie Diets and Intermittent Fasting in the Treatment of Obesity and Type-2 Diabetes. J. Physiol. Pharmacol. 2018, 69, 663–683. [Google Scholar] [CrossRef]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.; Mainous, A.G.; Leeuwenburgh, C.; Mattson, M.P. Flipping the Metabolic Switch: Understanding and Applying Health Benefits of Fasting. Obesity 2018, 26, 254–268. [Google Scholar] [CrossRef]
- Harvie, M.; Wright, C.; Pegington, M.; McMullan, D.; Mitchell, E.; Martin, B.; Cutler, R.G.; Evans, G.; Whiteside, S.; Maudsley, S.; et al. The Effect of Intermittent Energy and Carbohydrate Restriction v. Daily Energy Restriction on Weight Loss and Metabolic Disease Risk Markers in Overweight Women. Br. J. Nutr. 2013, 110, 1534–1547. [Google Scholar] [CrossRef]
- Catenacci, V.A.; Pan, Z.; Ostendorf, D.; Brannon, S.; Gozansky, W.S.; Mattson, M.P.; Martin, B.; MacLean, P.S.; Melanson, E.L.; Troy Donahoo, W. A Randomized Pilot Study Comparing Zero-Calorie Alternate-Day Fasting to Daily Caloric Restriction in Adults with Obesity. Obesity 2016, 24, 1874–1883. [Google Scholar] [CrossRef]
- Hoddy, K.K.; Kroeger, C.M.; Trepanowski, J.F.; Barnosky, A.; Bhutani, S.; Varady, K.A. Meal Timing during Alternate Day Fasting: Impact on Body Weight and Cardiovascular Disease Risk in Obese Adults. Obesity 2014, 22, 2524–2531. [Google Scholar] [CrossRef]

| Periodontal Disease Parameters | Metabolic Disease Parameters | Study Design | Author & Year | The Outcome |
|---|---|---|---|---|
| PD, CAL, ABL, tooth Mobility | BP, TG, FPG, HDL, and WC. | Longitudinal study Study duration: 33 years sample size: 760 | Kaye et al., 2016 [72] | PD may be exacerbated or developed as a result of MetS. |
| PD and ABL | HDL, BP, WC, FPG, and TG, | Longitudinal study Study duration: 15 years Sample size: 1964 | Tegelberg et al., 2019 [73] | PD was linked to MetS in an exposure-dependent manner. |
| PD (CPI) | BMI, BP, TG, HDL, TC, and FPG. | Longitudinal study Study duration: 4 years Sample size: 1964 | Morita et al., 2010 [74] | PD was linked to greater conversion of MetS components. |
| CPI | BP, FPG, TG, HDL, and WC. | Longitudinal study Study duration: 2 years Sample size: 390 | Sakurai et al., 2019 [75] | Positive MetS components were more prevalent in those with progressive PD than in those without/improved PD. |
| BOP, PD, Plaque, Recession | CRP, FPG, TG, TC, LDL, Pregnancy, weight, BMI, BP, HbA1c, and HDL | Longitudinal study Study duration: 3 years Sample size: 188 | Bullon et al., 2009 [76] | PD and MetS are linked. |
| CPI | HDL, BP, WC, FPG, and TG. | Longitudinal study Study duration: 1 year Sample size: 136 | Adachi et al., 2020 [77] | The development of the MetS did not appear to be connected to periodontitis. |
| CAL, BOP, PD | TG, WC, FPG, HDL, and BP. | Longitudinal study Study duration: 8/16 year Sample size: 539 | Nascimento et al., 2019 [78] | MetS and PD showed a favourable link when latent variables were used to account for the many aspects of each disease. In terms of observable characteristics, MetS and PD were not linked. |
| CPI | HDL, and FPG Abd obesity, BP, and TG, | Longitudinal study Study duration: 3 years. Sample size:125 | Iwasaki et al., 2015 [79] | The MetS have been linked to a higher risk of PD in older Japanese adults. |
| PD, CAL, BOP, and PI | HDL, Abd obesity, FPG/or T2DM, TG, and BP | Case-control No of patients: 208 Age of the patients: 37 to 78 | Li et al., 2009 [80] | PD was known to be correlated with MetS even when other risk factors were treated in patients with the condition. |
| CPI | FPG, dyslipidemia, BP, and BMI | Cross-sectional No pf patients: 1315 Age of the patients: 30 to 92 | Borges et al., 2007 [81] | PD patients had a higher prevalence of MetS, although the difference was not statistically significant. |
| CAL, GI, PD, and PI | HDL, TG TC, BP, FPG, and WC | Case-control No of patients:156 Age of the patients: ≥25 or above | Khader et al., 2008 [82] | Compared to patients without MetS, patients with MetS had more frequent and severe periodontitis. |
| CAL and PD. | TG, BP, FPG, Abd obesity, and HDL | Case-control No of patients: 584 Age of the patients:40 to 79 | Shimazaki et al., 2007 [70] | MetS increases the risk of PD. |
| PD and BOP | HDL. BP, TG, WC, and insulin resistance | Cross-sectional No of patients: 13,677 Age of the patients: ≥17 | D’Aiuto et al., 2008 [83] | Severe PD has been connected to MetS in adults in their mid-twenties. |
| PD. | TG, HDL, FPG/or Med, Abd obesity and B.P./or Med. | Cross-sectional No of patients: 7431 Age of the patients:20 to 90 | Andriankaja et al., 2007 [84] | In females, this research revealed a substantial correlation between MetS and periodontitis. It was found that both sexes were affected by abdominal obesity as a metabolic factor. |
| CPI | TG, WC, BP, HDL. TC, FPG, BMI, and HbA1c. | Cross-sectional No of patients: 2478 Age of the patients: 24 to 60 | Morita et al., 2010 [74] | In Japanese employees aged 20 to 60, there was a link between periodontal disease and MetS. |
| CPI | BP, HDL, FPG, TG, and obesity | Cross-sectional No of patients: 1070 Age of the patients: 40 to 70 | Kushiyama et al., 2009 [85] | The more MetS components, the worse the situation. and the greater the risk of developing severe periodontitis. |
| CAL, PD, PI, and GI | BP, HDL, WC, TG, and FPG | Cross-sectional No of patients: 276 Age of the patients:35 to 74 | Benguigui et al., 2010 [86] | Diabetes and PD are linked, with insulin resistance playing a significant role. |
| PD | BP, Insulin resistance, dyslipidemia and Abd obesity | Cross-sectional No of patients: 20 & 50 Age of the patients: 30 to 64 | Timonen et al., 2010 [87] | Numerous components of the MetS were shown to be weakly linked to periodontal disease and dental caries in this research. |
| PD, BOP, and calculus | FPG, BP, Abd obesity, TG, and HDL | Cross-sectional No of patients: 1046 Age of the patients: ≥18 | Han et al., 2010 [88] | PD and MetS may be linked. Age, gender, and smoking all played a significant role. The MetS with elevated glucose and hypertension had a more substantial impact. |
| BOP, CAL, and PD | FNG, HDL, T.G., W.C., and B.P. | Cross-sectional No of patients: 2370 Age of the patients: 40 to 79 | Furuta et al., 2013 [89] | There appear to be gender disparities in PD and MetS. As a result of MetS, women may be more susceptible to developing PD compared to men |
| GI, PI, and PDI | WC, BP, TG, HDL, FPG, or T2DM and TC. | Cross-sectional No of patients: 253 Age of the patients: >18 | Chen et al., 2011 [90] | In haemodialysis patients, moderate-to-severe PD is linked to MetS. |
| ABL | TG, FPG, WC, and BP. | Cross-sectional No of patients: 190 Age of the patients: mean: 56.8 ± 12 | Nesbitt et al., 2010 [91] | Individuals with symptomatic PD had a 2.5 times higher chance of developing MetS. |
| CAL, and PD | BP, FPG, TG, and HDL and obesity | Cross-sectional No of patients: 6421 Age of the patients: 34 to 77 | Fukui et al., 2012 [92] | Periodontal health is linked to MetS, especially in people suspected of having an untreated, periodontal disease. |
| PD | BP, HDL, WC, FPG, and TG. | Cross-sectional No of patients: 7178 Age of the patients: ≥19 | Kwon et al., 2011 [93] | PD had a 1.55 odds ratio of being related to MetS. |
| PD, CAL, and ABL | TG, FPG or Med, HDL, Abd obesity, BP or Med, | Cross-sectional No of patients: 657 Age of the patients: 50 to 79 | LaMonte et al., 2014 [94] | In this group of postmenopausal women, there was no consistent relationship in terms of MetS and periodontitis. |
| CAL and PD. | BP, dyslipidemia, BP and WC | Cross-sectional No of patients: 234 Age of the patients: ≥80 | Minagawa et al., 2015 [95] | The researchers discovered a link between PD and MetS. |
| CAL, BOP, and PD | BP, HDL, WC, FPG, and TG. | Cross-sectional No of patients: 419 Age of the patients: 24 to 89 | Gomes-Filho et al., 2016 [96] | The findings of this study suggest that severe PD is associated with MetS and vice versa. |
| BOP GI, PI, PD, and CAL | BP Glucose tolerance TG, HDL, and BMI | Case-control No of patients: 651 Age of the patients: | Jaramillo et al., 2017 [97] | PD and MetS have a positive relationship. The adjusted odds ratio is 2.72. Glucose sensitivity is a strongly related factor. |
| CAL and PD. | TG, HDL, BMI, WC, BP, and FPG | Cross-sectional No of patients: 5078 Age of the patients: 50 to 94 | Kim et al., 2018 [98] | The MetS was shown to be more common among Korean people whose PD had worsened. |
| CAL, PI, BOP, and PD | TGs LDL, BP and/ or WC | Case-control No of patients: Case: 122 Controls: 366 Age of the patients: | Campos et al., 2020 [99] | There is a greater prevalence, severity, and development of PD among persons with MetS. |
| BOP, Plaque, PD, and CAL | BP, FPG (OGTT), HDL, TG, and Abd obesity | Cross-sectional No of patients: 283 Age of the patients: 26 to 87 | Sora et al., 2013 [100] | The MetS are associated with the severity of PD in this Gullah group of people with type 2 diabetes. |
| CAL, BOP, and PD | HDL, BP, FPG, WC, and TG, | Cross-sectional No of patients: 125 Age of the patients: 35 to 76 | Thanakun et al., 2014 [101] | Severe PD was connected to MetS in this Thai population. |
| CPI | FPG, HDL, Abd obesity, BP, and TG. | Cross-sectional No of patients: 125 Age of the patients: 35 to 76 | Chen et al., 2010 [88] | MetS was prevalent enough to be deemed a medical disorder, and it was associated with PD. |
| BOP. PI, GI, PD, and CAL | TG, BP HDL, FPG and WC | Cross sectional No of patients: 363 Age of the patients: 18 to 81 | Musskopf et al., 2017 [102] | PD and MetS have a weak relationship. The correlation is seen in people between the ages of 41 and 60. |
| CPI | HDL, TG and Med, FPG/and BP and Med | Cross-sectional No of patients: 1856 Age of the patients: mean: 66.4 | Kikui et al., 2017 [103] | PD is linked to MetS and low HDL cholesterol. PD was found to be more common in people who had two or more MetS components. |
| BOP, CAL, PI, GI, and PD | FPG, BMI, WC, HDL, and BP. | Cross-sectional No of patients: 412 Age of the patients: mean: 57.8 ± 5.7 | Pham et al., 2018 [104] | The severity and extent of PD raised with the number of MetS components. Periodontal variables were connected to increased MetS risk. |
| CPI | HDL TG, Obesity, BP and FPG | Cross-sectional No of patients: 1070 Age of the patients: 40 to 70 | Kushiyama et al., 2019 [85] | The more components of the MetS present, the greater the chance of developing severe PD. |
| CAL of ≥ 3mm and ≥4 teeth with ≥ 4mm | HDL, Abd obesity, TG, FPG and BP. | Longitudinal study Duration: 1 year No of patients: 165 Age of the patients: 35 to 65 | Lopez et al., 2012 [105] | MetS patients who underwent root planning, systemic antibiotics, plaque removal, and subgingival scaling after nine months had lower CRP levels. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parveen, S.; Alhazmi, Y.A. Impact of Intermittent Fasting on Metabolic Syndrome and Periodontal Disease—A Suggested Preventive Strategy to Reduce the Public Health Burden. Int. J. Environ. Res. Public Health 2022, 19, 14536. https://doi.org/10.3390/ijerph192114536
Parveen S, Alhazmi YA. Impact of Intermittent Fasting on Metabolic Syndrome and Periodontal Disease—A Suggested Preventive Strategy to Reduce the Public Health Burden. International Journal of Environmental Research and Public Health. 2022; 19(21):14536. https://doi.org/10.3390/ijerph192114536
Chicago/Turabian StyleParveen, Sameena, and Yaser Ali Alhazmi. 2022. "Impact of Intermittent Fasting on Metabolic Syndrome and Periodontal Disease—A Suggested Preventive Strategy to Reduce the Public Health Burden" International Journal of Environmental Research and Public Health 19, no. 21: 14536. https://doi.org/10.3390/ijerph192114536
APA StyleParveen, S., & Alhazmi, Y. A. (2022). Impact of Intermittent Fasting on Metabolic Syndrome and Periodontal Disease—A Suggested Preventive Strategy to Reduce the Public Health Burden. International Journal of Environmental Research and Public Health, 19(21), 14536. https://doi.org/10.3390/ijerph192114536






